Therapeutic Effect of Activated Carbon-Induced Constipation Mice with Lactobacillus fermentum Suo on Treatment
Abstract
:1. Introduction
2. Results
2.1. Biological Barriers and the Ability of Hydrophobicity of Lactic Acid Bacteria
| Stain | Survival in pH 3.0 Manmade Gastric Juice (%) | Hydrophobic Property (%) | Growth in Bile Salt (%) | ||
|---|---|---|---|---|---|
| 0.3% | 0.5% | 1.0% | |||
| LF-Suo | 91.33 ± 5.46 | 70.31 ± 4.28 | 27.46 ± 2.23 | 22.51 ± 1.72 | 18.22 ± 1.21 |
| LB | 27.81 ± 3.41 | 25.56 ± 2.71 | 2.61 ± 0.34 | 1.57 ± 0.37 | 1.31 ± 0.22 |
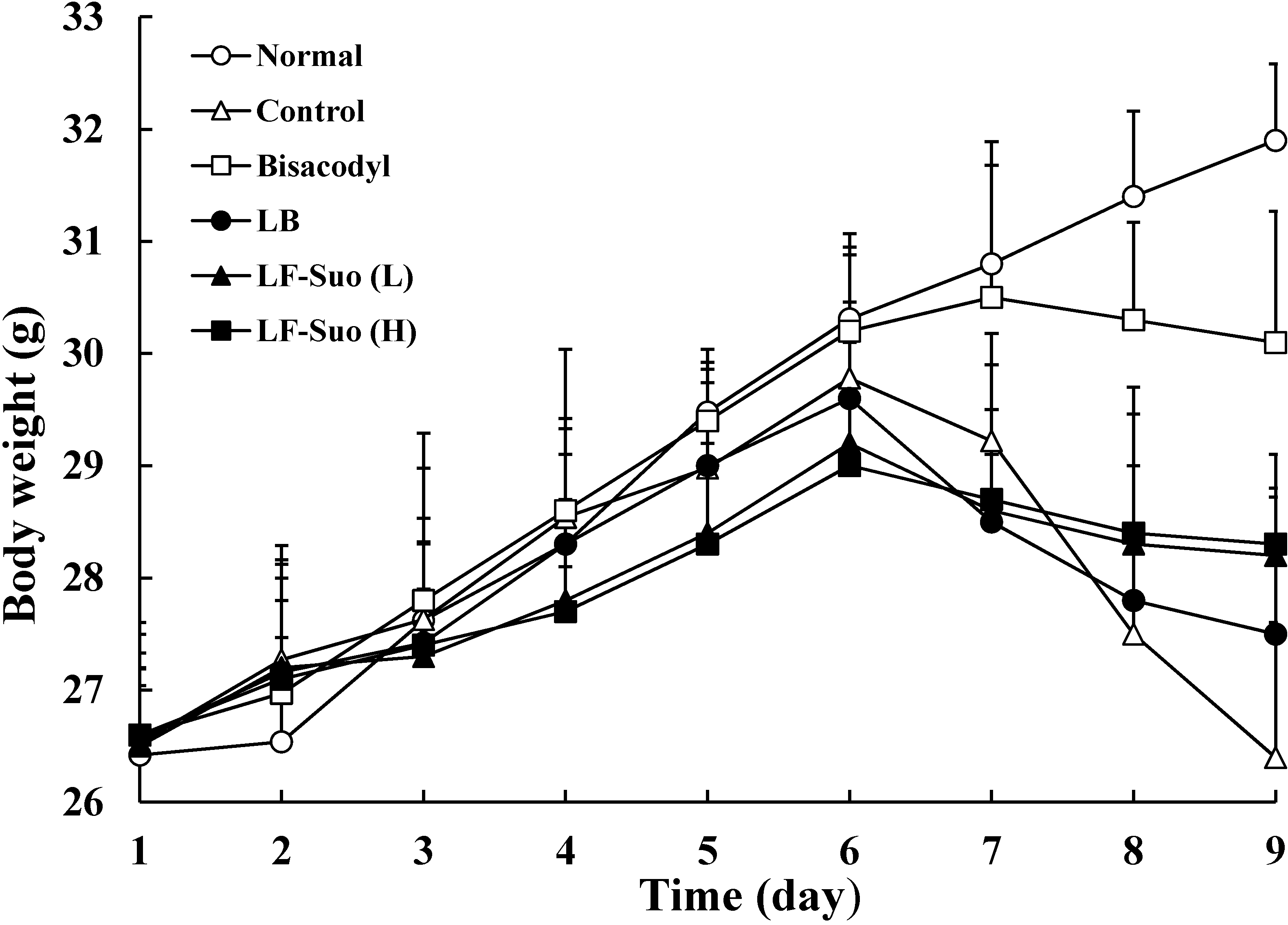
2.2. Body Weight during the Experiment
2.3. Effects of Lactic Acid Bacteria on Diet Uptake and Amount of Water Drinking
| Treatment | Normal (g) | Control (g) | Bisacodyl (g) | Lactobacillus bulgaricus (g) | LF-Suo (×109 CFU/kg b.w.) | |
|---|---|---|---|---|---|---|
| 0.1 (g) | 1.0 (g) | |||||
| 1 Day | 2.69 ± 0.12 | 2.66 ± 0.17 | 2.72 ± 0.14 | 2.74 ± 0.12 | 2.64 ± 0.21 | 2.69 ± 0.16 |
| 2 Day | 2.73 ± 0.13 | 2.71 ± 0.21 | 2.77 ± 0.12 | 2.75 ± 0.16 | 2.74 ± 0.17 | 2.78 ± 0.20 |
| 3 Day | 2.90 ± 0.11 | 2.84 ± 0.20 | 2.86 ± 0.20 | 2.79 ± 0.14 | 2.88 ± 0.15 | 2.93 ± 0.14 |
| 4 Day | 3.04 ± 0.15 | 3.01 ± 0.16 | 3.02 ± 0.15 | 3.00 ± 0.12 | 3.09 ± 0.22 | 3.14 ± 0.12 |
| 5 Day | 3.08 ± 0.18 | 3.06 ± 0.15 | 3.07 ± 0.10 | 3.12 ± 0.11 | 3.12 ± 0.15 | 3.15 ± 0.13 |
