Bioaccumulation of Arsenic Species in Rays from the Northern Adriatic Sea
Abstract
:1. Introduction
2. Results
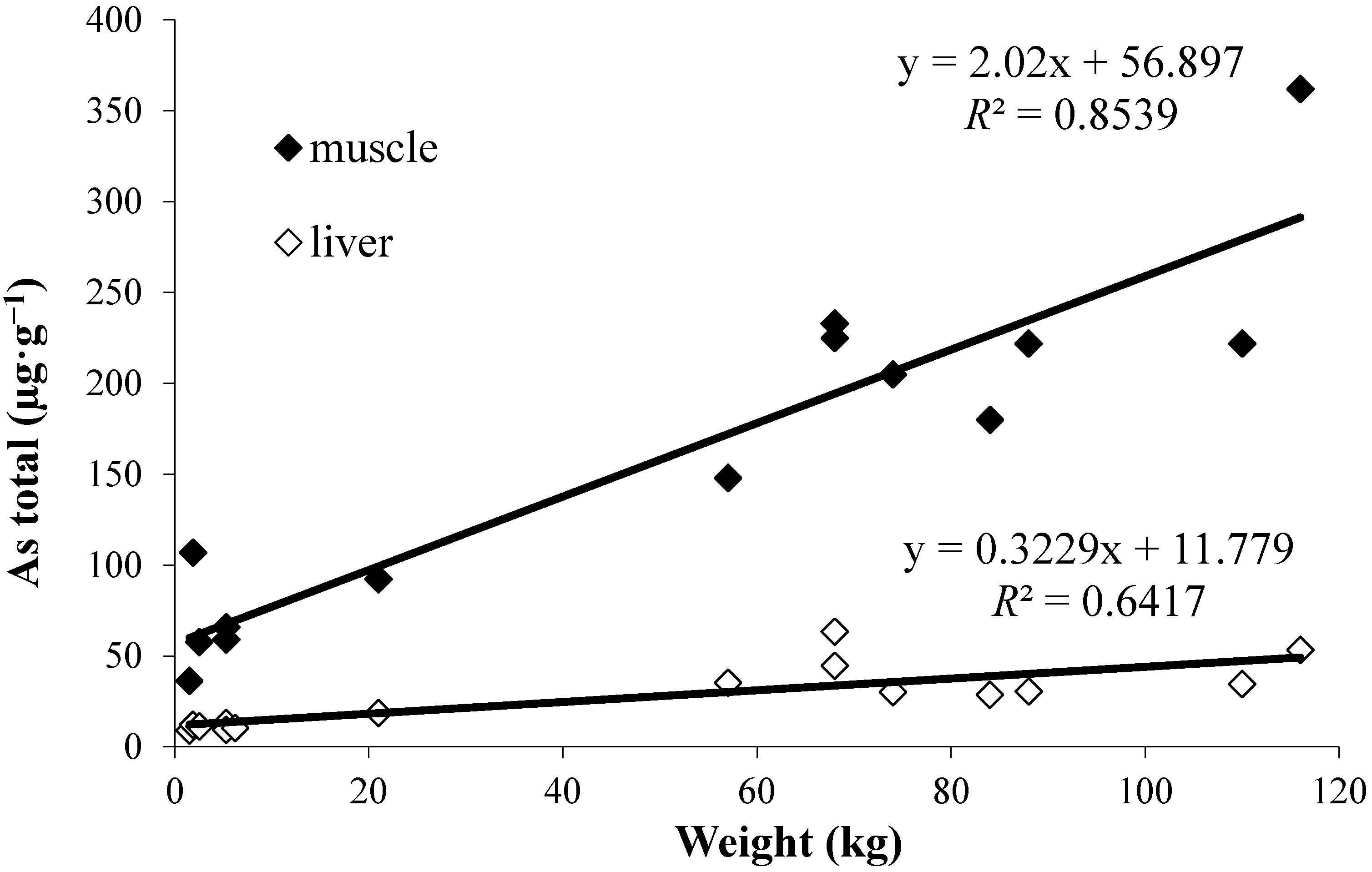
2.1. Total Arsenic
| Species/Max. DW * | Sample | DW * (cm) | DL # (cm) | TL ** (cm) | W ## (kg) | Sex | As (liver, µg·g−1) | As (muscle, µg·g−1) |
|---|---|---|---|---|---|---|---|---|
| Pteromylaeus bovinus/male cca. 90 cm, female max. 222 cm | 029PB | 52.8 | 30.2 | 87.5 | 1.50 | juv m | 9.1 ± 0.3 | 36.3 ± 0.5 |
| 034PB | 73.5 | 43.5 | 121.0 | 5.28 | m | 13.2 | 65.9 ± 0.8 | |
| 052PB | 113.5 | 71.5 | 194.0 | 21 | m | 18.7 | 92.3 | |
| 053PB | 163.0 | 108.0 | 196.0 | 74 | f | 30.2 ± 4.7 | 205 ± 5 | |
| 054PB | 188.5 | 118.0 | 224.0 | 110 | f | 34.6 | 222 ± 1 | |
| 055PB | 156.9 | 108.2 | 169.0 | 68 | f | 44.8 | 225 | |
| 056PB | 154.0 | 108.0 | 250.0 | 57 | f | 35.3 ± 1.9 | 148 ± 2 | |
| 057PB | 191.0 | 132.0 | 294.0 | 116 | f | 53.4 | 362 ± 10 | |
| 058PB | 222.0 | 115.5 | 267.0 | 88 | f | 30.6 | 222 ± 5 | |
| 060PB & | 53.5 | 12.4 | 87.9 | 1.88 | juv m | 12.2 | 107 ± 1 | |
| 062PB & | 57.1 | 33.5 | 86.0 | 2.52 | juv m | 11.3 | 57.8 ± 1.3 | |
| 063PB | 75.0 | 39.5 | 126.1 | 5.28 | f | 9.4 | 59.2 ± 0.8 | |
| 064PB | 77.5 | 41.1 | 125.6 | 6.20 | m | 10.5 ± 0.1 | - | |
| 066PB | 171.0 | 112.5 | 263.0 | 84 | f | 28.7 ± 2.4 | 180 ± 1 | |
| 067PB | 159.0 | 110.0 | 266.0 | 68 | f | 63.5 | 233 ± 1 | |
| Min | - | 52.8 | 12.4 | 86.0 | 1.5 | - | 9.1 | 36.3 |
| Max | - | 222.0 | 132.0 | 294.0 | 116 | - | 63.5 | 362 |
| Myliobatis aquila/cca. 150 cm | 023MA | - | - | - | - | - | - | 51.4 |
| 025MA & | 38.0 | 22.5 | 67.0 | 0.98 | f | 19.9 ± 1.0 | 69.8 | |
| 028MA & | 34.5 | 21.2 | 64.0 | 0.62 | juv | 21.3 ± 1.7 | - | |
| 035MA & | 27.7 | 16.0 | 52.5 | 0.30 | m | - | 32.4 | |
| 044MA & | 27.3 | 14.5 | 49.5 | 0.26 | juv | - | 47.1 | |
| 061MA & | 27.5 | 14.2 | 52.5 | 0.32 | juv | - | 36.5 | |
| Min | - | 27.3 | 14.2 | 49.5 | 0.26 | - | - | 32.4 |
