Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure
Abstract
:1. Evolution of Artificial Illumination
2. The Functional Organization of the Human Circadian System
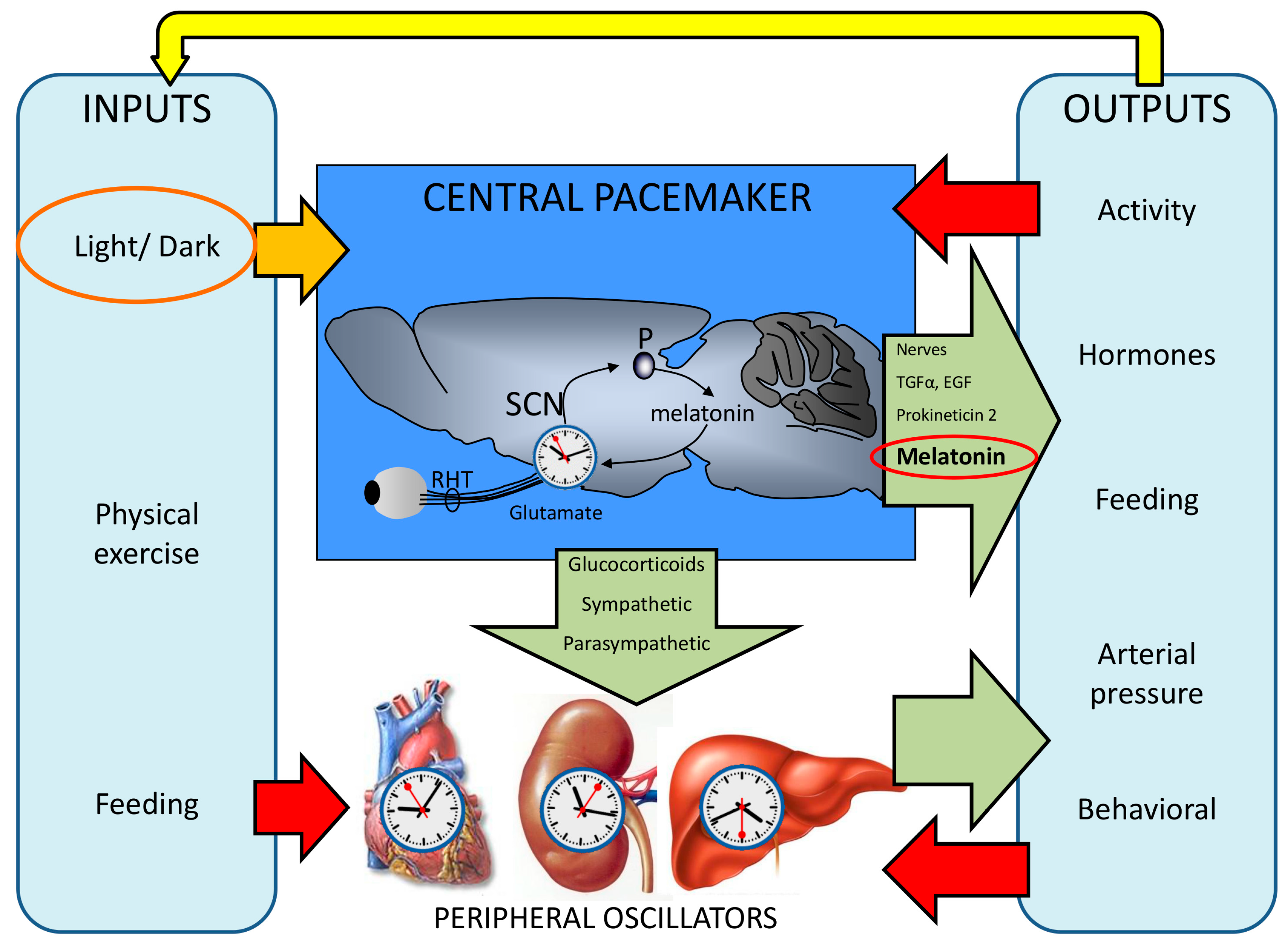
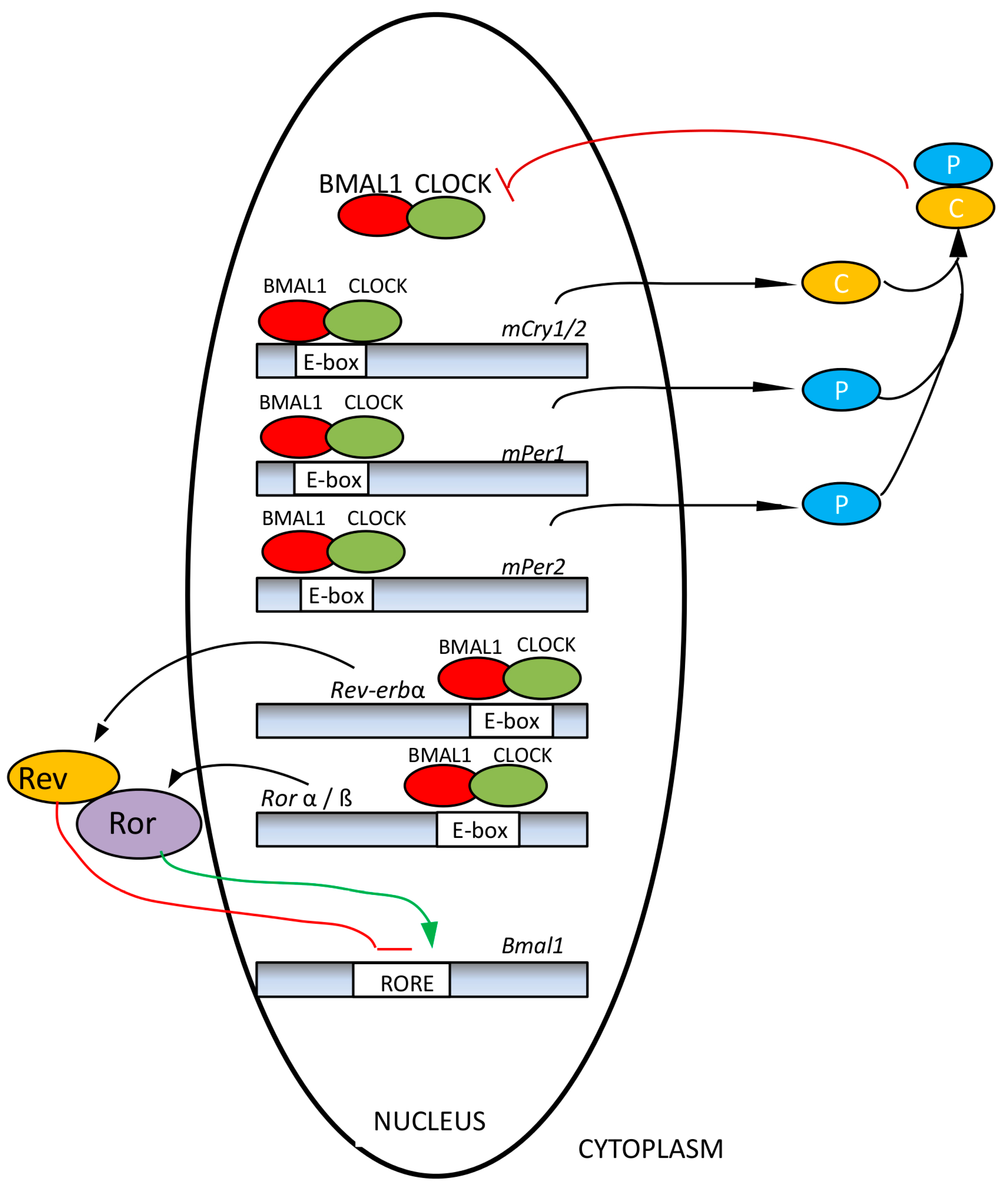
3. Influence of Unfavorable Illumination on Human Health
3.1. Latitudinal Influence on Light-Dark Cycle
3.2. Shift Work
3.3. Jet Lag/Social Jet Lag
3.4. Chronodisruption
4. Light Input Pathways
4.1. Intrinsically Photosensible Retinal Ganglion Cells (ipRGC)
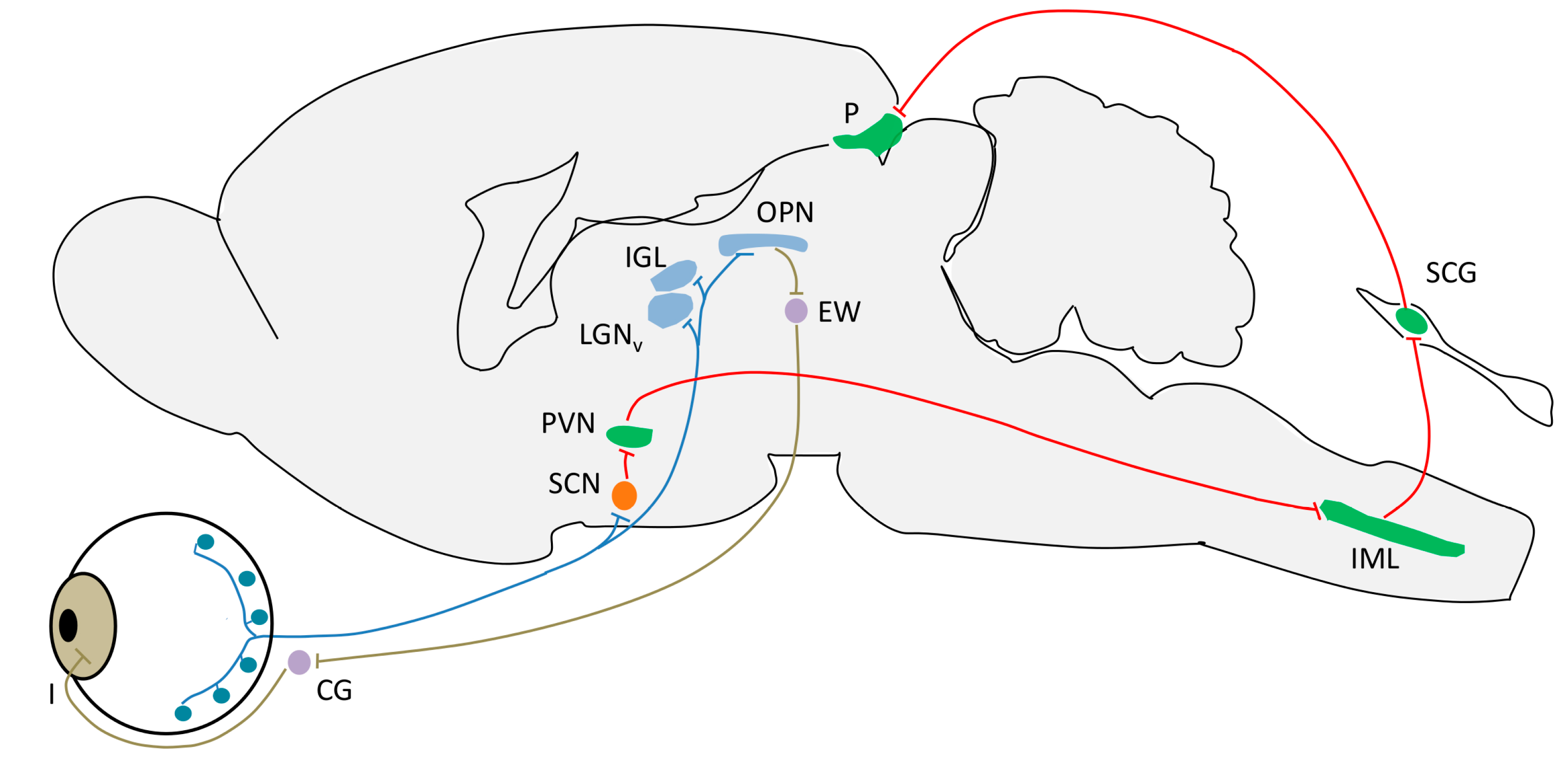
- Circadian photoentrainment. It is known that the circadian rhythm of “blind” patients that have lost image vision owing to rod/cone degeneration, but not ipRGCs, can still be photoentrained (and therefore can recover from jet lag, for example) [139,140]. Studies in melanopsin-null mice given a light pulse at the beginning of their rest or active period demonstrate the importance of ipRGCs in this process. The phase-shift induced was lower in these mice as compared to the wild-type, indicating that the contribution of rods and cones to this process is no higher than 50% [141].