| 6 Day | 3.10 ± 0.16 | 3.09 ± 0.14 | 3.12 ± 0.11 | 3.15 ± 0.16 | 3.16 ± 0.12 | 3.16 ± 0.17 |
| 7 Day | 3.14 ± 0.10 | 2.60 ± 0.22 | 2.88 ± 0.22 | 2.63 ± 0.12 | 2.71 ± 0.12 | 2.80 ± 0.10 |
| 8 Day | 3.15 ± 0.13 | 2.19 ± 0.15 | 2.78 ± 0.17 | 2.40 ± 0.15 | 2.57 ± 0.18 | 2.66 ± 0.14 |
| 9 Day | 3.22 ± 0.13 | 2.02 ± 0.09 | 2.70 ± 0.14 | 2.22 ± 0.13 | 2.40 ± 0.15 | 2.48 ± 0.08 |
| Treatment | Normal (mL) | Control (mL) | Bisacodyl Control (mL) | Lactobacillus bulgaricus Control (mL) | LF-Suo (×109 CFU/kg b.w.) | |
|---|---|---|---|---|---|---|
| 0.5 (mL) | 1.0 (mL) | |||||
| 1 Day | 6.24 ± 0.20 | 6.25 ± 0.20 | 6.24 ± 0.20 | 6.24 ± 0.12 | 6.24 ± 0.20 | 6.24 ± 0.12 |
| 2 Day | 6.26 ± 0.21 | 6.28 ± 0.15 | 6.30 ± 0.20 | 6.22 ± 0.16 | 6.24 ± 0.17 | 6.26 ± 0.20 |
| 3 Day | 6.27 ± 0.12 | 6.24 ± 0.16 | 6.30 ± 0.16 | 6.26 ± 0.12 | 6.26 ± 0.16 | 6.28 ± 0.17 |
| 4 Day | 6.25 ± .020 | 6.25 ± 0.20 | 6.29 ± 0.15 | 6.28 ± 0.16 | 6.28 ± 0.20 | 6.27 ± 0.21 |
| 5 Day | 6.32 ± 0.20 | 6.31 ± 0.15 | 6.32 ± 0.20 | 6.30 ± 0.13 | 6.31 ± 0.27 | 6.29 ± 0.20 |
| 6 Day | 6.34 ± 0.20 | 6.28 ± 0.21 | 6.34 ± 0.18 | 6.32 ± 0.10 | 6.27 ± 0.15 | 6.28 ± 0.20 |
| 7 Day | 6.37 ± 0.21 | 6.14 ± 0.17 | 6.28 ± 0.15 | 6.17 ± 0.13 | 6.25 ± 0.17 | 6.26 ± 0.15 |
| 8 Day | 6.38 ± 0.18 | 5.75 ± 0.18 | 6.25 ± 0.15 | 5.93 ± 0.14 | 6.08 ± 0.16 | 6.14 ± 0.12 |
| 9 Day | 6.40 ± 0.22 | 5.63 ± 0.20 | 6.21 ± 0.17 | 5.75 ± 0.12 | 6.01 ± 0.07 | 6.12 ± 0.12 |