| Max | - | 38.0 | 22.5 | 52.5 | 0.98 | - | 69.8 | |
| Pteroplatytrygon violacea/cca. 60 cm | 006PV | 60.0 | 54.9 | 139.2 | 7.56 | f | 26.3 ± 0.1 | 90.4 |
| 007PV | 56.2 | 42.0 | 137.5 | 5.44 | f | 34.8 | 48.3 | |
| 010PV | 55.0 | 42.5 | 129.0 | 5.22 | f | 40.7 | 82.4 ± 1.6 | |
| 038PV | 54.1 | 39.3 | 128.1 | 5.48 | f | 17.3 | 141 ± 4 | |
| 039PV | 44.5 | 34.0 | 107.0 | 2.64 | m | 23.8 ± 0.5 | 37.4 | |
| 049PV | 52.1 | 40.0 | 77.6 | 3.74 | m | 35.4 | 64.7 ± 0.2 | |
| 050PV | 58.8 | 45.0 | 126.2 | 6.12 | f | 34.4 ± 2.6 | 97.3 | |
| 051PV | 43.7 | 35.4 | 101.0 | 2.40 | m | 40.4 | 44.0 | |
| Min | - | 43.7 | 34.0 | 77.6 | 2.4 | - | 17.3 | 37.4 |
| Max | - | 60.0 | 54.9 | 139.2 | 7.56 | - | 40.7 | 97.3 |
| Species | Sample | Astotal (µg·g−1) | Sum of Species (%) | AsIII (µg·g−1) | AsV (µg·g−1) | AB (µg·g−1) | DMA (µg·g−1) | Ether Extractable (µg·g−1) |
|---|---|---|---|---|---|---|---|---|
| Pteromylaeus bovinus | 029PB | 9.1 ± 0.3 | 40.6 | Traces ** | - | 1.57 ± 0.28 | 0.67 ± 0.08 | 1.44 |
| 034PB | 13.2 | 42.0 | traces | traces | 1.30 ± 0.17 | 1.47 ± 0.14 | 2.73 | |
| 052PB | 18.7 | 73.3 | traces | - | 6.75 ± 1.48 | 1.30 ± 0.13 | 5.64 | |
| 053PB | 30.2 ± 4.7 | 50.2 | traces | traces | 9.94 ± 0.76 | 1.79 ± 0.20 | 3.35 | |
| 054PB | 34.6 | 64. 6 | 0.04 ± 0.01 | - | 15.2 ± 2.8 | 1.33 ± 0.16 | 5.77 | |
| 055PB | 44.8 | 52.2 | 0.05 ± 0.01 | - | 12.0 ± 2.7 | 1.60 ± 0.16 | 9.76 | |
| 056PB | 35.3 ± 1.9 | 60.5 | 0.04 ± 0.01 | 0.16±0.08 | 17.2 ± 2.0 | 1.35 ± 0.15 | 2.60 | |
| 057PB | 53.4 | 69.2 | 0.04 ± 0.00 | - | 25.8 ± 0.3 | 2.22 ± 0.25 | 8.91 | |
| 058PB | 30.6 | 63.7 | 0.03 ± 0.01 | - | 11.0 ± 1.4 | 2.03 ± 0.34 | 6.44 | |
| 060PB | 12.2 | 44.4 | traces | - | 2.13 ± 0.14 | 0.89 ± 0.22 | 2.39 | |
| 062PB | 11.3 | 48.1 | traces | - | 1.58 ± 0.34 | 0.76 ± 0.02 | 3.09 | |
| 063PB | 9.4 | 45.5 | traces | - | 1.41 ± 0.20 | 0.84 ± 0.16 | 2.01 | |
| 064PB | 10.5 ± 0.2 | 47.1 | 0.03 ± 0.01 | - | 1.83 ± 0.16 | 1.27 ± 0.09 | 1.82 | |