- Negative masking. Light reduces locomotion in mice and other nocturnal mammals. This effect is called “negative masking” and it has been demonstrated that melanopsin is required for a maximal and sustained response. Low intensity light promotes a “positive masking” (increase of locomotion), and it has been demonstrated that while rods and cones drive positive masking at very low intensities (this could imply that the image-forming system helps guide locomotion, or it may simply be due to the rod to ipRGC connections), these photoreceptors, together with ipRGCs, drive negative masking at higher light intensities [142].
- Sleep regulation. In nocturnal rodents, a pulse of light during the dark period induces sleep and c-fos expression in the VLPO nucleus [143], a sleep promoting brain area, while a pulse of darkness administered during the light period can induce awakening [143,144]. Melanopsin-null mice lack these effects and they show perturbations in sleep homeostasis [143]. These findings can be applied to diurnal organisms, but light would promote awakening and darkness would facilitate sleep [145].
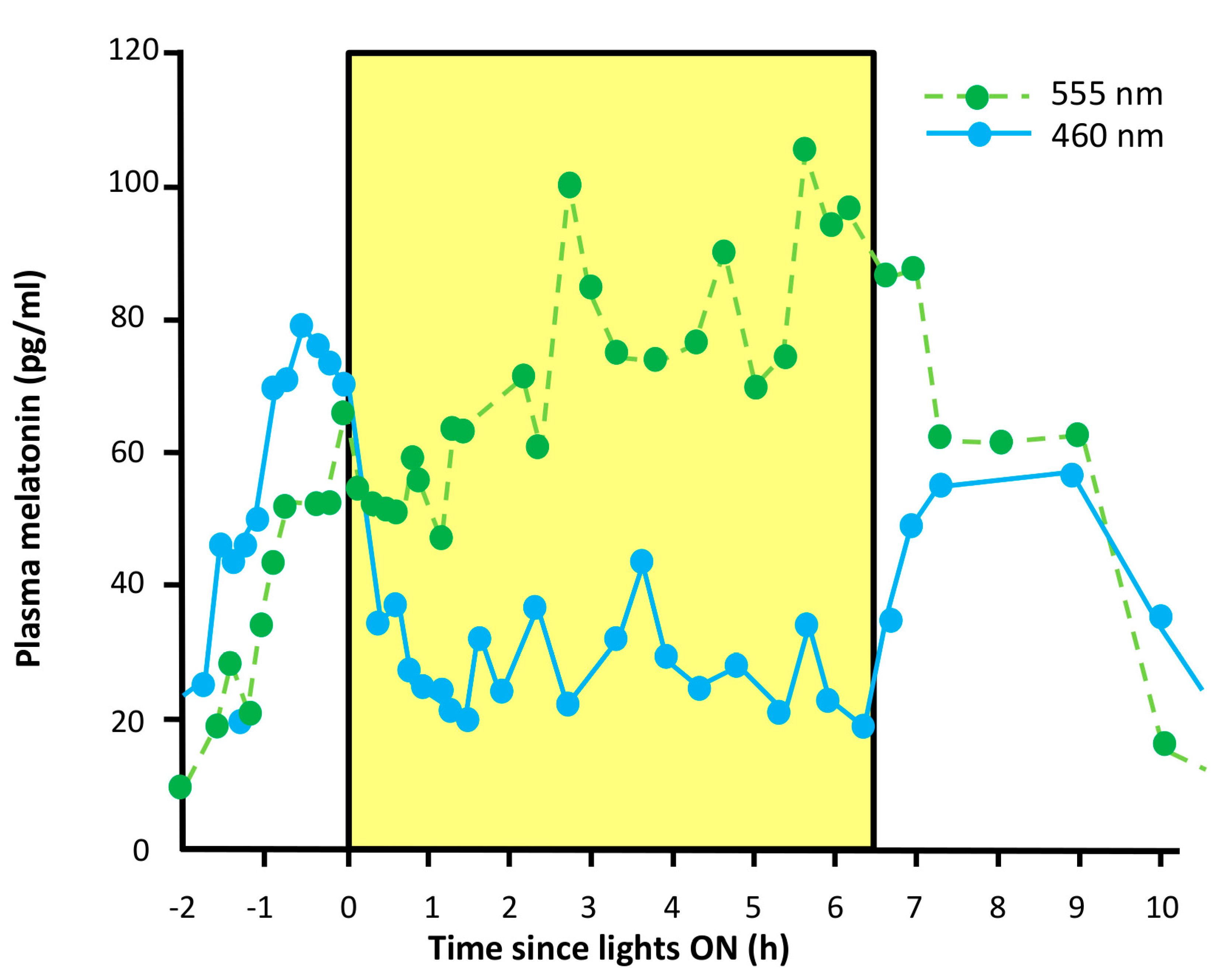
- Suppression of pineal melatonin. In rodless/coneless mice, melatonin suppression is complete under high light intensity [146,147], a process which requires melanopsin. Moreover, some people who suffer from blindness as the result of a severe loss of rods and cones also show this melatonin suppression, with a spectral sensitivity consistent with melanopsin signaling [148,149,150].
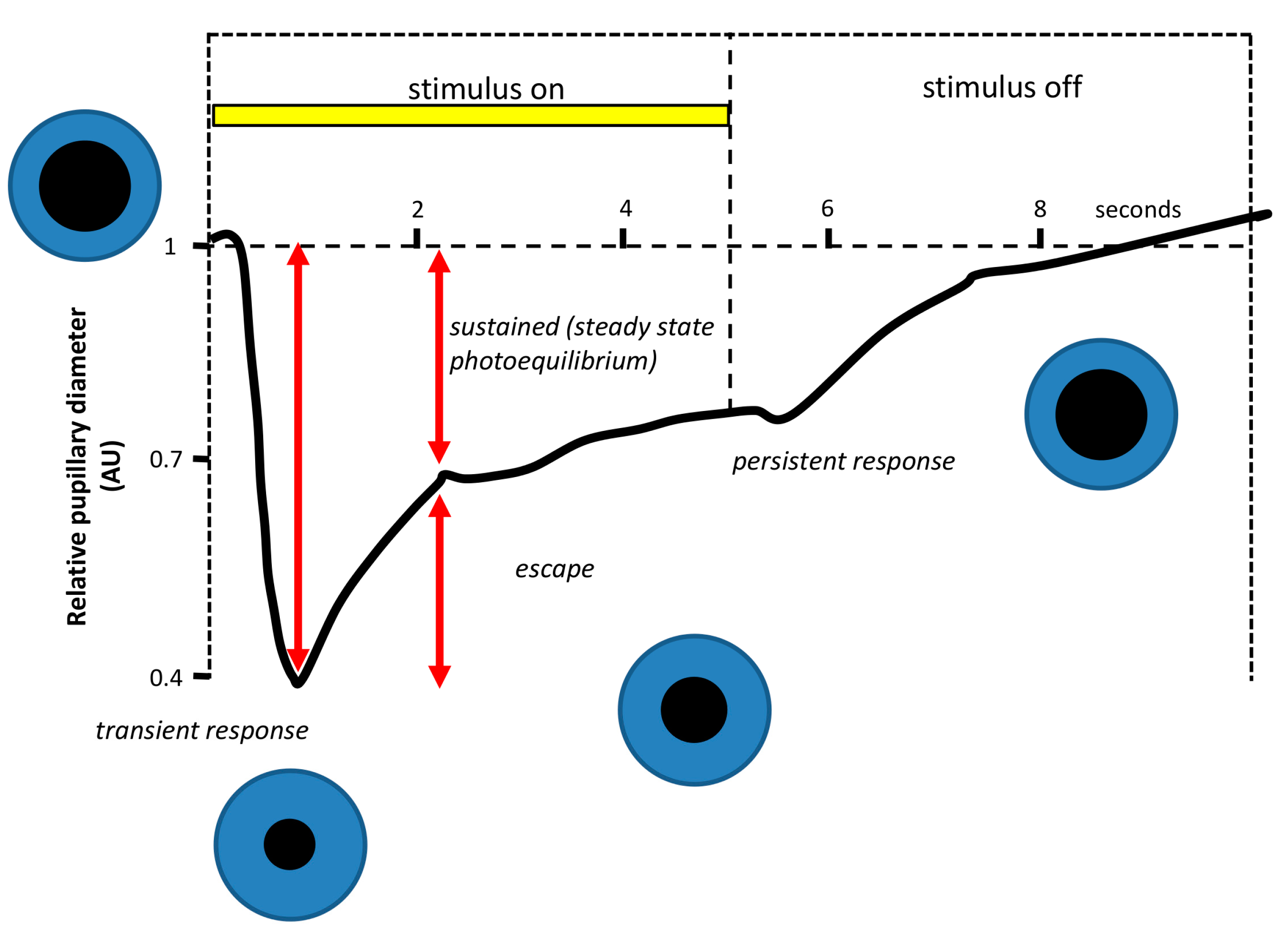
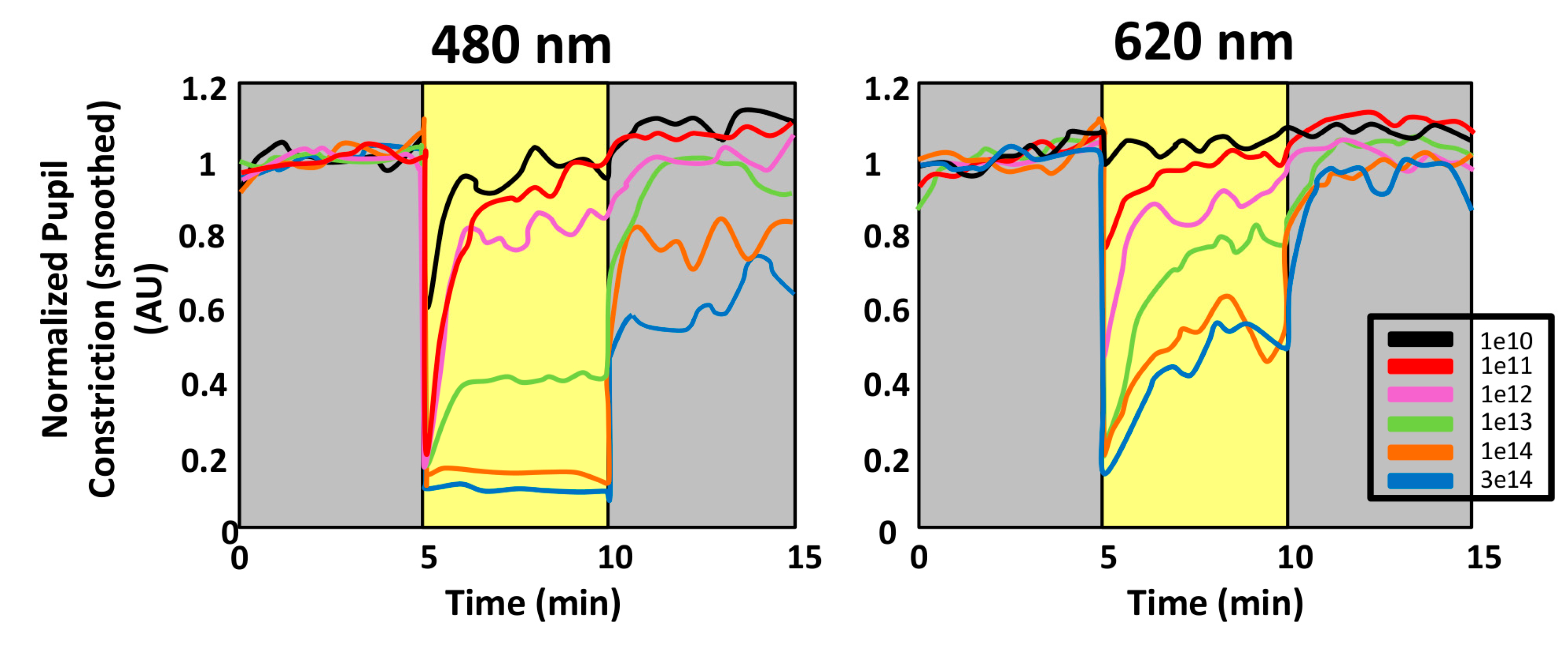
- Pupillary light reflex. The pupillary light reflex (PLR) allows reducing the rod and cone saturation by light, and improves resolution by increasing the depth of field. Because of its immediacy, the PLR is the most readily quantifiable behavior driven by ipRGCs. It is known that ipRGCs are necessary for reaching the maximal pupil constriction and for sustained constriction for long durations (perhaps to compensate for light adaptation in the rods and cones) [151]. In rodless/coneless mice, the PLR begins only in bright light, but it is driven until completion [146,152,153,154].