2.4. Effect of Lactic Acid Bacteria on Defecation Status of Mice
| Treatment | Normal | Control | Bisacodyl | Lactobacillus bulgaricus | LF-Suo (×109 CFU/kg b.w.) | |
|---|---|---|---|---|---|---|
| 0.1 (g) | 1.0 (g) | |||||
| 1–6 Days (Dose with samples) | ||||||
| Defecation weight (g) | 0.90 ± 0.09 | 0.94 ± 0.11 | 1.13 ± 0.07 | 0.91 ± 0.05 | 0.92 ± 0.08 | 0.91 ± 0.05 |
| Particle counts of defecation | 35 ± 4 | 36 ± 7 | 49 ± 6 | 36 ± 2 | 36 ± 5 | 37 ± 5 |
| Water content of defecation (%) | 47 ± 4 | 47 ± 5 | 55 ± 5 | 49 ± 4 | 45 ± 5 | 46 ± 6 |
| 7–9 Days (High concentration dose with samples and activated carbon) | ||||||
| Defecation weight (g) | 0.91 ± 0.05 | 0.37 ± 0.06 | 0.74 ± 0.15 | 0.40 ± 0.05 | 0.58 ± 0.04 | 0.67 ± 0.09 |
| Particle counts of defecation | 36 ± 3 | 19 ± 6 | 38 ± 5 | 21 ± 3 | 27 ± 5 | 31 ± 4 |
| Water content of defecation (%) | 46 ± 5 | 16 ± 3 | 40 ± 3 | 23 ± 2 | 29 ± 3 | 36 ± 4 |
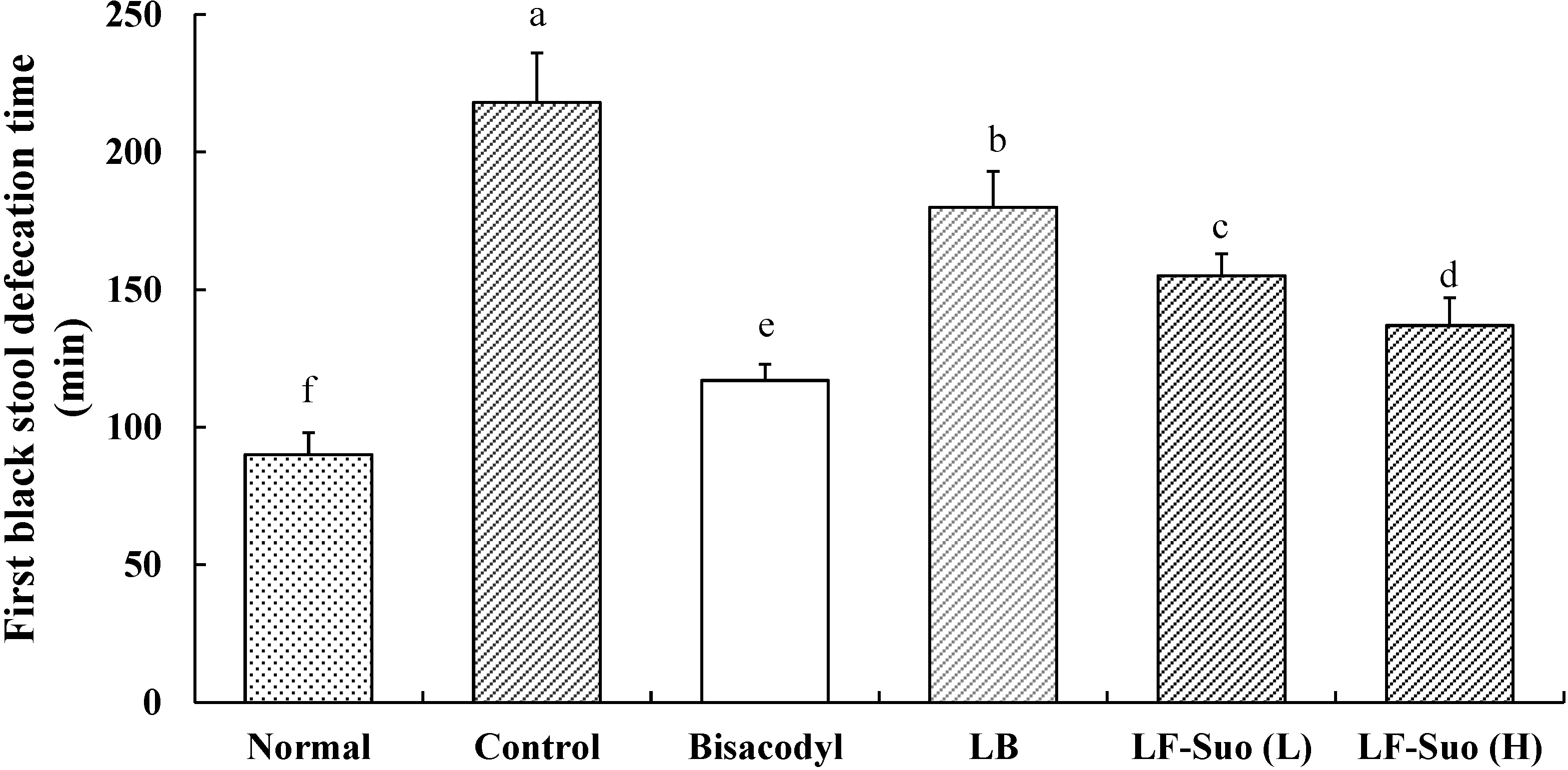
2.5. Time to the First Black Stool Defecation
2.6. GI (Gastrointestinal) Transit

| Treatment | Normal | Control | Bisacodyl | Lactobacillus bulgaricus | LF-Suo (×109 CFU/kg b.w.) | |
|---|---|---|---|---|---|---|
| 0.1 (g) | 1.0 (g) | |||||
| Small intestine length (cm) | 47.9 ± 2.6 a | 44.6 ± 4.2 a | 47.2 ± 2.2 a | 46.4 ± 3.2 a | 47.0 ± 3.8 a | 47.1 ± 2.5 a |