| 066PB | 28.7 ± 2.4 | 69.0 | 0.03 ± 0.02 | 0.45 ± 0.40 | 12.4 ± 2.0 | 2.60 ± 0.22 | 4.33 | |
| 067PB | 63.5 | 70.9 | 0.05 ± 0.01 | - | 31.5 ± 3.3 | 2.06 ± 0.23 | 11.4 | |
| Min | - | 9.1 | 40.6 | traces | - | 1.30 | 0.67 | 1.44 |
| Max | - | 63.5 | 70.9 | 0.05 | - | 31.5 | 2.60 | 11.4 |
| Myliobatis aquila | 025MA | 19.9 ± 1.0 | 46.9 | - | - | 3.13 ± 1.05 | 0.87 ± 0.11 | 5.33 |
| 028MA | 21.3 ± 1.7 | 43.7 | - | - | 4.36 ± 0.37 | 1.35 ± 0.07 | 3.60 | |
| Pteroplatytrygon violacea | 006PV | 26.3 ± 0.2 | 65.4 | - | - | 9.91 ± 1.84 | 1.52 ± 0.19 | 5.78 |
| 007PV | 34.8 | 61.2 | - | - | 9.06 ± 0.39 | 3.09 ± 0.13 | 9.15 | |
| 010PV | 40.7 | 69.4 | traces | - | 17.5 ± 1.9 | 2.64 ± 0.26 | 8.09 | |
| 038PV | 17.3 | 79.1 | - | - | 6.10 ± 0.55 | 1.20 ± 0.17 | 6.38 | |
| 039PV | 23.8 ± 0.5 | 67.5 | - | - | 3.79 ± 0.96 | 1.75 ± 0.18 | 10.5 | |
| 049PV | 35.4 | 77.1 | traces | - | 14.8 ± 0.6 | 2.02 ± 0.19 | 10.5 | |
| 050PV | 34.4 ± 2.6 | 69.8 | traces | - | 13.6 ± 1.1 | 2.55 ± 0.38 | 7.84 | |
| 051PV | 40.4 | 47.6 | - | - | 10.4 ± 1.0 | 1.97 ± 0.1 | 6.86 | |
| Min | - | 17.3 | 47.6 | 0.00 | - | 3.79 | 1.2 | 5.78 |
| Max | - | 40.7 | 79.1 | traces | - | 17.5 | 3.09 | 10.5 |
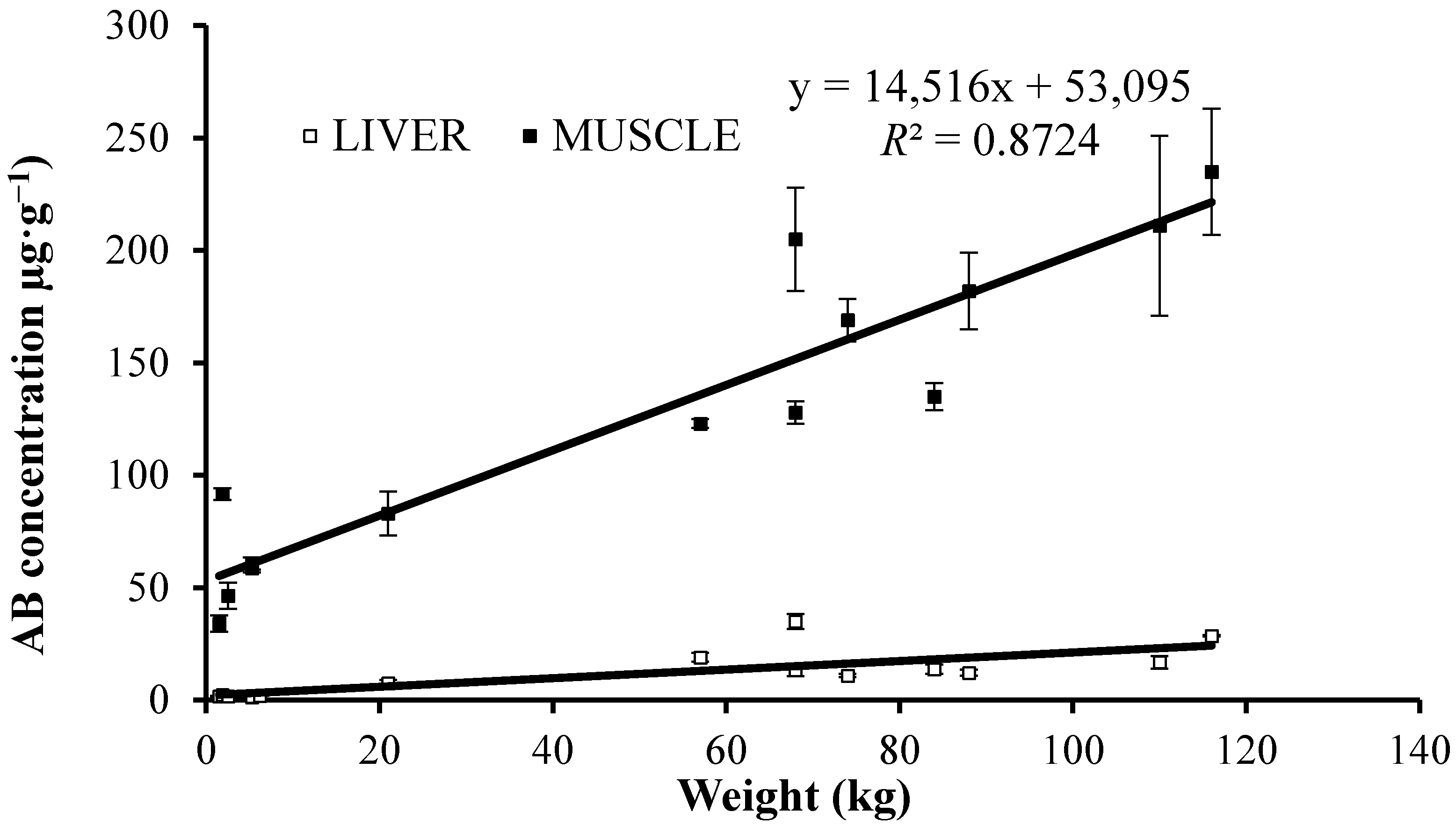
2.2. Arsenic Speciation in Tissues
| Species | Sample | Astotal | Astotal in Extract (%) | AsIII (µg/g) | AB (µg/g) |
|---|---|---|---|---|---|
| Pteromylaeus bovinus | 029PB | 36.3 ± 0.5 | 100 ± 6.0 | - | 30.7 ± 3.7 |
| 034PB | 65.9 ± 0.8 | 95.4 ± 2.1 | - | 54.8 ± 2.7 | |
| 052PB | 92.3 | 88.2 ± 1.5 | - | 74.9 ± 9.8 | |
| 053PB | 205 ± 4 | 78.5 ± 3.1 | traces ** | 152 ± 9.5 | |
| 054PB | 222 ± 2 | 79.2 ± 3.4 | traces | 190 ± 40 | |
| 055PB | 225 | 80.4 ± 2.8 | traces | 115 ± 5 | |
| 056PB | 148 ± 2 | 75.0 ± 4.9 | traces | 111 ± 2.0 | |
| 057PB | 362 ± 9 | 69.9 ± 5.1 | traces | 212 ± 28 | |
| 058PB | 222 ± 5 | 79.3 ± 4.5 | traces | 164 ± 17 | |
| 060PB | 107 ± 2 | 88.6 ± 1.7 | - | 82.6 ± 2.6 | |