4.1.1. Why Is PLR a Reliable Method to Assess Photoreceptor Contribution?
4.1.2. Circadian Rhythmicity in the Retina: Its Role in the Circadian System
4.2. Daytime Light Exposure Effects Mediated by Skin
5. Output Pathways: Melatonin
6. Impaired Retinal Light Input
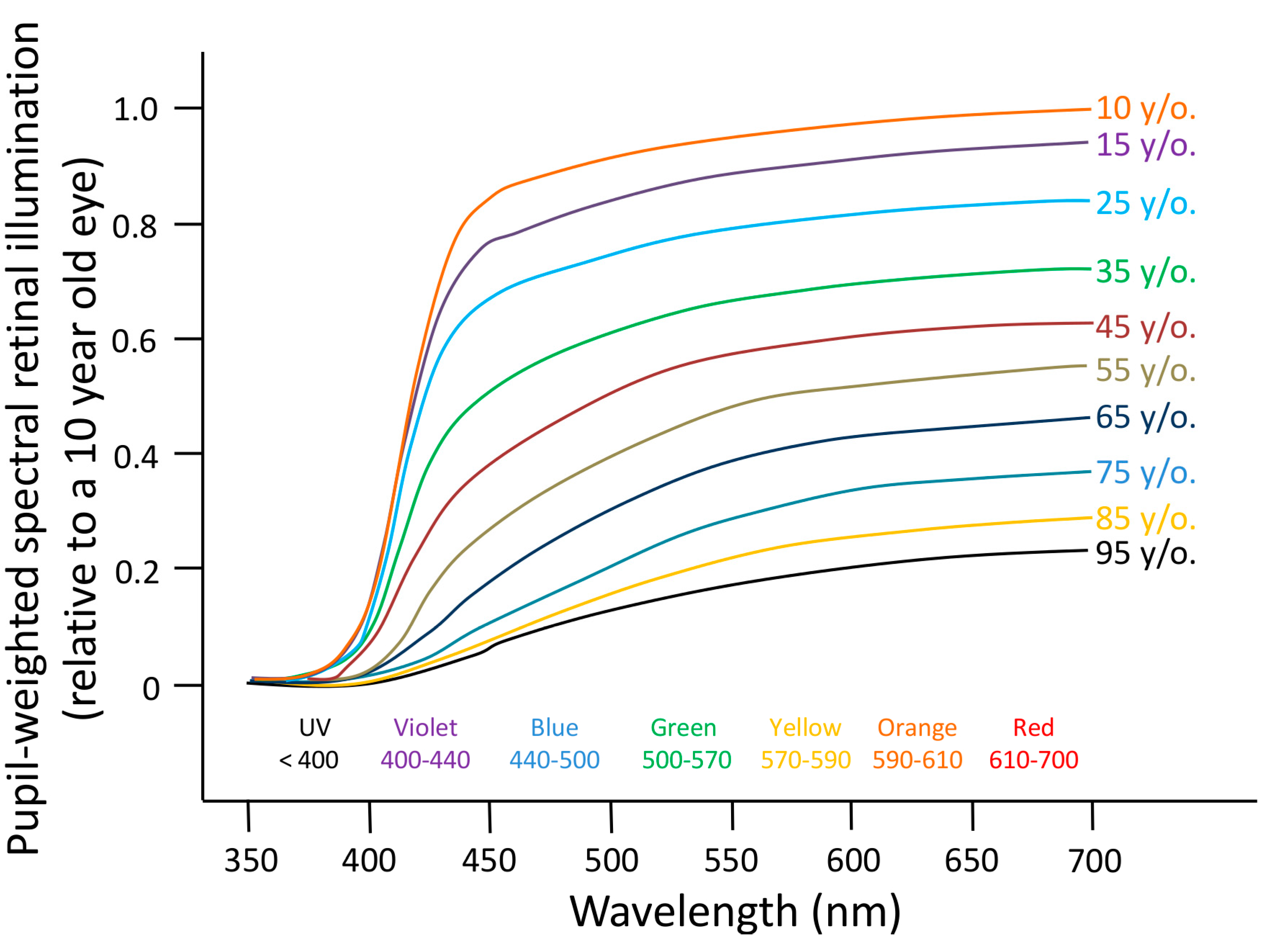
6.1. Aging
6.2. Blindness
- (a)
- Pupillary light reflex. Blind people with PCCP will retain the pupillary light reflex under short wavelength light stimulus.
- (b)
- Melatonin suppression. Totally blind people suffering from a PCCP will respond to a pulse of light with the suppression of melatonin production.
7. Circadian Healthy Light
8. Conclusions
- Humans have altered the natural light-dark cycle contrast by increasing light at night and spending most of their time indoors, with low light intensity exposure during the daytime.
- In order to maintain the health of our circadian system, appropriate lighting levels during the day should be recommended, even in certain cases of blindness (always under the supervision of an ophthalmologist).
- In addition, diurnal lighting should not be poor in wavelengths in the 460–480 nm range, since maximum human circadian spectral sensitivity occurs in this part of the spectrum.
- On the other hand, darkness during the night is desirable, and when illumination is a must, the abovementioned specifically active wavelengths should be avoided, shifting to a more reddish spectrum. Interestingly, bluish wavelengths are the ones that most interfere with astronomical observations, and “whiter” light is likely to increase the potential range of environmental impacts on other living organisms. Thus, reducing light pollution would have positive effects, not only on human health, but also in terms of cultural and environmental aspects.
- Since our recently acquired lifestyle habits seem to require illumination at night, new lighting technologies using the favorable spectrum and intensity should be developed to preserve circadian system functioning both at night and during the day inside buildings.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haim, A.; Portnov, B.A. Light Pollution as a New Risk Factor for Human Breast and Prostate Cancers; Springer Netherlands: Dordrecht, The Netherlands, 2013. [Google Scholar]
- De Beaune, S.A.; White, R. Ice Age Lamps. Sci. Am. 1993, 206, 108–113. [Google Scholar] [CrossRef]
- Nordhaus, W.D.; Nordhaus, W.D. Do real-output and real-wage measures capture reality? The history of lighting suggests not. In The Economics of New Goods; Brenahan, T.F., Gordon, J., Eds.; The University of Chicago Press: Chicago, IL, USA, 1994; pp. 27–70. [Google Scholar]
- Cinzano, P.; Falchi, F.; Elvidge, C.D. The first world atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001, 328, 689–707. [Google Scholar] [CrossRef]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Pauley, S.M. Lighting for the human circadian clock: Recent research indicates that lighting has become a public health issue. Med. Hypothes. 2004, 63, 588–596. [Google Scholar] [CrossRef]
- Mills, P.R.; Tomkins, S.C.; Schlangen, L.J.M. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J. Circadian Rhythm. 2007, 5, 2. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J. Light Hygiene: Time to make preventive use of insights -old and new- into the nexus of the drug light, melatonin, clocks, chronodisruption and public health. Med. Hypothes. 2009, 73, 537–541. [Google Scholar] [CrossRef]
- Roenneberg, T.; Daan, S.; Merrow, M. The art of entrainment. J. Biol. Rhythm. 2003, 18, 183–194. [Google Scholar] [CrossRef]
- Schmidt, C.; Collette, F.; Cajochen, C.; Peigneux, P. A time to think: Circadian rhythms in human cognition. Cogn. Neuropsychol. 2007, 24, 755–789. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Madrid, J.A. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv. Drug Deliv. Rev. 2010, 62, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B.; Takahashi, J.S.; Bass, J. The meter of metabolism. Cell 2008, 134, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, M.; Schibler, U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J. Biol. Rhythm. 2006, 21, 494–506. [Google Scholar] [CrossRef]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef] [PubMed]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 2013, 217, 3–27. [Google Scholar] [PubMed]
- Goriki, A.; Hatanaka, F.; Myung, J.; Kim, J.K.; Yoritaka, T.; Tanoue, S.; Abe, T.; Kiyonari, H.; Fujimoto, K.; Kato, Y.; et al. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol. 2014, 12, e1001839. [Google Scholar]
- Hardeland, R.; Madrid, J.A.; Tan, D.-X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.-K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, G. PPARs Integrate the mammalian clock and energy metabolism. PPAR Res. 2014, 2014, 653017. [Google Scholar] [PubMed]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, B.; Nakahata, Y.; Kaluzova, M.; Masubuchi, S.; Sassone-Corsi, P. Chromatin remodeling, metabolism and circadian clocks: The interplay of CLOCK and SIRT1. Int. J. Biochem. Cell Biol. 2009, 41, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, E.-K. AMP-activated protein kinase as a key molecular link between metabolism and clockwork. Exp. Mol. Med. 2013, 45, e33. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.S.; Boyault, C.; Knutti, D.; Padmanabhan, K.; Weitz, C.J. Identification of RACK1 and protein kinase Calpha as integral components of the mammalian circadian clock. Science 2010, 327, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Boo, K.; Kim, D.; Han, D.-H.; Choe, H.K.; Kim, C.R.; Sun, W.; Kim, H.; Kim, K.; Lee, H.; et al. Phosphorylation of LSD1 by PKCα is crucial for circadian rhythmicity and phase resetting. Mol. Cell 2014, 53, 791–805. [Google Scholar]
- Hardeland, R. Melatonin and circadian oscillators in aging—A dynamic approach to the multiply connected players. Interdisc. Top. Gerontol. 2014, 40, 128–140. [Google Scholar]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [PubMed]
- Yang, X.; Wood, P.A.; Ansell, C.M.; Quiton, D.F.T.; Oh, E.-Y.; Du-Quiton, J.; Hrushesky, W.J.M. The circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiol. Int. 2009, 26, 1323–1339. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Pelicano, H.; Liu, J.; Huang, P.; Lee, C.C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002, 111, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.A.; Yang, X.; Taber, A.; Oh, E.-Y.; Ansell, C.; Ayers, S.E.; Al-Assaad, Z.; Carnevale, K.; Berger, F.G.; Peña, M.M.O.; et al. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol. Cancer Res. 2008, 6, 1786–1793. [Google Scholar]
- Yang, X.; Wood, P.A.; Oh, E.-Y.; Du-Quiton, J.; Ansell, C.M.; Hrushesky, W.J.M. Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res. Treat. 2009, 117, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Mullenders, J.; Fabius, A.W.M.; Madiredjo, M.; Bernards, R.; Beijersbergen, R.L. A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS One 2009, 4, e4798. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-H.; Kim, E.M.; Park, J.K.; Hwang, S.-G.; Moon, S.-K.; Kim, W.-J.; Um, H.-D. Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol. Rep. 2013, 29, 2109–2113. [Google Scholar] [PubMed]
- Chini, C.C.S.; Escande, C.; Nin, V.; Chini, E.N. DBC1 (Deleted in Breast Cancer 1) modulates the stability and function of the nuclear receptor Rev-erbα. Biochem. J. 2013, 451, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Dacey, D.M.; Liao, H.-W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.-W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Jusuf, P.R.; Lee, S.C.S.; Hannibal, J.; Grünert, U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur. J. Neurosci. 2007, 26, 2906–2921. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W.; Riemersma-van Der Lek, R.F. Live to the rhythm, slave to the rhythm. Sleep Med. Rev. 2007, 11, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.; Cheng, M.Y. Prokineticin 2 and circadian clock output. FEBS J. 2005, 272, 5703–5709. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; la Fleur, S.E.; Wortel, J.; van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Neural control of the pineal gland. Behav. Brain Res. 1996, 73, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Poeggeler, B.; Balzer, I.; Behrmann, G. A hypothesis on the evolutionary origins of photoperiodism based on circadian rhythmicity of melatonin in phylogenetically distant organisms. In Chronobiology & Chronomedicine; Gutenbrunner, C., Hildebrandt, G., Moog, R., Eds.; Lang: Frankfurt, Germany, 1993; pp. 113–120. [Google Scholar]
- La Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buijs, R.M. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000, 871, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Chun, S.J.; Niijima, A.; Romijn, H.J.; Nagai, K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 2001, 431, 405–423. [Google Scholar] [PubMed]
- Kalsbeek, A.; Fliers, E.; Franke, A.N.; Wortel, J.; Buijs, R.M. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 2000, 141, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, R.C.; Kripke, D.F.; Ancoli-Israel, S.; Mowen, M.A.; Mason, W.J.; Fell, R.L.; Klauber, M.R.; Kaplan, O.J. Low illumination experienced by San Diego adults: Association with atypical depressive symptoms. Biol. Psychiatry 1994, 35, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Hébert, M.; Dumont, M.; Paquet, J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol. Int. 1998, 15, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Okawa, M.; Shimizu, T.; Hishikawa, Y. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. J. Clin. Endocrinol. Metab. 2001, 86, 129–134. [Google Scholar] [PubMed]
- Savides, T.J.; Messin, S.; Senger, C.; Kripke, D.F. Natural light exposure of young adults. Physiol. Behav. 1986, 38, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nicolas, A.; Ortiz-Tudela, E.; Madrid, J.A.; Rol, M.A. Crosstalk between environmental light and internal time in humans. Chronobiol. Int. 2011, 28, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Bishop, L.; Luke, C.; Middleton, B.; Williams, P.; Arendt, J. Sleep during the Antarctic winter: Preliminary observations on changing the spectral composition of artificial light. J. Sleep Res. 2008, 17, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Mottram, A.R.; Svenson, J.E. Rhythm disturbances. Emerg. Med. Clin. N. Am. 2011, 29, 729–746. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Korkmaz, A.; Erren, T.C.; Piekarski, C.; Tamura, H.; Manchester, L.C. Light at night, chronodisruption, melatonin suppression, and cancer risk: A review. Crit. Rev. Oncog. 2007, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J. Biological rhythms during residence in polar regions. Chronobiol. Int. 2012, 29, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, K.A.; Reiter, R.J. Melatonin rhythms in Arctic urban residents. J. Pineal Res. 1994, 16, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Piekarski, C. Does winter darkness in the Artic protect against cancer? The melatonin hypothesis revisited. Med. Hypothes. 1999, 53, 1–5. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Rodriguez, C.; Tan, D.X.; Lopez-Burillo, S.; Reiter, R.J. Melatonin and cell death: Differential actions on apoptosis in normal and cancer cells. Cell Mol. Life Sci. 2003, 60, 1407–1426. [Google Scholar] [CrossRef] [PubMed]
- Leon-Blanco, M.M.; Guerrero, J.M.; Reiter, R.J.; Calvo, J.R.; Pozo, D. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J. Pineal Res. 2003, 35, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Mascia, F.; Gualano, L.; di Bella, L. Melatonin anticancer effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, J.; Leja-Szpak, A. Melatonin influences pancreatic cancerogenesis. Histol. Histopathol. 2014, 29, 423–431. [Google Scholar] [PubMed]
- Cutando, A.; López-Valverde, A.; de Vicente, J.; Gimenez, J.L.; Carcía, I.A.; de Diego, R.G. Action of melatonin on squamous cell carcinoma and other tumors of the oral cavity (Review). Oncol. Lett. 2014, 7, 923–926. [Google Scholar] [PubMed]