| Distance traveled (cm) | 47.9 ± 2.6 a | 18.8 ± 3.2 f | 44.7 ± 1.3 b | 25.6 ± 2.5 e | 34.0 ± 3.1 d | 40.3 ± 1.9 c |
| GI transit (%) | 100.0 ± 0.0 a | 42.1 ± 5.6 f | 94.6 ± 6.7 b | 55.2 ± 4.6 e | 72.3 ± 6.5 d | 85.5 ± 6.3 c |
2.7. MTL (Motilin), Gas (Gastrin), ET (Endothelin), SS (Somatostatin), AChE (Acetylcholinesterase), SP (Substance P) and VIP (Vasoactive Intestinal Peptide) Levels in Serum
| Level (pg/mL) | Normal | Control | Bisacodyl | Lactobacillus bulgaricus | LF-Suo (×109 CFU/kg b.w.) | |
|---|---|---|---|---|---|---|
| 0.5 (g) | 1.0 (g) | |||||
| MTL | 173.2 ± 12.6 a | 101.3 ± 9.7 f | 155.4 ± 8.7 b | 119.7 ± 7.7 e | 137.5 ± 5.7 d | 150.5 ± 6.8 c |
| Gas | 80.2 ± 3.2 a | 44.3 ± 2.7 f | 73.6 ± 2.6 b | 50.2 ± 2.1 e | 63.8 ± 2.0 d | 70.3 ± 2.3 c |
| ET | 13.9 ± 0.4 a | 7.1 ± 0.3 f | 12.1 ± 0.3 b | 8.4 ± 0.3 e | 9.9 ± 0.2 d | 10.9 ± 0.2 c |
| SS | 33.2 ± 1.9 f | 61.8 ± 1.6 a | 40.3 ± 2.0 e | 50.2 ± 1.9 b | 47.2 ± 1.2 c | 43.5 ± 1.3 d |
| AchE | 31.1 ± 1.2 a | 12.7 ± 0.9 f | 27.8 ± 0.9 b | 15.9 ± 0.8 e | 22.4 ± 0.9 d | 25.6 ± 0.9 c |
| SP | 63.2 ± 2.8 a | 37.2 ± 1.9 f | 55.3 ± 1.7 b | 41.3 ± 0.5 e | 47.7 ± 0.8 d | 53.1 ± 0.7 c |
| VIP | 52.3 ± 1.9 a | 30.6 ± 1.0 f | 47.1 ± 1.1 b | 33.6 ± 0.9 e | 40.3 ± 0.4 d | 45.2 ± 0.8 c |
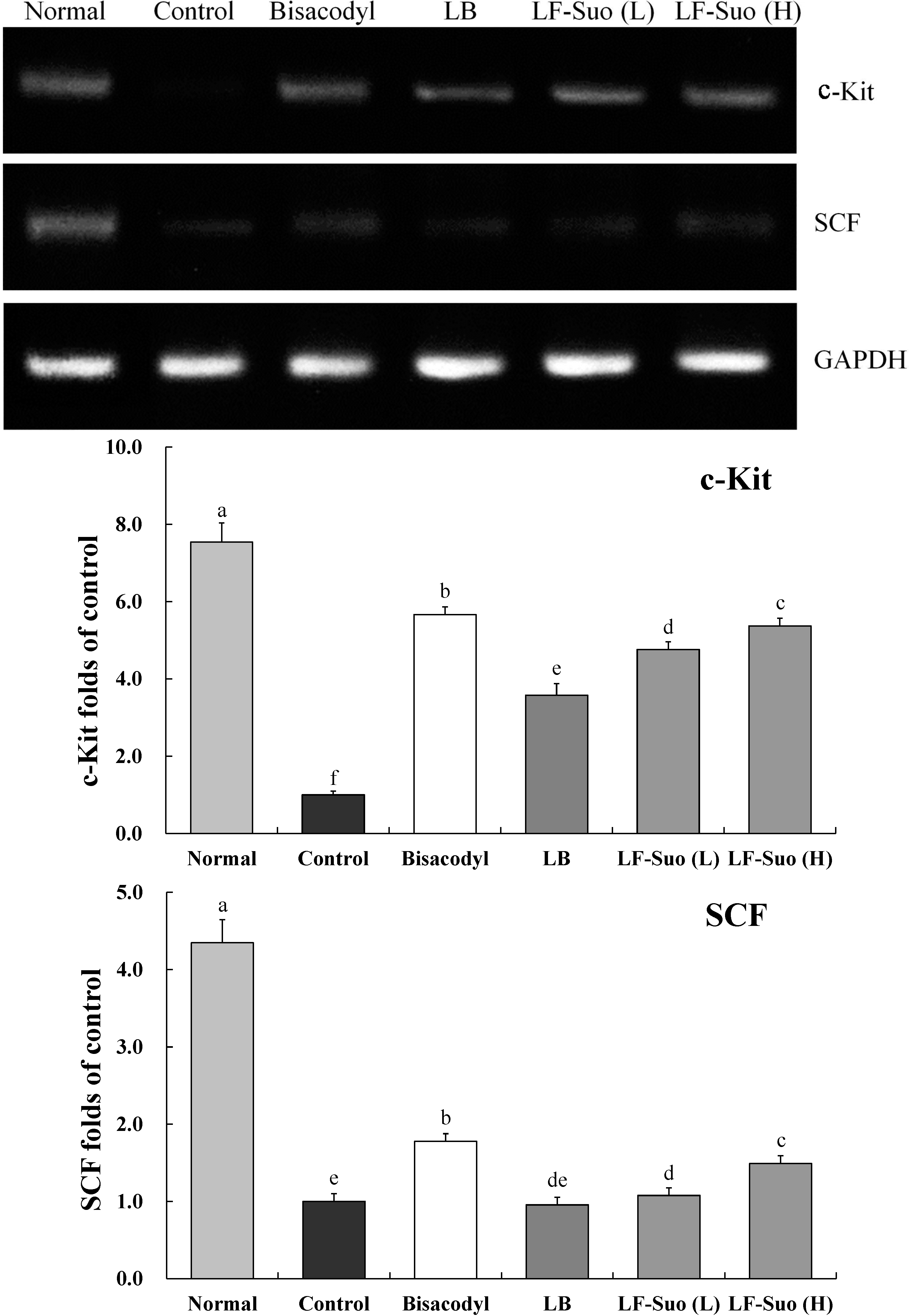
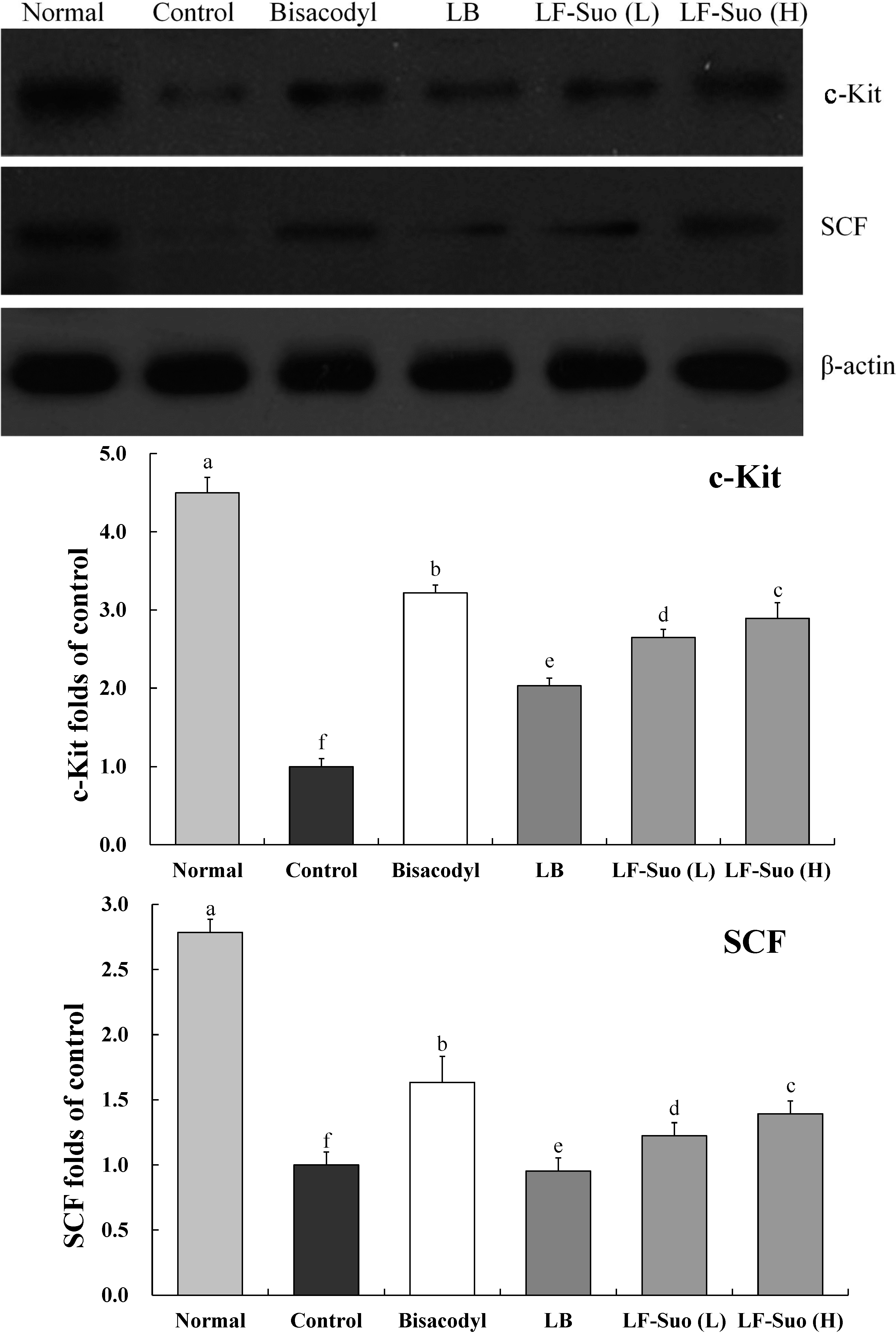
2.8. Gene Expressions of c-Kit and SCF (Stem Cell Factor)
2.9. Gene Expression of TRPV1, GDNF and NOS