| 062PB | 57.8 ± 1.3 | 82.9 ± 3.8 | - | 41.9 ± 5.8 | |
| 063PB | 59.2 ± 0.8 | 95.9 ± 3.9 | - | 53.0 ± 2.0 | |
| 066PB | 180 ± 0.5 | 81.1 ± 3.4 | traces | 122 ± 6 | |
| 067PB | 233 ± 2 | 79.8 ± 2.7 | traces | 185 ± 23 | |
| Min | - | 36.3 | - | 0.00 | 30.7 |
| Max | - | 362 | - | traces | 212 |
| Myliobatis aquila | 023MA | 51.4 | 87.5 ± 3.3 | - | 33.2 ± 3.8 |
| 025MA | 69.8 | - | traces | 34.6 ± 3.4 | |
| 035MA | 32.4 | 84.6 ± 2.2 | - | 25.3 ± 2.0 | |
| 044MA | 47.1 | 85.8 ± 3.0 | traces | 27.8 ± 0.7 | |
| 061MA | 36.5 | 86.3 ± 2.5 | - | 29.4 ± 8.6 | |
| Min | - | 32.4 | - | 0.00 | 25.3 |
| Max | - | 69.8 | - | traces | 34.6 |
| Pteroplatytrygon violacea | 006PV | 90.4 | 91.9 ± 8.0 | 0.03 | 80.6 ± 10.4 |
| 007PV | 48.3 | 69.6 ± 3.0 | - | 30.9 ± 0.3 | |
| 010PV | 82.4 ± 1.6 | 88.2 ± 2.1 | - | 75.3 ± 9.8 | |
| 038PV | 141 ± 4 | 73.8 ± 3.8 | 0.03 | 109 ± 10 | |
| 039PV | 37.4 | 88.8 ± 6.4 | traces | 30.1 ± 2.7 | |
| 049PV | 64.7 ± 0.5 | 82.8 ± 4.5 | traces | 39.1 ± 3.9 | |
| 050PV | 97.3 | 88.0 ± 3.4 | traces | 42.3 ± 3.7 | |
| 051PV | 44.0 | 88.9 ± 2.6 | - | 33.4 ± 1.2 | |
| Min | - | 37.4 | - | 0.00 | 30.1 |
| Max | - | 141 | - | 0.03 | 109 |
| Variables | DW | DL | TL | W | As-Muscle | As-Liver | AB-Liver |
|---|---|---|---|---|---|---|---|
| As-muscle | 0.8862 * | 0.8948 * | 0.8555 * | 0.9254 * | - | 0.6112 * | - |
| AB-muscle | 0.9006 * | 0.8942 * | 0.8817 * | 0.9206 * | 0.9577 * | - | 0.6698 * |
| As-liver | 0.4902 * | 0.6218 * | 0.5797 * | 0.5724 * | - | - | - |
| AB-liver | 0.5684 * | 0.6871 * | 0.6964 * | 0.6346 * | - | 0.9370 * | - |
| DMA-liver | 0.2261 | 0.3253 | 0.3756 | 0.2661 | - | 0.6641 * | 0.5815 * |
| Ether extract-liver | 0.1148 | 0.2375 | 0.2034 | 0.1910 | - | 0.7578 * | 0.6276 * |
| Unextracted -liver | 0.6464 | 0.6236 | 0.5370 | - | −0.2178 | 0.1573 | 0.0760 |
3. Discussion
3.1. Distribution and Speciation of Arsenic
| Species/Life Style | Source of Data | Age | Liver As (µg·g−1) | Muscle As (µg·g−1) | Individual Ratio Muscle to Liver |