- Rosenthal, N.E.; Sack, D.A.; Gillin, J.C.; Lewy, A.J.; Goodwin, F.K.; Davenport, Y.; Mueller, P.S.; Newsome, D.A.; Wehr, T.A. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch. Gen. Psychiatry 1984, 41, 72–80. [Google Scholar]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; el Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, F.C.; Rao, A.; Maguire, A. Circadian molecular clocks and cancer. Cancer Lett. 2014, 342, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Paquet, J. Progressive decrease of melatonin production over consecutive days of simulated night work. Chronobiol. Int. 2014, 15, 1–8. [Google Scholar]
- Erren, T.C.; Reiter, R.J. Defining chronodisruption. J. Pineal Res. 2009, 46, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Mirick, D.K.; Stevens, R.G. Night shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 2001, 93, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Kroenke, C.H.; Laden, F.; Hankinson, S.E. Night work and risk of breast cancer. Epidemiology 2006, 17, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Reiter, R.J. A generalized theory of carcinogenesis due to chronodisruption. Neuro Endocrinol. Lett. 2008, 29, 815–21. [Google Scholar] [PubMed]
- Schernhammer, E.S.; Speizer, F.E.; Walter, C.; Hunter, D.J.; Fuchs, C.S.; Colditz, G.A. Night-shift work and risk of colorectal cancer in the nurses’ health study. J. Natl. Cancer Inst. Cancer Inst. 2003, 95, 825–828. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Hankinson, S.E.; Schernhammer, E.S. Night shift work and the risk of endometrial cancer. Cancer Res. 2007, 67, 10618–10622. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, R. Chronodisruption, cell cycle checkpoints and DNA repair. Indian J. Exp. Biol. 2014, 52, 399–403. [Google Scholar] [PubMed]
- Ben-Shlomo, R.; Kyriacou, C.P. Light pulses administered during the circadian dark phase alter expression of cell cycle associated transcripts in mouse brain. Cancer Genet. Cytogenet. 2010, 197, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Escribano, B.M.; Moreno, A.; Tasset, I.; Túnez, I. Impact of light/dark cycle patterns on oxidative stress in an adriamycin-induced nephropathy model in rats. PLoS One 2014, 9, e97713. [Google Scholar] [CrossRef] [PubMed]
- Megdal, S.P.; Kroenke, C.H.; Laden, F.; Pukkala, E.; Schernhammer, E.S. Night work and breast cancer risk: A systematic review and meta-analysis. Eur. J. Cancer 2005, 41, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G. Electric power use and breast cancer: A hypothesis. Am. J. Epidemiol. 1987, 125, 556–561. [Google Scholar] [PubMed]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.E.; Yamazaki, S.; Pendergast, J.S.; Landry, G.J.; Takumi, T.; Nakamura, W. Comment on “Differential rescue of light- and food-entrainable circadian rhythms”. Science 2008, 322, 675. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.M.F.; Abreu, A.C.B.; Barbosa, M.F.dos S.; Teixeira, J.E.M. The relationship between shift work and sleep patterns in nurses. Cien. Saude Colet. 2013, 18, 763–768. [Google Scholar]
- Aasm International Classification of Sleep Disorders: Diagnostic and Coding Manual. (ICSD-2); Thorpy, M.J. (Ed.) American Academy of Sleep Medicine (Publisher): Westchester, NY, USA, 2005.
- Winget, C.M.; DeRoshia, C.W.; Markley, C.L.; Holley, D.C. A review of human physiological and performance changes associated with desynchronosis of biological rhythms. Aviat. Space Environ. Med. 1984, 55, 1085–1096. [Google Scholar] [PubMed]
- Waterhouse, J.; Reilly, T.; Atkinson, G.; Edwards, B. Jet lag: Trends and coping strategies. Lancet 2007, 369, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Melatonin and its relevance to jet lag. Travel Med. Infect. Dis. 2009, 7, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.; Rea, M.S.; Plitnick, B.; Figueiro, M.G. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl. Ergon. 2013, 44, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Lanaj, K.; Johnson, R.E.; Barnes, C.M. Beginning the workday yet already depleted? Consequences of late-night smartphone use and sleep. Organ. Behav. Hum. Decis. Process. 2014, 124, 11–23. [Google Scholar] [CrossRef]
- Matricciani, L.; Olds, T.; Petkov, J. In search of lost sleep: Secular trends in the sleep time of school-aged children and adolescents. Sleep Med. Rev. 2012, 16, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, J. A newly proposed disease condition produced by light exposure during night: Asynchronization. Brain Dev. 2009, 31, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Z.; Jia, C.; Buysse, D.J. Sleep patterns and problems among chinese adolescents. Pediatrics 2008, 121, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Roberts, C.R.; Chan, W. Persistence and change in symptoms of insomnia among adolescents. Sleep 2008, 31, 177–184. [Google Scholar] [PubMed]
- García-Jiménez, M.A.; Salcedo-Aguilar, F.; Rodríguez-Almonacid, F.M.; Redondo-Martínez, M.P.; Monterde-Aznar, M.L.; Marcos-Navarro, A.I.; Torrijos-Martínez, M.P. The prevalence of sleep disorders among adolescents in Cuenca, Spain. Rev. Neurol. 2004, 39, 18–24. [Google Scholar] [PubMed]
- Calamaro, C.J.; Mason, T.B.A.; Ratcliffe, S.J. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics 2009, 123, e1005–e1010. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.I.; Gazda, C.J.; Burgess, H.J.; Crowley, S.J.; Fogg, L.F. Advancing circadian rhythms before eastward flight: A strategy to prevent or reduce jet lag. Sleep 2005, 28, 33–44. [Google Scholar] [PubMed]
- Houpt, T.A.; Boulos, Z.; Moore-Ede, M.C. MidnightSun: Software for determining light exposure and phase-shifting schedules during global travel. Physiol. Behav. 1996, 59, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Revell, V.L.; Eastman, C.I. How to trick mother nature into letting you fly around or stay up all night. J. Biol. Rhythm. 2005, 20, 353–365. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Bortman, G.P.; Liotta, G.; Pérez Lloret, S.; Albornoz, L.E.; Cutrera, R.A.; Batista, J.; Ortega Gallo, P. A multifactorial approach employing melatonin to accelerate resynchronization of sleep-wake cycle after a 12 time-zone westerly transmeridian flight in elite soccer athletes. J. Pineal Res. 2002, 32, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Brun, J.; David, M.; Sassolas, G.; Chazot, G. Melatonin and jet lag: Confirmatory result using a simplified protocol. Biol. Psychiatry 1992, 32, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Skene, D.J.; Middleton, B.; Lockley, S.W.; Deacon, S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J. Biol. Rhythm. 1997, 12, 604–617. [Google Scholar] [CrossRef]
- Chen, S.-K.; Badea, T.C.; Hattar, S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 2011, 476, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.; Dawson, A.G.; Thompson, L.; Brook, R. A double-blind trial of melatonin as a treatment for jet lag in international cabin crew. Biol. Psychiatry 1993, 33, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Herxheimer, A.; Petrie, K.J. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst. Rev. 2002. [Google Scholar] [CrossRef]
- Vinzio, S.; Ruellan, A.; Perrin, A.-E.; Schlienger, J.-L.; Goichot, B. Actigraphic assessment of the circadian rest-activity rhythm in elderly patients hospitalized in an acute care unit. Psychiatry Clin. Neurosci. 2003, 57, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Missildine, K.; Bergstrom, N.; Meininger, J.; Richards, K.; Foreman, M.D. Sleep in hospitalized elders: A pilot study. Geriatr. Nurs. 2010, 31, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Boyko, Y.; Ording, H.; Jennum, P. Sleep disturbances in critically ill patients in ICU: How much do we know? Acta Anaesthesiol. Scand. 2012, 56, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Ramos, M.I.; Arroyo-Novoa, C.M.; Lee, K.A.; Padilla, G.; Puntillo, K.A. Sleep and delirium in ICU patients: A review of mechanisms and manifestations. Intensiv. Care Med. 2009, 35, 781–795. [Google Scholar] [CrossRef]
- Perras, B.; Meier, M.; Dodt, C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensiv. Care Med. 2007, 33, 1954–1958. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J. Revisiting chronodisruption: When the physiological nexus between internal and external times splits in humans. Naturwissenschaften 2013, 100, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.Á,; Madrid, J.A. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar]
- Ortiz-Tudela, E.; Iurisci, I.; Beau, J.; Karaboue, A.; Moreau, T.; Rol, M.A.; Madrid, J.A.; Lévi, F.; Innominato, P.F. The circadian rest-activity rhythm, a potential safety pharmacology endpoint of cancer chemotherapy. Int. J. Cancer 2014, 134, 2717–2225. [Google Scholar] [CrossRef] [PubMed]
- Zornoza-Moreno, M.; Fuentes-Hernández, S.; Sánchez-Solis, M.; Rol, M.Á.; Larqué, E.; Madrid, J.A. Assessment of circadian rhythms of both skin temperature and motor activity in infants during the first 6 months of life. Chronobiol. Int. 2011, 28, 330–337. [Google Scholar]
- Corbalán-Tutau, M.D.; Madrid, J.A.; Ordovás, J.M.; Smith, C.E.; Nicolás, F.; Garaulet, M. Differences in daily rhythms of wrist temperature between obese and normal-weight women: Associations with metabolic syndrome features. Chronobiol. Int. 2011, 28, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Falaturi, P.; Reiter, R.J. Research into the chronodisruption-cancer theory: The imperative for causal clarification and the danger of causal reductionism. Neuro Endocrinol. Lett. 2010, 31, 1–3. [Google Scholar] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Korkmaz, A.; Ma, S. Obesity and metabolic syndrome: Association with chronodisruption, sleep deprivation, and melatonin suppression. Ann. Med. 2012, 44, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Knutsson, A.; Bøggild, H. Shiftwork and cardiovascular disease: Review of disease mechanisms. Rev. Environ. Health 2000, 15, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Ennaceur, A.; Cole, J.C.; Suh, C.K. Chronic jet lag produces cognitive deficits. J. Neurosci. 2000, 20, RC66. [Google Scholar] [PubMed]
- Tri Hoang Do, M.; Yau, K. Intrinsically Photosensitive Retinal Ganglion Cells. Physiol. Rev. 2010, 90, 1547–1581. [Google Scholar] [CrossRef] [PubMed]
- McDougal, D.H.; Gamlin, P.D. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010, 50, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.W.; Kamp, C.W. Dopaminergic amacrine neurons of rat retinas with photoreceptor degeneration continue to respond to light. Life Sci. 1980, 26, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Shanahan, T.L.; Klerman, E.B.; Martens, H.; Brotman, D.J.; Emens, J.S.; Klein, T.; Rizzo, J.F. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 1995, 332, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Foster, R.G. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology 1999, 140, 1520–1524. [Google Scholar] [PubMed]
- Freedman, M.S.; Lucas, R.J.; Soni, B.; von Schantz, M.; Muñoz, M.; David-Gray, Z.; Foster, R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 1999, 284, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Douglas, R.H.; Foster, R.G. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 2001, 4, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Jiang, G.; de Grip, W.J.; Hayes, W.P.; Rollag, M.D. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA 1998, 95, 340–5. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A novel human opsin in the inner retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [PubMed]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Belenky, M.A.; Smeraski, C.A.; Provencio, I.; Sollars, P.J.; Pickard, G.E. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J. Comp. Neurol. 2003, 460, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Fain, G.L.; Hardie, R.; Laughlin, S.B. Phototransduction and the evolution of photoreceptors. Curr. Biol. 2010, 20, R114–R124. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.-W.; Hardie, R.C. Phototransduction motifs and variations. Cell 2009, 139, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J.; Falktoft, B.; Georg, B.; Rask, L. N-linked deglycosylated melanopsin retains its responsiveness to light. Biochemistry 2009, 48, 5142–5148. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Kubokawa, K.; Tsukamoto, H.; Shichida, Y.; Terakita, A. Cephalochordate melanopsin: Evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 2005, 15, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S.; Cornut, P.-L.; Rieux, C.; Drouyer, E.; Denis, P.; Gronfier, C.; Cooper, H.M. Melanopsin bistability: A fly’s eye technology in the human retina. PLoS One 2009, 4, e5991. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Lu, J.; Fischer, D.; Saper, C.B. A broad role for melanopsin in nonvisual photoreception. J. Neurosci. 2003, 23, 7093–7106. [Google Scholar] [PubMed]