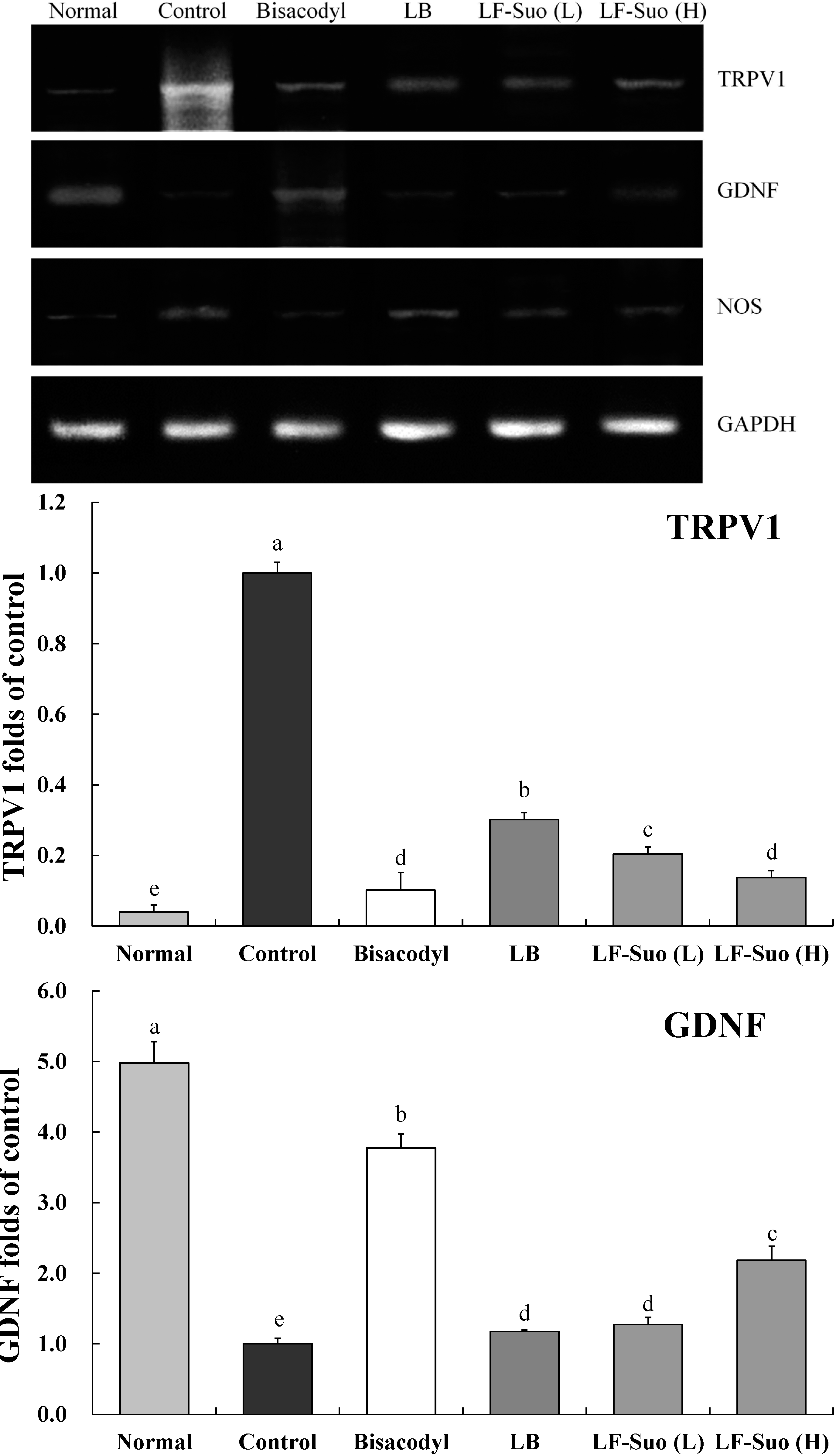
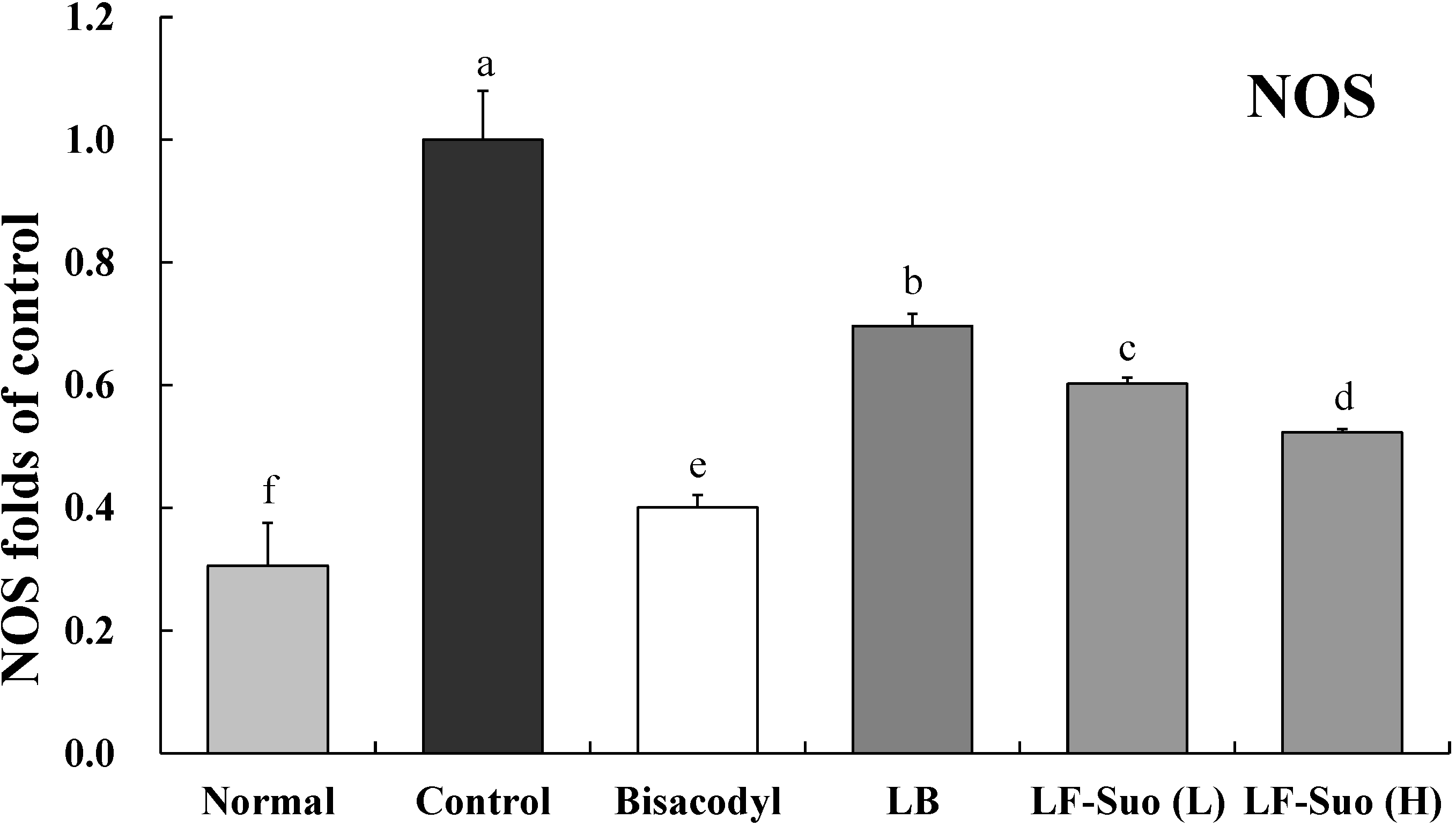

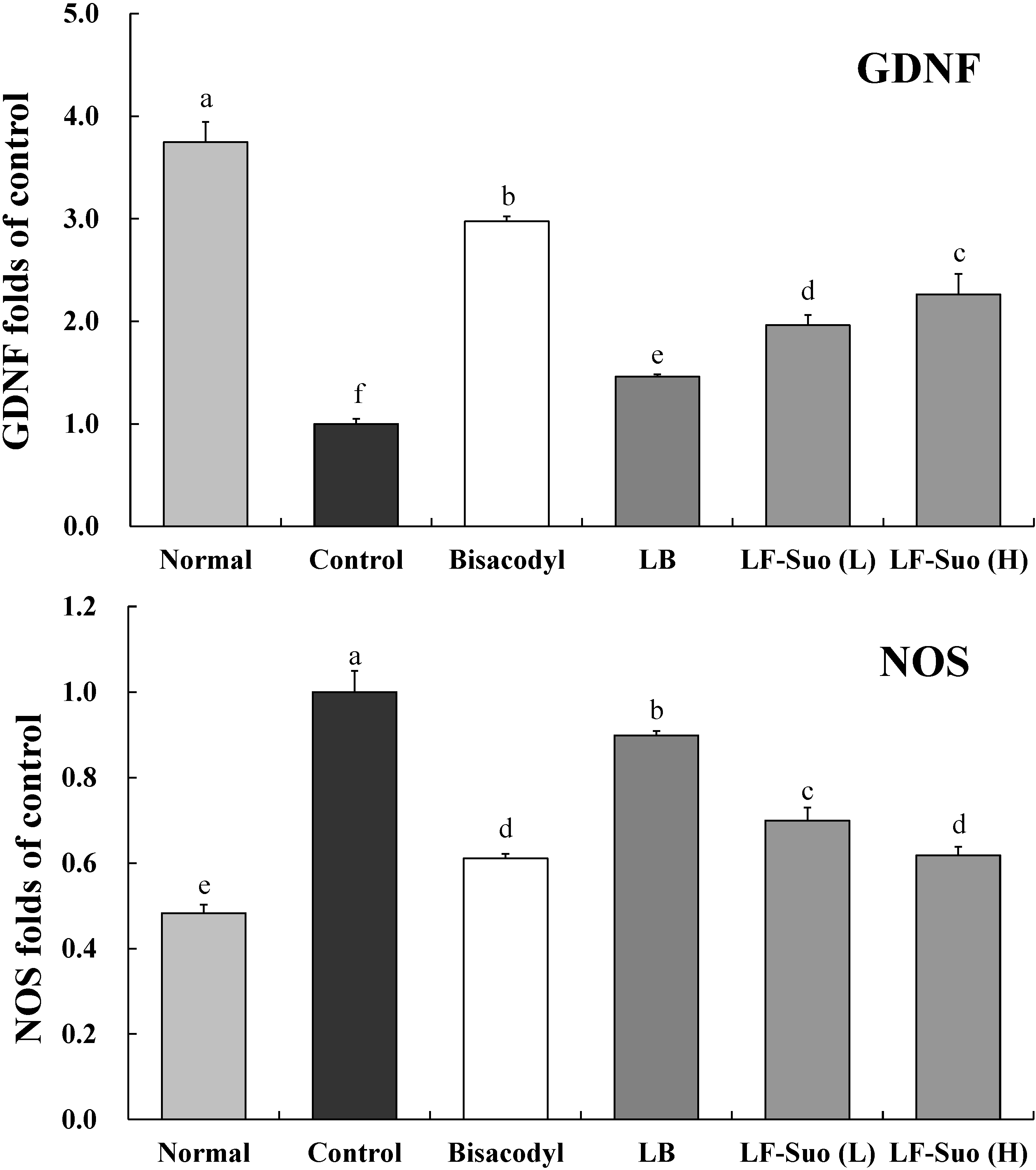
3. Discussion
4. Materials and Methods
4.1. Microorganism Strains
4.2. Animals
4.3. Endurance Capacity of Lactic Acid Bacteria to pH 3.0 Manmade Gastric Juice
4.4. Determination of Bacterial Tolerance of Bile Salt (Oxgall) in Different Concentration
4.5. Determination the Hydrophobic Property of Lactic Acid Bacteria
4.6. Induction of Constipation in Mice
4.7. Measurement Defecation Status of Mice
4.8. GI Transit and Defecation Time
4.9. MTL, Gas, ET, SS, AChE, SP and VIP Levels in Serum
4.10. RT-PCR Assay
4.11. Western Blot Assay
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, C.S.; Li, J.; Qian, Y.; Suo, H.Y. Research progress of yak milk and fermented yak milk and their nutritional value. J. Dairy Sci. Technol. 2012, 35, 43–46. [Google Scholar]
- Wu, C.S.; Shu, C.; Li, J.; Qian, Y.; Suo, H.Y. The research progress and prospect of yak yogurt lactic acid bacteria. Food Ind. 2012, 33, 129–133. [Google Scholar]
- Ueki, A.; Otsuka, M. Life style risks of Parkinson’s disease: Association between decreased water intake and constipation. J. Neurol. 2004, 251, 18–23. [Google Scholar] [CrossRef]