|---|---|---|---|---|---|
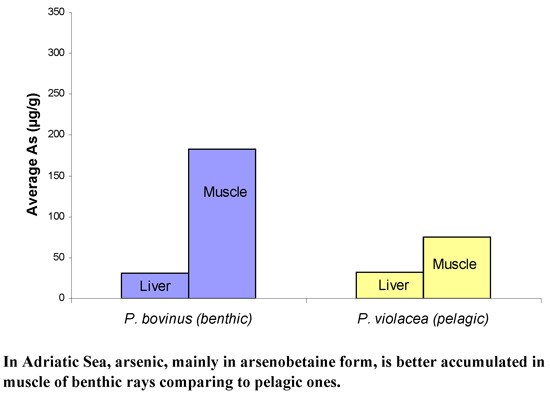
| P. bovinus-benthic | This study | Juvenile | 10.9 ± 1.6 (n = 3) | 67.0 ± 36.2 (n = 3) | 5.96 ± 2.50 (n = 3) |
| Mature | 31.1 ± 16.9 (n = 12) | 183 ± 88 (n = 12) | 5.69± 1.18 (n = 12) | ||
| M. Aquila-benthic | This study | Juvenile | 20.6 (n = 2) | 47.4 ± 14.7 (n = 5) | 3.50 (n = 1) |
| P. violacea-pelagic | This study | Mature | 31.7 ± 8.3 (n = 8) | 75.7 ± 34.5 (n = 8) | 2.02 ± 0.83 * (n = 7) |
| Rhinoptera steindachneri-benthic | [33] | Juvenile | 21.0–54.2 | 5.0–33.0 | - |
| Mature | 27.2–101.8 | 15.1–99.2 | |||
| Raja clavata-benthic | [34] | - | cca. 10–19 # (n = 10) | cca. 27–157 # (n = 10) | - |
| Manta birostris-pelagic | [35] | - | cca. 0.4–0.5 # | cca. 0.7–2.6 # | - |
3.2. Bioaccumulation of Arsenic with Proposed Biomagnification of AB
4. Experimental Section
4.1. Samples
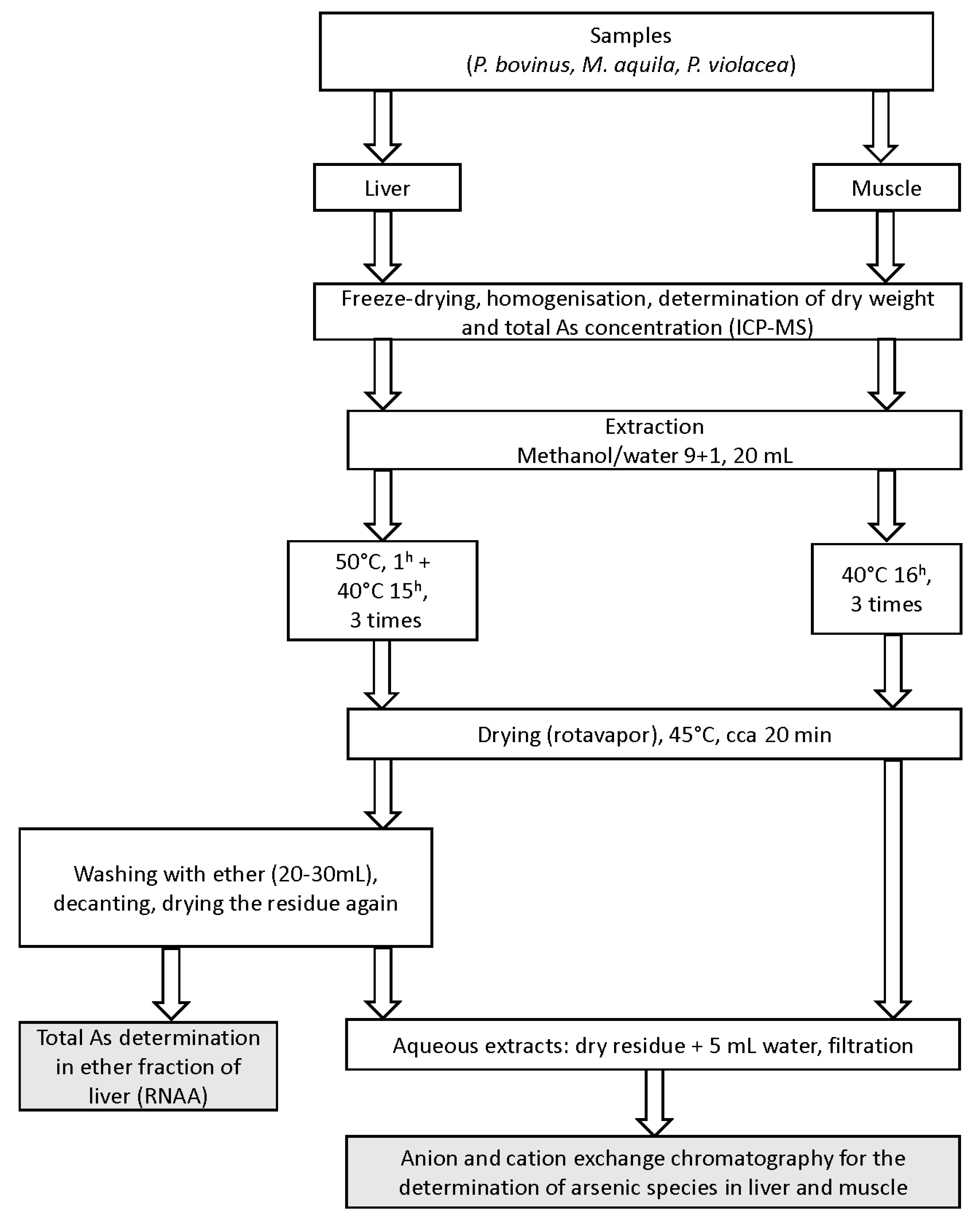
4.2. Sample Preparation
4.3. Reagents and Standards
4.4. Preparation of Extracts (Figure 3)
4.5. Arsenic Speciation Using HPLC-HGAFS
4.6. Total Arsenic Determination Using ICP-MS
4.7. Total Arsenic Determination Using Instrumental Neutron Activation Analysis (INAA)
4.8. Quality Assurance and Quality Control
5. Conclusions
- High arsenic concentrations in liver and especially in muscle of all three ray species studied were found.
- Higher arsenic levels were observed in muscle of both benthic species (Pteromylaeus bovinus, Myliobatis aquila) in comparison with the pelagic one (Pteroplatytrygon violacea).
- The main arsenic compounds found in muscle was AB and in liver AB, DMA and arsenolipids.
- The good correlations found between the length or weight of the fish and total arsenic, or AB, concentrations for muscle reflect important accumulation of AB with age, and according to wider knowledge of local arsenic/AB distribution also its biomagnification in the benthic food chains.