- Hannibal, J.; Fahrenkrug, J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 2004, 316, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.-W.; Berson, D.M. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M. Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci. 2003, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Klerman, E.B.; Shanahan, T.L.; Brotman, D.J.; Rimmer, D.W.; Emens, J.S.; Rizzo, J.F.; Czeisler, C.A. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J. Biol. Rhythm. 2002, 17, 548–555. [Google Scholar] [CrossRef]
- Zaidi, F.H.; Hull, J.T.; Peirson, S.N.; Wulff, K.; Aeschbach, D.; Gooley, J.J.; Brainard, G.C.; Gregory-Evans, K.; Rizzo, J.F.; Czeisler, C.A.; et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr. Biol. 2007, 17, 2122–2128. [Google Scholar]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Foster, R.G.; Stone, E.M.; Sheffield, V.C.; Mrosovsky, N. Classical and melanopsin photoreception in irradiance detection: Negative masking of locomotor activity by light. Eur. J. Neurosci. 2008, 27, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.W.; Hannibal, J.; Hagiwara, G.; Colas, D.; Ruppert, E.; Ruby, N.F.; Heller, H.C.; Franken, P.; Bourgin, P. Melanopsin as a sleep modulator: Circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4 (−/−) mice. PLoS Biol. 2009, 7, e1000125. [Google Scholar] [CrossRef] [PubMed]
- Altimus, C.M.; Güler, A.D.; Villa, K.L.; McNeill, D.S.; Legates, T.A.; Hattar, S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc. Natl. Acad. Sci. USA 2008, 105, 19998–20003. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Piggins, H.D. Electrophysiology of the suprachiasmatic circadian clock. Prog. Neurobiol. 2007, 82, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Provencio, I.; Tu, D.C.; Pires, S.S.; Rollag, M.D.; Castrucci, A.M.; Pletcher, M.T.; Sato, T.K.; Wiltshire, T.; Andahazy, M.; et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 2003, 301, 525–527. [Google Scholar]
- Lucas, R.J.; Freedman, M.S.; Muñoz, M.; Garcia-Fernández, J.M.; Foster, R.G. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 1999, 284, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Sliney, D.; Hanifin, J.P.; Glickman, G.; Byrne, B.; Greeson, J.M.; Jasser, S.; Gerner, E.; Rollag, M.D. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J. Biol. Rhythm. 2008, 23, 379–386. [Google Scholar] [CrossRef]
- Lockley, S.W.; Brainard, G.C.; Czeisler, C.A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003, 88, 4502–4505. [Google Scholar] [CrossRef] [PubMed]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tu, D.C.; Denner, D.; Shane, T.; Fitzgerald, C.M.; van Gelder, R.N. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1268–1275. [Google Scholar] [CrossRef]
- Lucas, R.J.; Hattar, S.; Takao, M.; Berson, D.M.; Foster, R.G.; Yau, K.-W. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 2003, 299, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Semo, M.; Peirson, S.; Lupi, D.; Lucas, R.J.; Jeffery, G.; Foster, R.G. Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. Eur. J. Neurosci. 2003, 17, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.R.; Appleford, J.M.; Sekaran, S.; Chinthapalli, K.; Jenkins, A.; Seeliger, M.; Biel, M.; Humphries, P.; Douglas, R.H.; Wenzel, A.; et al. Residual photosensitivity in mice lacking both rod opsin and cone photoreceptor cyclic nucleotide gated channel 3 alpha subunit. Vis. Neurosci. 2004, 21, 675–683. [Google Scholar]
- Kawasaki, A.; Kardon, R.H. Intrinsically photosensitive retinal ganglion cells. J. Neuroophthalmol. 2007, 27, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Mien, I.H.; Hilaire, M.A.S.; Yeo, S.; Chua, E.C.; Reen, E.; van Hanley, C.J.; Hull, J.T.; Czeisler, C.A.; Lockley, S.W. Melanopsin and rod—cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J. Neurosci. 2012, 32, 14242–14253. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nelms, J.L.; Nguyen, M.; Silver, R.; Lehman, M.N. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat. Neurosci. 2003, 6, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ribelayga, C.P. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One 2012, 7, e50602. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Wong, K.Y.; Berson, D.M. Circadian modulation of melanopsin-driven light response in rat ganglion-cell photoreceptors. J. Biol. Rhythm. 2009, 24, 391–402. [Google Scholar] [CrossRef]
- Van Hook, M.J.; Wong, K.Y.; Berson, D.M. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur. J. Neurosci. 2012, 35, 507–518. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.G.; Iuvone, P.M.; Tosini, G. Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog. Retin. Eye Res. 2014, 39, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Menaker, M. The pineal complex and melatonin affect the expression of the daily rhythm of behavioral thermoregulation in the green iguana. J. Comp. Physiol. A 1996, 179, 135–142. [Google Scholar] [CrossRef]
- Tosini, G.; Menaker, M. The clock in the mouse retina: Melatonin synthesis and photoreceptor degeneration. Brain Res. 1998, 789, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Besharse, J.C.; Iuvone, P.M. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature 1983, 305, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Nir, I.; Haque, R.; Iuvone, P.M. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000, 870, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.E.; McIvor, W.E.; Menaker, M. Circadian rhythmicity in dopamine content of mammalian retina: Role of the photoreceptors. J. Neurochem. 2002, 83, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.E.; Grace, M.S.; McIvor, W.; Menaker, M. Circadian rhythms of dopamine in mouse retina: The role of melatonin. Vis. Neurosci. 2002, 19, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Jaliffa, C.O.; Saenz, D.; Resnik, E.; Keller Sarmiento, M.I.; Rosenstein, R.E. Circadian activity of the GABAergic system in the golden hamster retina. Brain Res. 2001, 912, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.R.; Hattar, S.; Hankins, M.W.; Lucas, R.J. Melanopsin regulates visual processing in the mouse retina. Curr. Biol. 2006, 16, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Storch, K.-F.; Paz, C.; Signorovitch, J.; Raviola, E.; Pawlyk, B.; Li, T.; Weitz, C.J. Intrinsic circadian clock of the mammalian retina: Importance for retinal processing of visual information. Cell 2007, 130, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Teirstein, P.S.; Goldman, A.I.; O’Brien, P.J. Evidence for both local and central regulation of rat rod outer segment disc shedding. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1268–1273. [Google Scholar]
- Von Schantz, M.; Lucas, R.J.; Foster, R.G. Circadian oscillation of photopigment transcript levels in the mouse retina. Brain Res. Mol. Brain Res. 1999, 72, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.; Organisciak, D.; Wong, P. Factors underlying circadian dependent susceptibility to light induced retinal damage. Adv. Exp. Med. Biol. 2006, 572, 411–416. [Google Scholar] [PubMed]
- Organisciak, D.T.; Darrow, R.M.; Barsalou, L.; Kutty, R.K.; Wiggert, B. Circadian-dependent retinal light damage in rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3694–3701. [Google Scholar]
- Ogilvie, J.M.; Speck, J.D. Dopamine has a critical role in photoreceptor degeneration in the rd mouse. Neurobiol. Dis. 2002, 10, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Pozdeyev, N.; Mazzoni, F.; Contreras-Alcantara, S.; Liu, C.; Kasamatsu, M.; Martinez-Merlos, T.; Strettoi, E.; Iuvone, P.M.; Tosini, G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 15043–1508. [Google Scholar] [CrossRef] [PubMed]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Sancar, A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc. Natl. Acad. Sci. USA 1998, 95, 6097–6102. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.-X.; Zhang, D.-Q.; Zhou, T.; Yamazaki, S.; McMahon, D.G. Circadian organization of the mammalian retina. Proc. Natl. Acad. Sci. USA 2006, 103, 9703–9708. [Google Scholar] [CrossRef] [PubMed]
- Dorenbos, R.; Contini, M.; Hirasawa, H.; Gustincich, S.; Raviola, E. Expression of circadian clock genes in retinal dopaminergic cells. Vis. Neurosci. 2007, 24, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Iuvone, P.M. Melatonin synthesis in chicken retina: Effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci. Lett. 1992, 135, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.B.; Tigges, M.; Iuvone, P.M. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 1993, 601, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fukuhara, C.; Wessel, J.H.; Iuvone, P.M.; Tosini, G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004, 315, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Davidson, A.J.; Fukuhara, C.; Kasamatsu, M.; Castanon-Cervantes, O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007, 21, 3866–3871. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Pozdeyev, N.; Sakamoto, K.; Iuvone, P.M. The circadian clock system in the mammalian retina. Bioessays 2008, 30, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Liu, C.; Tosini, G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J. Neurosci. 2004, 24, 9693–9697. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Liu, C.; Kasamatsu, M.; Pozdeyev, N.V; Iuvone, P.M.; Tosini, G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur. J. Neurosci. 2005, 22, 3129–3136. [Google Scholar]
- Mathes, A.; Engel, L.; Holthues, H.; Wolloscheck, T.; Spessert, R. Daily profile in melanopsin transcripts depends on seasonal lighting conditions in the rat retina. J. Neuroendocrinol. 2007, 19, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Zele, A.J.; Feigl, B.; Smith, S.S.; Markwell, E.L. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One 2011, 6, e17860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Q.; Wong, K.Y.; Sollars, P.J.; Berson, D.M.; Pickard, G.E.; McMahon, D.G. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 14181–14186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Q.; Belenky, M.A.; Sollars, P.J.; Pickard, G.E.; McMahon, D.G. Melanopsin mediates retrograde visual signaling in the retina. PLoS One 2012, 7, e42647. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Slominski, A.; Zbytek, B.; Szczesniewski, A.; Semak, I.; Kaminski, J.; Sweatman, T.; Wortsman, J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E701–E706. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. Cutaneous glucocorticosteroidogenesis: Securing local homeostasis and the skin integrity. Exp. Dermatol. 2014, 23, 369–74. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Richter, H.G.; Rojas-García, P.; Vergara, M.; Forcelledo, M.L.; Valladares, L.E.; Torrealba, F.; Valenzuela, G.J.; Serón-Ferré, M. mt1 Melatonin receptor in the primate adrenal gland: Inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 2003, 88, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Richter, H.G.; Germain, A.M.; Valenzuela, G.J.; Campino, C.; Rojas-García, P.; Forcelledo, M.L.; Torrealba, F.; Serón-Ferré, M. Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J. Physiol. 2004, 554, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Serón-Ferré, M.; Dinet, V.; Korf, H.-W. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: Differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal Res. 2006, 40, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Fuller, P.M.; Gooley, J.J.; Saper, C.B. Neurobiology of the sleep-wake cycle: Sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythm. 2006, 21, 482–493. [Google Scholar] [CrossRef]