- Wexner, S.D.; Beck, D.E.; Baron, T.H.; Fanelli, R.D.; Hyman, N.; Hyman, N.; Shen, B.; Wasco, K.E. A consensus document on bowel preparation before colonoscopy: Prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest. Endosc. 2006, 63, 894–909. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Wang, Q.; Qian, Y.; Zhou, Y.; Wang, R.; Zhao, X. Component analysis of Pu-erh and its anti-constipation effects. Mol. Med. Rep. 2014, 9, 2003–2009. [Google Scholar] [PubMed]
- Farrugia, G.; Lei, S.; Lin, X.; Miller, S.M.; Nath, K.A.; Ferris, C.D.; Levitt, M.; Szurszewski, J.H. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2003, 100, 8567–8570. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Reed, D.; Sarr, M.G.; Farrugia, G.; Szurszewski, J.H. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol. Motil. 2001, 13, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Farrugia, G.; Miller, S.M.; Ferris, C.D.; Snyder, S.H.; Szurszewski, J.H. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: Evidence from genomic deletion of biosynthetic enzymes. Proc. Natl. Acad. Sci. USA 2000, 97, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Battish, R.; Cao, G.Y.; Lynn, R.B.; Chakder, S.; Rattan, S. Heme oxygenase-2 distribution in anorectum: Colocalization with neuronal nitric oxide synthase. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, 148–155. [Google Scholar]