- Since the content and relations between specific osmoregulators in these ray species are not known (Yancey, personal communication) our finding of high AB levels point on favorable retention of glycine betaine and coincidentally also AB for the purpose of osmoregulation; this hypothesis needs further investigation. In fact we are missing basic biological (or better physiological) data for particular ray species.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ščančar, J.; Zuliani, T.; Turk, T.; Milačič, R. Organotin compounds and selected metals in the marine environment of Northern Adriatic Sea. Environ. Monit. Assess. 2007, 127, 271–282. [Google Scholar]
- Horvat, M.; Covelli, S.; Faganeli, J.; Logar, M.; Fajon, V.; Rajar, R.; Širca, A.; Žagar, D. Mercury in contaminated coastal environments; a case study: The Gulf of Trieste. Sci. Total Environ. 1999, 237, 43–56. [Google Scholar]
- Hines, M.E.; Horvat, M.; Faganeli, J.; Bonzongo, J.C.J.; Barkay, T.; Major, E.B.; Scott, K.J.; Bailey, E.A.; Warwick, J.J.; Lyons, W.B. Mercury biogeochemistry in the Idrija River, Slovenia, from above the mine into the Gulf of Trieste. Environ. Res. 2000, 83, 129–139. [Google Scholar]
- Stravisi, F. The vertical structure annual cycle of the mass field parameters in the Gulf of Trieste. Boll. Oceanol. Teor. Appl. 1983, 1, 239–250. [Google Scholar]
- Covelli, S.; Faganeli, J.; de Vittor, C.; Predonzani, S.; Acquavita, A.; Horvat, M. Benthic fluxes of mercury species in a lagoon environment (Grado Lagoon, Northern Adriatic Sea, Italy). Appl. Geochem. 2008, 23, 529–546. [Google Scholar]
- Šlejkovec, Z.; Faganeli, J.; Falnoga, I. Jožef Stefan Institute: Ljubljana; Arsenic in northern Adriatic Sea. unpublished work. 2012–2014.
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar]
- Kosta, L.; Ravnik, V.; Byrne, A.R.; Štirn, J.; Dermelj, M.; Stegnar, P. Some trace elements in the waters, marine organisms and sediments of the Adriatic by neutron activation analysis. J. Radioanal. Chem. 1978, 44, 317–332. [Google Scholar]
- Acquavita, A.A.; Predonzani, S.; Matassi, G.; Rossin, P.; Tamberlich, F.; Falomo, T.; Valic, I. Heavy metal contents and distribution in coastal sediments of the gulf of trieste (Northern Adriatic Sea, Italy). Water Air Soil Pollut. 2010, 211, 95–111. [Google Scholar]
- Conklin, S.D.; Creed, P.A.; Creed, J.T. Detection and quantification of a thio-arsenosugar in marine molluscs by IC-ICP-MS with an emphasis on the interaction of arsenosugars with sulfide as a function of pH. J. Anal. At. Spectrom. 2006, 21, 869–875. [Google Scholar]
- Phillips, D.J.H. Arsenic in aquatic organisms: a review emphasizing chemical speciation. Aquat. Toxicol. 1990, 16, 151–186. [Google Scholar]
- Maher, W.A.; Foster, S.D.; Taylor, A.M.; Krikowa, F.; Duncan, E.G.; Chariton, A.A. Arsenic distribution and species in two Zostera capricorni seagrass ecosystems, New South Wales, Australia. Environ. Chem. 2011, 8, 9–18. [Google Scholar]
- Goessler, W.; Maher, W.; Irgolic, K.J.; Kuehnelt, D.; Schlagenhaufen, C.; Kaise, T. Arsenic compounds in a marine food chain. Fresenius J. Anal. Chem. 1997, 359, 434–437. [Google Scholar]
- Bilandžić, N.; Đokić, M.; Sedak, M. Metal content determination in four fish species from the Adriatic Sea. Food Chem. 2011, 124, 1005–1010. [Google Scholar]
- Ghidini, S.; Delbono, G.; Campanini, G. Cd, Hg and As concentrations in fish caught in the North Adriatic Sea. Vet. Res. Commun. 2003, 27, 297–299. [Google Scholar]
- Šlejkovec, Z.; Kápolna, E.; Ipolyi, I.; van Elteren, J.T. Arsenosugars and other arsenic compounds in littoral zone algae from the Adriatic Sea. Chemosphere 2006, 63, 1098–1105. [Google Scholar]
- Lin, H.T.; Chen, S.W.; Shen, C.J.; Chu, C. Arsenic speciation in fish on the market. J. Food Drug Anal. 2008, 16, 70–75. [Google Scholar]
- Caumette, G.; Koch, I.; Reimer, K.J. Arsenobetaine formation in plankton: A review of studies at the base of the aquatic food chain. J. Environ. Monit. 2012, 14, 2841–2853. [Google Scholar]
- Craig, S.A.S. Betaine in human nutrition. Review article. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar]
- Cullen, W.R.; Nelson, J.C. The biotransformation of monomethylaronate and dimethylarsinate into arsenobetaine in seawater and mussels. Appl. Organomet. Chem. 1993, 7, 319–327. [Google Scholar]
- Francesconi, K.A.; Edmonds, J.S. Arsenic and marine organisms. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: San Diego, CA, USA, 1996; pp. 147–189. [Google Scholar]
- Rahman, M.A.; Hasegawa, H.; Lim, R.P. Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ. Res. 2012, 116, 118–135. [Google Scholar]
- Suhendrayatna, A.; Ohki, S.; Maeda, S. Biotransformation of arsenite in freshwater food-chain models. Appl. Organomet. Chem. 2001, 15, 277–284. [Google Scholar]
- Andreae, M.O. Organoarsenic compounds in the environment. In Organometallic Compounds in the Environment; Craig, P.J., Ed.; Longman: Harlow, UK, 1986; pp. 198–228. [Google Scholar]