- Hardeland, R. New approaches in the management of insomnia: Weighing the advantages of prolonged-release melatonin and synthetic melatoninergic agonists. Neuropsychiatr. Dis. Treat. 2009, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dong, Y.; Yang, Z.; Kato, H.; Ni, Y.; Fu, Z. Differential resetting process of circadian gene expression in rat pineal glands after the reversal of the light/dark cycle via a 24 h light or dark period transition. Chronobiol. Int. 2009, 26, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Wongchitrat, P.; Felder-Schmittbuhl, M.-P.; Govitrapong, P.; Phansuwan-Pujito, P.; Simonneaux, V. A noradrenergic sensitive endogenous clock is present in the rat pineal gland. Neuroendocrinology 2011, 94, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light suppresses melatonin secretion in humans. Science 1980, 210, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Bojkowski, C.J.; Aldhous, M.E.; English, J.; Franey, C.; Poulton, A.L.; Skene, D.J.; Arendt, J. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm. Metab. Res. 1987, 19, 437–440. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, I.M.; Norman, T.R.; Burrows, G.D.; Armstrong, S.M. Human melatonin suppression by light is intensity dependent. J. Pineal Res. 1989, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zeitzer, J.M.; Dijk, D.J.; Kronauer, R.; Brown, E.; Czeisler, C. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000, 526, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [PubMed]
- Yasukouchi, A.; Hazama, T.; Kozaki, T. Variations in the light-induced suppression of nocturnal melatonin with special reference to variations in the pupillary light reflex in humans. J. Physiol. Anthropol. 2007, 26, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Stehle, J.H.; von Gall, C.; Korf, H.-W. Melatonin: A clock-output, a clock-input. J. Neuroendocrinol. 2003, 15, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Ahmed, S.; Jackson, J.M.; Sack, R.L. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int. 1992, 9, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A. Clinical implications of the melatonin phase response curve. J. Clin. Endocrinol. Metab. 2010, 95, 3158–3160. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, K.V.; Verevkin, E.G.; Antyufeev, V.S.; Wirz-Justice, A.; Cajochen, C. The hockey-stick method to estimate evening dim light melatonin onset (DLMO) in humans. Chronobiol. Int. 2014, 31, 349–55. [Google Scholar] [CrossRef] [PubMed]
- Keijzer, H.; Smits, M.G.; Duffy, J.F.; Curfs, L.M.G. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med. Rev. 2014, 18, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sack, R.L.; Lewy, A.J. Melatonin as a chronobiotic: Treatment of circadian desynchrony in night workers and the blind. J. Biol. Rhythm. 1997, 12, 595–603. [Google Scholar] [CrossRef]
- Lewy, A.J.; Emens, J.; Jackman, A.; Yuhas, K. Circadian uses of melatonin in humans. Chronobiol. Int. 2006, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.M.; Markwald, R.R.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; Wright, K.P. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep 2013, 36, 1617–1624. [Google Scholar] [PubMed]
- Saxvig, I.W.; Wilhelmsen-Langeland, A.; Pallesen, S.; Vedaa, O.; Nordhus, I.H.; Bjorvatn, B. A randomized controlled trial with bright light and melatonin for delayed sleep phase disorder: Effects on subjective and objective sleep. Chronobiol. Int. 2014, 31, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Hardeland, R.; Rodenbeck, A.; Huether, G. Tissue retention and subcellular distribution of continuously infused melatonin in rats under near physiological conditions. J. Pineal Res. 1998, 25, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010, 48, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [PubMed]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.-X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Topal, T.; Tan, D.-X.; Reiter, R.J. Role of melatonin in metabolic regulation. Rev. Endocr. Metab. Disord. 2009, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ates, O.; Cayli, S.; Gurses, I.; Yucel, N.; Altinoz, E.; Iraz, M.; Kocak, A.; Yologlu, S. Does pinealectomy affect the recovery rate after spinal cord injury? Neurol. Res. 2007, 29, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ozler, M.; Simsek, K.; Ozkan, C.; Akgul, E.O.; Topal, T.; Oter, S.; Korkmaz, A. Comparison of the effect of topical and systemic melatonin administration on delayed wound healing in rats that underwent pinealectomy. Scand. J. Clin. Lab. Investig. 2010, 70, 447–452. [Google Scholar] [CrossRef]
- Tasdemir, S.; Samdanci, E.; Parlakpinar, H.; Polat, A.; Tasdemir, C.; Cengiz, N.; Sapmaz, H.; Acet, A. Effects of pinealectomy and exogenous melatonin on the brains, testes, duodena and stomachs of rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 860–866. [Google Scholar] [PubMed]
- Kus, M.A.; Sarsilmaz, M.; Karaca, O.; Acar, T.; Gülcen, B.; Hismiogullari, A.A.; Ogetürk, M.; Kus, I. Effects of melatonin hormone on hippocampus in pinealectomized rats: An immunohistochemical and biochemical study. Neuro Endocrinol. Lett. 2013, 34, 418–425. [Google Scholar] [PubMed]
- DeCoursey, P.J. Effect of light on the circadian activity rhythm of the flying squirrel, Glaucomys volans. Zeitschrift für Vergleichende Physiol. 1961, 44, 331–354. [Google Scholar] [CrossRef]
- Tembrock, G.; Winfree, A.T. The Geometry of Biological Time. (Biomathematics, Vol. 8.). Springer-Verlag, Berlin-Heidelberg-New York 1980, xiv, 530 S., 290 Abb., DM 59.50. Biom. J. 1982, 24, 320. [Google Scholar]
- Honma, K.; Honma, S.; Wada, T. Phase-dependent shift of free-running human circadian rhythms in response to a single bright light pulse. Experientia 1987, 43, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Minors, D.S.; Waterhouse, J.M.; Wirz-Justice, A. A human phase-response curve to light. Neurosci. Lett. 1991, 133, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Sturis, J.; Byrne, M.M.; Blackman, J.D.; Leproult, R.; Ofek, G.; L’Hermite-Balériaux, M.; Refetoff, S.; Turek, F.W.; van Reeth, O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am. J. Physiol. 1994, 266, E953–E963. [Google Scholar] [PubMed]
- Khalsa, S.B.S.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Kronauer, R.E.; Allan, J.S.; Duffy, J.F.; Jewett, M.E.; Brown, E.N.; Ronda, J.M. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 1989, 244, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Jewett, M.E.; Kronauer, R.E.; Czeisler, C.A. Phase-amplitude resetting of the human circadian pacemaker via bright light: A further analysis. J. Biol. Rhythm. 1994, 9, 295–314. [Google Scholar] [CrossRef]
- Rüger, M.; St Hilaire, M.A.; Brainard, G.C.; Khalsa, S.-B.S.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Human phase response curve to a single 6.5 h pulse of short-wavelength light. J. Physiol. 2013, 591, 353–363. [Google Scholar]
- Wang, X.-S.; Armstrong, M.E.G.; Cairns, B.J.; Key, T.J.; Travis, R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. (Lond.) 2011, 61, 78–89. [Google Scholar] [CrossRef]
- Szosland, D. Shift work and metabolic syndrome, diabetes mellitus and ischaemic heart disease. Int. J. Occup. Med. Environ. Health 2010, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, S.; Viitasalo, K.; Härmä, M. The relationship between current and former shift work and the metabolic syndrome. Scand. J. Work. Environ. Health 2012, 38, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.H.; Jeong, J.U.; Jeon, M.J.; Sakong, J. The association between shift work and the metabolic syndrome in female workers. Ann. Occup. Environ. Med. 2013, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Otsuka, T. Effect of shift work on the development of metabolic syndrome after 3 years in Japanese male workers. Arch. Environ. Occup. Health 2014, 69, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Zhang, Y.; Zhang, B.; He, Y.; Xie, S.; Li, M.; Miao, X.; Li, Z.; Yu, I.T.-S.; et al. Meta-analysis on night shift work and risk of metabolic syndrome. Occup. Environ. Med. 2014, 71, A78. [Google Scholar]
- Kawabe, Y.; Nakamura, Y.; Kikuchi, S.; Murakami, Y.; Tanaka, T.; Takebayashi, T.; Okayama, A.; Miura, K.; Okamura, T.; Ueshima, H. Relationship between shift work and clustering of the metabolic syndrome diagnostic components. J. Atheroscler. Thromb. 2014, 21, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Holmbäck, U.; van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S. Shift work is associated with increased risk of type 2 diabetes, study shows. BMJ 2014, 349, g4804. [Google Scholar] [CrossRef]
- Gan, Y.; Yang, C.; Tong, X.; Sun, H.; Cong, Y.; Yin, X.; Li, L.; Cao, S.; Dong, X.; Gong, Y.; et al. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup. Environ. Med. 2014. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hsiao, T.-J.; Chen, P.-C. Shift work aggravates metabolic syndrome development among early-middle-aged males with elevated ALT. World J. Gastroenterol. 2009, 15, 5654–5661. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.; Levandovski, R.; Dantas, G.; Caumo, W.; Hidalgo, M.P. Obesity and shift work: Chronobiological aspects. Nutr. Res. Rev. 2010, 23, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Lowden, A.; Moreno, C.; Holmbäck, U.; Lennernäs, M.; Tucker, P. Eating and shift work—Effects on habits, metabolism and performance. Scand. J. Work. Environ. Health 2010, 36, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-D.; Lin, Y.-C.; Hsiao, S.-T. Obesity and high blood pressure of 12-hour night shift female clean-room workers. Chronobiol. Int. 2010, 27, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Esquirol, Y.; Perret, B.; Ruidavets, J.B.; Marquie, J.C.; Dienne, E.; Niezborala, M.; Ferrieres, J. Shift work and cardiovascular risk factors: New knowledge from the past decade. Arch. Cardiovasc. Dis. 2011, 104, 636–668. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Scheer, F.A.J.L. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr. Opin. Endocrinol. Diabetes. Obes. 2014, 21, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.M.; Hallschmid, M.; Schultes, B. The metabolic burden of sleep loss. Lancet. Diabetes Endocrinol. 2014. [Google Scholar] [CrossRef]
- Bayon, V.; Leger, D.; Gomez-Merino, D.; Vecchierini, M.-F.; Chennaoui, M. Sleep debt and obesity. Ann. Med. 2014, 46, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Graubard, B.I. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am. J. Clin. Nutr. 2014, 100, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, A.; Gemma, C.; Gianotti, T.F.; Sookoian, S.; Pirola, C.J. Increased levels of resistin in rotating shift workers: A potential mediator of cardiovascular risk associated with circadian misalignment. Atherosclerosis 2010, 210, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Association between light exposure at night and insomnia in the general elderly population: The HEIJO-KYO cohort. Chronobiol. Int. 2014, 31, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Pallesen, S.; Glozier, N.; Bjorvatn, B.; Salo, P.; Tell, G.S.; Ursin, R.; Øverland, S. Midlife insomnia and subsequent mortality: The Hordaland health study. BMC Public Health 2014, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Coto-Montes, A. New vistas on oxidative damage and aging. Open Biol. J. 2010, 3, 39–52. [Google Scholar] [CrossRef]
- Aschoff, J.; von Saint Paul, U.; Wever, R. Lifetime of flies under influence of time displacement. Naturwissenschaften 1971, 58, 574. [Google Scholar] [CrossRef] [PubMed]
- Penev, P.D.; Kolker, D.E.; Zee, P.C.; Turek, F.W. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am. J. Physiol. 1998, 275, H2334–H2337. [Google Scholar] [PubMed]
- Kasai, H.; Iwamoto-Tanaka, N.; Miyamoto, T.; Kawanami, K.; Kawanami, S.; Kido, R.; Ikeda, M. Life style and urinary 8-hydroxydeoxyguanosine, a marker of oxidative dna damage: Effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn. J. Cancer Res. 2001, 92, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Hung, H.-C.; Pan, S.-M.; Wu, M.-T. The change of oxidative DNA damage in nurses with shift work. Occup. Environ. Med. 2014, 71, A97. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Morfeld, P. Shift work and cancer research: A thought experiment into a potential chronobiological fallacy of past and perspectives for future epidemiological studies. Neuro Endocrinol. Lett. 2013, 34, 282–286. [Google Scholar] [PubMed]