- Emmanuel, A.V.; Tack, J.; Quigley, E.M.; Talley, N.J. Pharmacological management of constipation. Neurogastroenterol. Motil. 2009, 21, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Duan, P.B.; Du, S.Z.; Sun, J.F.; Mei, S.J.; Wang, X.Q.; Zhang, Y.Y. Efficacy of auriculotherapy for constipation in adults: A systematic review and meta-analysis of randomized controlled trials. J. Altern. Complement. Med. 2014, 20, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Walia, R.; Mahajan, L.; Steffen, R. Recent advances in chronic constipation. Curr. Opin. Pediatr. 2009, 21, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Lissner, S. Fixed combination of oxycodone with naloxone: A new way to prevent and treat opioid-induced constipation. Adv. Ther. 2010, 27, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Mercenier, A.; Pavan, S.; Pot, B. Probiotics as biotherapeutic agents: Present knowledge and future prospects. Curr. Pharm. Des. 2003, 9, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Song, S.H.; Huang, J.Q.; Huang, Y.; Xie, M.Y. Tolerance of Lactobacillus plantarum NCU116 in stimulated digestive environments. Food Sci. 2011, 32, 114–117. [Google Scholar]
- Bekkali, N.; Bongers, M.E.J.; van den Berg, M.M.; Liem, O.; Benninga, M.A. The role of a probiotics mixture in the treatment of childhood constipation: A pilot study. Nutr. J. 2007, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 28, 197–215. [Google Scholar] [CrossRef]