- Hayase, D.; Agusa, T.; Toyoshima, S.; Takahashi, S.; Horai Hirata, S.; Itai, T.; Omori, K.; Nishida, S.; Tanabe, S. Biomagnification of arsenic species in the deep-sea ecosystem of the Sagami bay, Japan. Interdiscip. Stud. Environ. Chem. 2010, 4, 199–204. [Google Scholar]
- Pettine, M.; Mastroianni, D.; Camusso, M.; Guzzi, L.; Martinotti, W. Distribution of As, Cr and V species in the Po-Adriatic mixing area, (Italy). Mar. Chem. 1997, 58, 335–349. [Google Scholar]
- Degobbis, D.; Precali, R.; Ferrari, C.R.; Djakovac, T.; Rinaldi, A.; Ivancic, I.; Gismondi, M.; Smodlaka, N. Changes in nutrient concentrations and ratios during mucilage events in the period 1999–2002. Sci. Total Environ. 2005, 353, 103–114. [Google Scholar]
- Hasegawa, H.; Rahman, M.A.; Kitahara, K.; Itaya, Y.; Maki, T.; Ueda, K. Seasonal changes of arsenic speciation in lake waters in relation to eutrophication. Sci. Total Environ. 2010, 408, 1684–1690. [Google Scholar]
- Horvat, M.; Degenek, N.; Lipej, L.; Snoj Tratnik, J.; Faganeli, J. Trophic transfer and accumulation of mercury in ray species in coastal waters affected by historic mercury mining (Gulf of Trieste, northern Adriatic Sea). Environ. Sci. Pollut. Res. 2014, 21, 4163–4176. [Google Scholar]
- Van Elteren, J.T.; Šlejkovec, Z. Ion-exchange separation of eight arsenic compounds with HPLC-UV-HG-AFS and stability testing of the compounds related to food treatment procedures. J. Chromatogr. A 1997, 789, 339–348. [Google Scholar]
- Goessler, W.; Pavkov, M. Accurate quantification and transformation of arsenic compounds during wet ashing with nitric acid and microwave assisted heating. Analyst 2003, 128, 796–802. [Google Scholar]
- Gutiérrez-Mejía, E.; Lares, L.; Sosa-Nishizaki, O. Mercury and arsenic in muscle and liver of the Golden cownose ray, Rhinoptera steindachneri, Evermann and Jenkins, 1891, from the Upper gulf of California, Mexico. Bull. Environ. Contam. Toxicol. 2009, 83, 230–234. [Google Scholar]
- De Gieter, M.; Leermakers, M.; van Ryssen, R.; Noyen, J.; Goeyens, L.; Baeyens, W. Total and toxic arsenic levels in North Sea fish. Arch. Environ. Contam. Toxicol. 2002, 43, 406–417. [Google Scholar]
- Essumang, D.K. Analysis and human health risk assessment of arsenic, cadmium and mercury in Manta birostris (Manta ray) caught along the Ghanian coastline. Hum. Ecol. Risk Assess. 2009, 15, 985–998. [Google Scholar]
- Lipej, L.; Mavrič, B.; Paliska, D.; Capapé, C. Feeding habits of the pelagic stingray Pteroplatytrygon violacea (Chondrichthyes: Dasyatidae) in the Adriatic Sea. J. Mar. Biol. Assoc. UK 2013, 93, 285–290. [Google Scholar]
- Kristan, U.; Kanduč, T.; Osterc, A.; Šlejkovec, Z.; Ramšak, A.; Stibilj, V. Assesment of pollution level using Mytilus galloprovincialis as a bioindicator species: The case of the Gulf of Trieste. Mar. Pollut. Bull. 2014. [Google Scholar] [CrossRef]
- Zeng, H.; Uthus, E.O.; Combs, G.F., Jr. Mechanistic aspects of the interaction between selenium and arsenic. J. Inorg. Biochem. 2005, 99, 1269–1274. [Google Scholar]
- Li, L.; Sun, J.; Li, B.; Li, Y.F.; Chen, C.Y.; Chai, Z.F.; Iida, A.S.; Gao, Y.X. Detection of mercury-, arsenic-, and selenium-containing proteins in fish liver from a mercury polluted area of Guizhou Province. J. Toxicol. Environ. Health Part A 2008, 71, 1266–1269. [Google Scholar]
- Gailer, J. Arsenic-selenium and mercury-selenium bonds in biology. Coord. Chem. Rev. 2007, 251, 234–254. [Google Scholar]
- Dobrovoljc, K.; Falnoga, I.; Tušek-Žnidarič, M.; Mazej, D.; Ščančar, J.; Bulog, B. Cd, Cu, Zn, Se, and Metallothioneins in Two Amphibians, Necturus maculosus (Amphibia, Caudata) and Bufo bufo (Amphibia, Anura). Biol. Trace Elem. Res. 2012, 150, 178–194. [Google Scholar]
- Hinojosa Reyes, L.; Guzmán Mar, J.L; Mizanur Rahman, G.M.; Seybert, B.; Fahrenholz, T.; Kingston, S.H.M. Simultaneous determination of arsenic and selenium species in fish tissues using microwave-assisted enzymatic extraction and ion chromatography—inductively coupled plasma mass spectrometry. Talanta 2009, 78, 983–990. [Google Scholar]
- Hanaoka, K.; Tanaka, Y.; Nagata, Y.; Yoshida, K.; Kaise, T. Water-soluble arsenic residues from several arsenolipids occuring in the tissues of the starspotted shark Musterus manazo. Appl. Organomet. Chem. 2001, 15, 299–305. [Google Scholar]
- Le, X.C.; Li, X.F.; Lai, V.; Ma, M.; Yalcin, S.; Feldmann, J. Simultaneous speciation of selenium and arsenic using elevated temperature liquid chromatography separation with inductively coupled plasma mass spectrometry detection. Spectrochim. Acta Part B At. Spectrosc. 1998, 53, 899–909. [Google Scholar]
- Šlejkovec, Z.; Ruelas-Inzunza, J.R. Jožef Stefan Institute: Ljubljana; Arsenic speciation in tuna fish samples. unpublished work. 2008. [Google Scholar]
- Storelli, M.M.; Marcotrigiano, G.O. Organic and inorganic arsenic and lead in fish from South Adriatic Sea, Italy. Food Addit. Contam. 2000, 17, 763–768. [Google Scholar]
- Goessler, W.; Kuehnelt, D.; Schlagenhaufen, C.; Šlejkovec, Z.; Irgolic, K.J. Arsenobetaine and other arsenic compounds in the National Research Council of Canada Certified Reference Materials DORM 1 and DORM 2. J. Anal. At. Spectrom. 1998, 13, 183–187. [Google Scholar]