- Hansen, J. Light at night, shiftwork, and breast cancer risk. J. Natl. Cancer Inst. 2001, 93, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, H.A. Nightshift work and risk of breast cancer and other cancers—A critical review of the epidemiologic evidence. Scand. J. Work Environ. Health 2008, 34, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Lassen, C.F. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup. Environ. Med. 2012, 69, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Stevens, R.G. Case-control study of shift-work and breast cancer risk in Danish nurses: Impact of shift systems. Eur. J. Cancer 2012, 48, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Verbeek, J.; Seidler, A.; Lindbohm, M.-L.; Ojajärvi, A.; Orsini, N.; Costa, G.; Neuvonen, K. Night-shift work and breast cancer—A systematic review and meta-analysis. Scand. J. Work Environ. Health 2013, 39, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, B.B.; Tergas, A.I.; Mateen, F.J.; Bhayani, N.H.; Oh, J. Night-shift work and risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 138, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, L.; Erren, T.C.; Glass, D.C.; Girschik, J.; Thomson, A.K.; Saunders, C.; Boyle, T.; El-Zaemey, S.; Rogers, P.; Peters, S.; et al. The association between different night shiftwork factors and breast cancer: A case-control study. Br. J. Cancer 2013, 109, 2472–2480. [Google Scholar]
- Tsc, L.A.S.; Wang, F.; Chan, W.C.; Wu, C.; Li, M.; Kwok, C.H.; Leung, S.L.; Yu, W.C.; Yu, I.T.-S. Long-term nightshift work and breast cancer risk in Hong Kong women: Results update. Occup. Environ. Med. 2014, 71, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E. Nighshift work and breast cancer risk—Good news, bad news ? Occup. Environ. Med. 2014, 71, A121. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Kogevinas, M.; Martin Sanchez, V.; Moreno, V.; Pollan, M.; Moleón, J.J.J.; Ardanaz, E.; Maltzibar, J.; Peiro, R.; Tardon, A.; et al. Colorectal cancer risk and shift work in a population-based case-control study in Spain (MCC-Spain). Occup. Environ. Med. 2014, 71, A5–A6. [Google Scholar]
- Carter, B.D.; Diver, W.R.; Hildebrand, J.S.; Patel, A.V.; Gapstur, S.M. Circadian disruption and fatal ovarian cancer. Am. J. Prev. Med. 2014, 46, S34–S41. [Google Scholar]
- Lahti, T.A.; Partonen, T.; Kyyrönen, P.; Kauppinen, T.; Pukkala, E. Night-time work predisposes to non-Hodgkin lymphoma. Int. J. Cancer 2008, 123, 2148–2151. [Google Scholar] [CrossRef] [PubMed]
- Ursi, M.; Noli, M.; Marras, F.; Aresti, C.; Mannetje, A.; Cocco, P. Risk of non-Hodgkin Lymphoma in health occupations. Occup. Environ. Med. 2014, 71, A113–A114. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [PubMed]
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110. [Google Scholar]
- Vinogradova, I.A.; Anisimov, V.N.; Bukalev, A.V.; Semenchenko, A.V.; Zabezhinski, M.A. Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in rats. Aging 2009, 1, 855–865. [Google Scholar] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Albares, J.; Segarra, F.; Campos, M.; Estivill, E.; Rol, M.A.; Madrid, J.A. Ambulatory circadian monitoring (ACM) based on thermometry, motor activity and body position (TAP): A comparison with polysomnography. Physiol. Behav. 2014, 126, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Circadian phase asessment by ambulatory monitoring in humans: Correlation with dim light. Melatonin Onset. 2013; 31, 37–51. [Google Scholar]
- Myers, B.L.; Badia, P. Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions. Neurosci. Biobehav. Rev. 1995, 19, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.L.; Mainster, M.A. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br. J. Ophthalmol. 2008, 92, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.P.; Chiquet, C.; Teikari, P.; Cornut, P.-L.; Claustrat, B.; Denis, P.; Cooper, H.M.; Gronfier, C. Aging of non-visual spectral sensitivity to light in humans: Compensatory mechanisms? PLoS One 2014, 9, e85837. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Thompson, K.; Burns, S.A. Pupil location under mesopic, photopic and pharmacologically dilated conditions. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2508–2512. [Google Scholar]
- Charman, W.N. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol. Opt. 2003, 23, 181–187. [Google Scholar] [PubMed]
- Revell, V.L.; Skene, D.J. Impact of age on human non-visual responses to light. Sleep Biol. Rhythm. 2010, 8, 84–94. [Google Scholar] [CrossRef]
- Herbst, K.; Sander, B.; Lund-Andersen, H.; Broendsted, A.E.; Kessel, L.; Hansen, M.S.; Kawasaki, A. Intrinsically photosensitive retinal ganglion cell function in relation to age: A pupillometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Harman, A.; Abrahams, B.; Moore, S.; Hoskins, R. Neuronal density in the human retinal ganglion cell layer from 16 to 77 years. Anat. Rec. 2000, 260, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Owsley, C.; Jackson, G.R. Spare the rods, save the cones in aging and age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2015–2018. [Google Scholar]
- Li, R.S.; Chen, B.-Y.; Tay, D.K.; Chan, H.H.L.; Pu, M.-L.; So, K.-F. Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2951–2958. [Google Scholar] [CrossRef]
- Roberts, D.E.; Killiany, R.J.; Rosene, D.L. Neuron numbers in the hypothalamus of the normal aging rhesus monkey: Stability across the adult lifespan and between the sexes. J. Comp. Neurol. 2012, 520, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Bottum, K.; Poon, E.; Haley, B.; Karmarkar, S.; Tischkau, S.A. Suprachiasmatic nucleus neurons display endogenous resistance to excitotoxicity. Exp. Biol. Med. (Maywood) 2010, 235, 237–246. [Google Scholar] [CrossRef]
- Gibson, E.M.; Williams, W.P.; Kriegsfeld, L.J. Aging in the circadian system: Considerations for health, disease prevention and longevity. Exp. Gerontol. 2009, 44, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Weinert, H.; Weinert, D.; Schurov, I.; Maywood, E.S.; Hastings, M.H. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol. Int. 2001, 18, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Yoshinobu, Y.; Kaneko, S.; Mori, A.; Nikaido, T.; Moriya, T.; Akiyama, M.; Shibata, S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J. Neurosci. Res. 2001, 66, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Kolker, D.E.; Fukuyama, H.; Huang, D.S.; Takahashi, J.S.; Horton, T.H.; Turek, F.W. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J. Biol. Rhythm. 2003, 18, 159–169. [Google Scholar] [CrossRef]
- Kunieda, T.; Minamino, T.; Katsuno, T.; Tateno, K.; Nishi, J.; Miyauchi, H.; Orimo, M.; Okada, S.; Komuro, I. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ. Res. 2006, 98, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Antoch, M.P. The clock proteins, aging, and tumorigenesis. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 477–482. [Google Scholar] [CrossRef]
- Dijk, D.J.; Duffy, J.F.; Riel, E.; Shanahan, T.L.; Czeisler, C.A. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J. Physiol. 1999, 516, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Kunz, D.; Schmitz, S.; Mahlberg, R.; Mohr, A.; Stöter, C.; Wolf, K.J.; Herrmann, W.M. A new concept for melatonin deficit: On pineal calcification and melatonin excretion. Neuropsychopharmacology 1999, 21, 765–72. [Google Scholar] [CrossRef] [PubMed]
- Schmid, H.A.; Requintina, P.J.; Oxenkrug, G.F.; Sturner, W. Calcium, calcification, and melatonin biosynthesis in the human pineal gland: A postmortem study into age-related factors. J. Pineal Res. 1994, 16, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Zeitzer, J.M.; Daniels, J.E.; Duffy, J.F.; Klerman, E.B.; Shanahan, T.L.; Dijk, D.J.; Czeisler, C.A. Do plasma melatonin concentrations decline with age? Am. J. Med. 1999, 107, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.R.A.; Luijpen, M.W.; van Someren, E.J.W.; Sergeant, J.A.; Scheltens, P.; Scherder, E.J.A. Peripheral electrical nerve stimulation and rest-activity rhythm in Alzheimer’s disease. J. Sleep Res. 2006, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Tozawa, T.; Satoh, K.; Matsumoto, Y.; Hishikawa, Y.; Okawa, M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep–waking. Biol. Psychiatry 1999, 45, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G. Introduction to Color Imaging Science [Book Review]. IEEE Signal Process. Mag. 2006, 23, 690. [Google Scholar] [CrossRef]
- Campbell, S.S.; Kripke, D.F.; Gillin, J.C.; Hrubovcak, J.C. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol. Behav. 1988, 42, 141–44. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Klauber, M.R.; Jones, D.W.; Kripke, D.F.; Martin, J.; Mason, W.; Pat-Horenczyk, R.; Fell, R. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep 1997, 20, 18–23. [Google Scholar] [PubMed]
- Anderiesen, H.; Scherder, E.J.A.; Goossens, R.H.M.; Sonneveld, M.H. A systematic review—Physical activity in dementia: The influence of the nursing home environment. Appl. Ergon. 2014, 45, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Arendt, J.; Skene, D.J. Visual impairment and circadian rhythm disorders. 2007; 9, 301–314. [Google Scholar]
- Hollwich, F.; Dieckhues, B. Circadian rhythm in the blind. J. Interdisiplinary Cycle Res. 1971, 2, 291–301. [Google Scholar] [CrossRef]
- Orth, D.N.; Island, D.P. Light synchronization of the circadian rhythm in plasma cortisol (17-OHCS) concentration in man. J. Clin. Endocrinol. Metab. 1969, 29, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Krieger, D.T.; Rizzo, F. Circadian periodicity of plasma 11-hydroxycorticosteroid levels in subjects with partial and absent light perception. Neuroendocrinology 1971, 8, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Bodenheimer, S.; Winter, J.S.; Faiman, C. Diurnal rhythms of serum gonadotropins, testosterone, estradiol and cortisol in blind men. J. Clin. Endocrinol. Metab. 1973, 37, 472–475. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, B.; Bellastella, A.; Esposito, V.; Colucci, C.F.; Montalbetti, N. Letter: Circadian rhythm of cortisol secretion in elderly and blind subjects. Br. Med. J. 1974, 2, 274. [Google Scholar] [CrossRef] [PubMed]
- Migeon, C.J.; Tyler, F.H.; Mahoney, J.P.; Florentin, A.A.; Castle, H.; Bliss, E.L.; Samuels, L.T. The diurnal variation of plasma levels and urinary excretion on 17-hydroxycorticosteroids in normal subjects, night workers and blind subjects. J. Clin. Endocrinol. Metab. 1956, 16, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, E.; Perlow, M.; Sassin, J.; Fukushima, D.; Burack, B.; Hellman, L. Persistence of the twenty-four hour pattern of episodic cortisol secretion and growth hormone release in blind subjects. Trans. Am. Neurol. Assoc. 1972, 97, 197–199. [Google Scholar]
- Scheving, L.E.; Kanabrocki, E.L.; Tsai, T.H.; Pauly, J.E. Circadian and other variation in epinephrine and norepinephrine among several human populations, including healthy blinded and sighted subjects and patients with leprosy. Prog. Clin. Biol. Res. 1987, 227A, 329–349. [Google Scholar]
- Lewy, A.J.; Newsome, D.A. Different types of melatonin circadian secretory rhythms in some blind subjects. J. Clin. Endocrinol. Metab. 1983, 56, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Sack, R.L.; Lewy, A.J.; Blood, M.L.; Keith, L.D.; Nakagawa, H. Circadian rhythm abnormalities in totally blind people: Incidence and clinical significance. J. Clin. Endocrinol. Metab. 1992, 75, 127–134. [Google Scholar] [PubMed]
- Tabandeh, H.; Lockley, S.W.; Buttery, R.; Skene, D.J.; Defrance, R.; Arendt, J.; Bird, A.C. Disturbance of sleep in blindness. Am. J. Ophthalmol. 1998, 126, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Lockley, S.W.; James, K.; Arendt, J. Correlation between urinary cortisol and 6-sulphatoxymelatonin rhythms in field studies of blind subjects. Clin. Endocrinol. (Oxf.) 1999, 50, 715–719. [Google Scholar] [CrossRef]
- Miles, L.; Wilson, M.A. High incidence of cyclic sleep/wake disorders in the blind. Sleep Res. 1977, 6, 192. [Google Scholar]
- Miles, L.E.; Raynal, D.M.; Wilson, M.A. Blind man living in normal society has circadian rhythms of 24.9 h. Science 1977, 198, 421–423. [Google Scholar]
- Martens, H.; Endlich, H.; Hildebrandt, G.; Moog, R. Sleep/wake distribution in blind subjects with and without sleep complaints. Sleep Res. 1990, 19, 398. [Google Scholar]
- Lockley, S.W.; Skene, D.J.; Arendt, J.; Tabandeh, H.; Bird, A.C.; Defrance, R. Relationship between melatonin rhythms and visual loss in the blind. J. Clin. Endocrinol. Metab. 1997, 82, 3763–3770. [Google Scholar] [PubMed]
- Skene, D.; Lockley, S.W.; Arendt, J. Melatonin in circadian sleep disorders in the blind. Biol. Signals Recept. 1999, 8, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Skene, D.J.; James, K.; Thapan, K.; Wright, J.; Arendt, J. Melatonin administration can entrain the free-running circadian system of blind subjects. J. Endocrinol. 2000, 164, R1–R6. [Google Scholar] [CrossRef] [PubMed]