- Besada, V.; Gonzalez, J.J.; Schultze, F. Mercury, cadmium, lead, arsenic, copper and zinc concentrations in albacore, yellowfin tuna and bigeye tuna from the Atlantic Ocean. Ciencias Mar. 2006, 32, 439–445. [Google Scholar]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Total and methylmercury in cartilaginous fish from Mediterranean Sea. Mar. Pollut. Bull. 2002, 44, 1354–1358. [Google Scholar]
- Wang, N-X.; Li, Y.; Deng, X-H.; Miao, A-J.; Ji, R.; Yang, L-Y. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res. 2013, 47, 2497–2506. [Google Scholar]
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F. The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta. Mar. Chem. 2013, 157, 78–85. [Google Scholar]
- De Vooys, C.G.N.; Geenevasen, J.A.J. Biosynthesis and role in osmoregulation of glycine-betaine in the Mediterranean mussel Mytilus galloprovincialis LMK. Comp. Biochem. Physiol. Part B 2002, 132, 409–414. [Google Scholar]
- Whaley-Martin, K.J.; Koch, I.; Moriarty, M.; Reimer, K.J. Arsenic speciation in Blue mussels (Mytilus edulis) along a highly contaminated arsenic gradient. Environ. Sci. Technol. 2012, 46, 3110–3118. [Google Scholar]
- Bedford, J.J.; Harper, J.L.; Leader, J.P.; Yancey, P.H.; Smith, R.A.J. Betaine is the principal counteracting osmolyte in tissues of the elephant fish, Callorhinus millii (Elasmobranchii, Holocephali). Comp. Biochem. Physiol. Part B 1998, 119, 512–526. [Google Scholar]
- Gailer, J.; Irgolic, K.J.; Francesconi, K.A.; Edmonds, J.S. Metabolism of arsenic compounds by the blue mussel Mytilus edulis after accumulation from seawater spiked with arsenic compounds. Appl. Organomet. Chem. 1995, 9, 341–355. [Google Scholar]
- Fujihara, J.; Kunito, T.; Kubota, R.; Tanabe, S. Arsenic accumulation in livers of pinnipeds, seabirds and sea turtles: subcellular distribution and interaction between arsenobetaine and glycine betaine. Comp. Biochem. Physiol. Part C 2003, 136, 287–296. [Google Scholar]
- Pierce, S.K.; Rowland-Faux, L.M.; O’Brien, S.M. Different salinity tolerance mechanisms in Atlantic and Chesapeake Bay conspecific oysters: Glycine betaine and amino acid pool variations. Mar. Biol. 1992, 113, 107–115. [Google Scholar]
- Price, A.; Maher, W.; Kirby, J.; Krikowa, F.; Duncan, E.; Taylor, A.; Potts, J. Distribution of arsenic species in an open seagrass ecosystem: relationship to trophic groups, habitats and feeding zones. Environ. Chem. 2012, 9, 77–88. [Google Scholar]
- Larsen, E.H.; Francesconi, K.A. Arsenic concentrations correlate with salinity for fish taken from the North Sea and Baltic waters. J. Mar. Biol. Assoc. UK 2003, 83, 283–284. [Google Scholar]
- Penrose, W.R.; Conacher, H.B.; Black, R.; Méranger, J.C.; Miles, W.; Cunningham, H.M.; Squires, W.R. Implications of inorganic/organic interconversion on fluxes of arsenic in marine food webs. Environ. Health Perspect. 1977, 19, 53–59. [Google Scholar]
- Chen, M.L.; Ma, L.Y.; Chen, X.W. New procedures for arsenic speciation: A review. Talanta 2014, 125, 78–86. [Google Scholar]
- Mavrič, B.; Jenko, R.; Makovc, T.; Lipej, L. On the occurrence of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832), in the Gulf of Trieste (Northern Adriatic). Ann. Ser. Hist. Nat. 2004, 14, 181–186. [Google Scholar]
- Dulčič, J.; Lipej, L.; Orlando Bonaca, M.; Jenko, R.; Grbec, B.; Guélorget, O.; Capapé, C. The bull ray, Pteromylaeus bovinus (Myliobatidae), in the northern Adriatic Sea. Cybium 2008, 32, 119–123. [Google Scholar]
- Šlejkovec, Z.; van Elteren, J.T. Determination of arsenic compounds in reference materials by HPLC-UV-HG-AFS. Talanta 1999, 49, 619–627. [Google Scholar]
- Liu, L.H.; Yun, Z.J.; He, B.; Jiang, G.B. Efficient interface for online coupling of capillary electrophoresis with inductively coupled plasma-mass spectrometry and its application in simultaneous speciation analysis of arsenic and selenium. Anal. Chem 2014, 86, 8167–8175. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šlejkovec, Z.; Stajnko, A.; Falnoga, I.; Lipej, L.; Mazej, D.; Horvat, M.; Faganeli, J. Bioaccumulation of Arsenic Species in Rays from the Northern Adriatic Sea. Int. J. Mol. Sci. 2014, 15, 22073-22091. https://doi.org/10.3390/ijms151222073
Šlejkovec Z, Stajnko A, Falnoga I, Lipej L, Mazej D, Horvat M, Faganeli J. Bioaccumulation of Arsenic Species in Rays from the Northern Adriatic Sea. International Journal of Molecular Sciences. 2014; 15(12):22073-22091. https://doi.org/10.3390/ijms151222073
Chicago/Turabian StyleŠlejkovec, Zdenka, Anja Stajnko, Ingrid Falnoga, Lovrenc Lipej, Darja Mazej, Milena Horvat, and Jadran Faganeli. 2014. "Bioaccumulation of Arsenic Species in Rays from the Northern Adriatic Sea" International Journal of Molecular Sciences 15, no. 12: 22073-22091. https://doi.org/10.3390/ijms151222073
APA StyleŠlejkovec, Z., Stajnko, A., Falnoga, I., Lipej, L., Mazej, D., Horvat, M., & Faganeli, J. (2014). Bioaccumulation of Arsenic Species in Rays from the Northern Adriatic Sea. International Journal of Molecular Sciences, 15(12), 22073-22091. https://doi.org/10.3390/ijms151222073