- Hack, L.M.; Lockley, S.W.; Arendt, J.; Skene, D.J. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J. Biol. Rhythm. 2003, 18, 420–429. [Google Scholar]
- Skene, D.J.; Arendt, J. Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med. 2007, 8, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Gronfier, C.; Wright, K.P.; Kronauer, R.E.; Czeisler, C.A. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Natl. Acad. Sci. USA 2007, 104, 9081–9086. [Google Scholar] [CrossRef] [PubMed]
- Aries, M. Human Lighting Demands; Eindhoven University Press: Eindhoven, The Netherlands, 2005. [Google Scholar]
- Van Bommel, W.J.M. Non-visual biological effect of lighting and the practical meaning for lighting for work. Appl. Ergon. 2006, 37, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.; Figueiro, M.; Bullough, J. Circadian photobiology: An emerging framework for lighting practice and research. Light. Res. Technol. 2002, 34, 177–190. [Google Scholar] [CrossRef]
- Van Bommel, W.; van den Beld, G. Lighting for work: A review of visual and biological effects. Light. Res. Technol. 2004, 36, 255–269. [Google Scholar] [CrossRef]
- Rea, M.; Figueiro, M.; Bierman, A.; Hamner, R. Modelling the spectral sensitivity of the human circadian system. Light. Res. Technol. 2011, 44, 386–396. [Google Scholar] [CrossRef]
- Godley, B.F.; Shamsi, F.A.; Liang, F.-Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi-Ueda, T.; Majima, H.J.; Watanabe, K.; Ueda, T.; Indo, H.P.; Suenaga, S.; Hisamitsu, T.; Ozawa, T.; Yasuhara, H.; Koide, R. Blue LED light exposure develops intracellular reactive oxygen species, lipid peroxidation, and subsequent cellular injuries in cultured bovine retinal pigment epithelial cells. Free Radic. Res. 2013, 47, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, E.; Bonnin-Arias, C.; Pérez-Carrasco, M.J.; Muñoz de Luna, J.; Vázquez, D.; Sánchez-Ramos, C. Effects of light-emitting diode radiations on human retinal pigment epithelial cells in vitro. Photochem. Photobiol. 2013, 89, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.-M.; Wang, G.-S.; Sliney, D.; Yang, C.-H.; Lee, L.-L. White light-emitting diodes (LEDs) at domestic lighting levels and retinal injury in a rat model. Environ. Health Perspect. 2014, 122, 269–276. [Google Scholar] [PubMed]
- Rahman, S.A.; Marcu, S.; Shapiro, C.M.; Brown, T.J.; Casper, R.F. Spectral modulation attenuates molecular, endocrine, and neurobehavioral disruption induced by nocturnal light exposure. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E518–E527. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Shapiro, C.M.; Wang, F.; Sinlay, H.; Kazmi, S.; Brown, T.J.; Casper, R.f. Effects of filtering visual short wavelengths during nocturnal shiftwork on sleep and performance. Chronobiol. Int. 2013, 30, 951–962. [Google Scholar]
- Sasseville, A.; Hébert, M. Using blue-green light at night and blue-blockers during the day to improves adaptation to night work: A pilot study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.S.; Terman, M.; Lewy, A.J.; Dijk, D.J.; Eastman, C.I.; Boulos, Z. Light treatment for sleep disorders: Consensus report. V. Age-related disturbances. J. Biol. Rhythm. 1995, 10, 151–154. [Google Scholar] [CrossRef]
- Fontana Gasio, P.; Kräuchi, K.; Cajochen, C.; Someren, E.; van Amrhein, I.; Pache, M.; Savaskan, E.; Wirz-Justice, A. Dawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderly. Exp. Gerontol. 2003, 38, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rodin, I. Seasonal Affective Disorder: Practice and Research; Partonen, T., Magnusson, A., Eds.; Oxford University: Oxford, UK, 2001; Volume 20, p. 311. [Google Scholar]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. Camb. Philos. Soc. 2013, 88, 912–927. [Google Scholar] [PubMed]
- Aschoff, J. The phase-angle difference in circadian periodicity. In Circadian Clocks; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1965; pp. 262–276. [Google Scholar]
- Wever, R. A mathematical model for circadian rhythms. In Circadian Clocks; Aschoff, J., Ed.; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1965; pp. 47–73. [Google Scholar]
- Terman, M.; Schlager, D.; Fairhurst, S.; Perlman, B. Dawn and dusk simulation as a therapeutic intervention. Biol. Psychiatry 1989, 25, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Terman, M.; Terman, J.S. Light therapy. In Principles and Practise of Sleep Medicine; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier: Philadelphia, PA, USA, 2005; pp. 1424–1442. [Google Scholar]
- Ohashi, Y.; Okamoto, N.; Uchida, K.; Iyo, M.; Mori, N.; Morita, Y. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol. Psychiatry 1999, 45, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in aging and disease-multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012, 3, 194–225. [Google Scholar] [PubMed]
- Mishima, K.; Hishikawa, Y.; Okawa, M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer’s type. Chronobiol. Int. 1998, 15, 647–54. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Gehrman, P.; Martin, J.L.; Shochat, T.; Marler, M.; Corey-Bloom, J.; Levi, L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav. Sleep Med. 2003, 1, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.A.; Hubbard, E.M.; Mastick, J.; Luxenberg, J.S.; Burr, R.L.; van Someren, E.J.W. Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int. Psychogeriatr. 2005, 17, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.A.; Burr, R.L.; van Someren, E.J.W.; Hubbard, E.M.; Luxenberg, J.S.; Mastick, J.; Cooper, B.A. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J. Am. Geriatr. Soc. 2008, 56, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.A.; Graf, C.L.; Hubbard, E.M.; Luxenberg, J.S. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. West. J. Nurs. Res. 2007, 29, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Yamadera, H.; Ito, T.; Suzuki, H.; Asayama, K.; Ito, R.; Endo, S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin. Neurosci. 2000, 54, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Swaab, D.F. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007, 8, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Yamadera, H.; Ito, T.; Suzuki, H.; Kudo, Y.; Endo, S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J. Nippon Med. Sch. 2003, 70, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef]
- Aoki, M.; Yokota, Y.; Hayashi, T.; Kuze, B.; Murai, M.; Mizuta, K.; Ito, Y. Disorder of the saliva melatonin circadian rhythm in patients with Meniere’s disease. Acta Neurol. Scand. 2006, 113, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Rohr, U.D.; Herold, J. Melatonin deficiencies in women. Maturitas 2002, 41, S85–S104. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Loisy, C.; Brun, J.; Beorchia, S.; Arnaud, J.L.; Chazot, G. Nocturnal plasma melatonin levels in migraine: A preliminary report. Headache 1989, 29, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Brun, J.; Geoffriau, M.; Zaidan, R.; Mallo, C.; Chazot, G. Nocturnal plasma melatonin profile and melatonin kinetics during infusion in status migrainosus. Cephalalgia 1997, 17, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Brugger, P.; Marktl, W.; Herold, M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet 1995, 345, 1408. [Google Scholar] [CrossRef] [PubMed]
- Girotti, L.; Lago, M.; Ianovsky, O.; Carbajales, J.; Elizari, M.V.; Brusco, L.I.; Cardinali, D.P. Low urinary 6-sulphatoxymelatonin levels in patients with coronary artery disease. J. Pineal Res. 2000, 29, 138–142. [Google Scholar]
- Sewerynek, E. Melatonin and the cardiovascular system. Neuro Endocrinol. Lett. 2002, 23, 79–83. [Google Scholar] [PubMed]
- Altun, A.; Yaprak, M.; Aktoz, M.; Vardar, A.; Betul, U.A.; Ozbay, G. Impaired nocturnal synthesis of melatonin in patients with cardiac syndrome X. Neurosci. Lett. 2002, 327, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Yaprak, M.; Altun, A.; Vardar, A.; Aktoz, M.; Ciftci, S.; Ozbay, G. Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int. J. Cardiol. 2003, 89, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Garcia-Gonzalez, M.J.; Samimi-Fard, S.; Kaski, J.C.; Reiter, R.J. Light/dark patterns of soluble vascular cell adhesion molecule-1 in relation to melatonin in patients with ST-segment elevation myocardial infarction. J. Pineal Res. 2008, 44, 65–69. [Google Scholar] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Reiter, R.J. Clinical aspects of melatonin in the acute coronary syndrome. Curr. Vasc. Pharmacol. 2009, 7, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Perras, B.; Kurowski, V.; Dodt, C. Nocturnal melatonin concentration is correlated with illness severity in patients with septic disease. Intensiv. Care Med. 2006, 32, 624–625. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Spence, D.W.; Kato, H.; Cardinali, D.P. Melatonin in septic shock: Some recent concepts. J. Crit. Care 2010, 25, 656 e1–e6. [Google Scholar] [PubMed]
- Grin, W.; Grünberger, W. A significant correlation between melatonin deficiency and endometrial cancer Gynecol. Obstet. Investig. 1998, 45, 62–65. [Google Scholar] [CrossRef]
- Hu, S.; Shen, G.; Yin, S.; Xu, W.; Hu, B. Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer. Adv. Ther. 2009, 26, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Puy, H.; Deybach, J.C.; Baudry, P.; Callebert, J.; Touitou, Y.; Nordmann, Y. Decreased nocturnal plasma melatonin levels in patients with recurrent acute intermittent porphyria attacks. Life Sci. 1993, 53, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bylesjö, I.; Forsgren, L.; Wetterberg, L. Melatonin and epileptic seizures in patients with acute intermittent porphyria. Epileptic Disord. 2000, 2, 203–208. [Google Scholar] [PubMed]
- O’Brien, I.A.; Lewin, I.G.; O’Hare, J.P.; Arendt, J.; Corrall, R.J. Abnormal circadian rhythm of melatonin in diabetic autonomic neuropathy. Clin. Endocrinol. (Oxf.) 1986, 24, 359–364. [Google Scholar] [CrossRef]
- Peschke, E.; Stumpf, I.; Bazwinsky, I.; Litvak, L.; Dralle, H.; Mühlbauer, E. Melatonin and type 2 diabetes—A possible link? J. Pineal Res. 2007, 42, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Vissel, B. Treatment implications of the altered cytokine-insulin axis in neurodegenerative disease. Biochem. Pharmacol. 2013, 86, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, J.-T.; Zhu, X.-C.; Tan, L. TREM2 in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 180–185. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Lourenco, M.V.; Ferreira, S.T. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement. 2014, 10, S26–S32. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Tong, M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.T.; Clarke, J.; Bomfim, T.; de Felice, F.G. Inflammation, defective insulin signaling, and neurological dysfunction in Alzheimer’s disease. Alzheimers Dement. 2014, 10, S76–S83. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Evans, E.E.; Scheer, F.A.J.L.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 2006, 29, 161–168. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonmati-Carrion, M.A.; Arguelles-Prieto, R.; Martinez-Madrid, M.J.; Reiter, R.; Hardeland, R.; Rol, M.A.; Madrid, J.A. Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. Int. J. Mol. Sci. 2014, 15, 23448-23500. https://doi.org/10.3390/ijms151223448
Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, Reiter R, Hardeland R, Rol MA, Madrid JA. Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. International Journal of Molecular Sciences. 2014; 15(12):23448-23500. https://doi.org/10.3390/ijms151223448
Chicago/Turabian StyleBonmati-Carrion, Maria Angeles, Raquel Arguelles-Prieto, Maria Jose Martinez-Madrid, Russel Reiter, Ruediger Hardeland, Maria Angeles Rol, and Juan Antonio Madrid. 2014. "Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure" International Journal of Molecular Sciences 15, no. 12: 23448-23500. https://doi.org/10.3390/ijms151223448
APA StyleBonmati-Carrion, M. A., Arguelles-Prieto, R., Martinez-Madrid, M. J., Reiter, R., Hardeland, R., Rol, M. A., & Madrid, J. A. (2014). Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. International Journal of Molecular Sciences, 15(12), 23448-23500. https://doi.org/10.3390/ijms151223448