- Sousa, C.; Rodrigues, D.; Oliveira, R.; Song, W.; Mano, J.F.; Azeredo, J. Superhydrophobic poly (l-lactic acid) surface as potential bacterial colonization substrate. AMB Express 2011, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Yang, B.C.; Jung, Y.R.; Lee, J.W.; Im, G.S.; Seong, H.H.; Park, J.K.; Kang, J.K.; Hwang, S. In vitro and in vivo genotoxic effects of somatic cell nuclear transfer cloned cattle meat. Food Chem. Toxicol. 2011, 49, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Hur, J.W.; Yu, M.A.; Cheigh, C.I.; Kim, K.N.; Hwang, J.K.; Pyun, Y.R. Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J. Food Prot. 2003, 66, 3–12. [Google Scholar] [PubMed]
- Li, G.J.; Qian, Y.; Sun, P.; Feng, X.; Zhu, K.; Zhao, X. Preventive effect of polysaccharide of Larimichthys crocea swimming bladder on activated carbon-induced constipation in mice. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 167–172. [Google Scholar] [CrossRef]
- Tang, X.G.; Wu, J.; Liu, F. Study the amount of GAS, MTL, SP in the blood of constipation rat model by biansetong mixture. Chin. Arch. Tradit. Chin. Med. 2011, 29, 1549–1551. [Google Scholar]
- Liu, Y.; Zhao, X.R.; Wang, R.; Qiu, G.Q.; Zhang, M. Effect of Zhizhuwan on gastrointestinal peptide concentrations in plasma of diabetic gastroenteropathy with constipation patients. China J. Chin. Mater. Med. 2008, 33, 2966–2968. [Google Scholar]
- Iijima, K.; Koike, T.; Abe, Y.; Shimosegawa, T. Cutoff serum pepsinogen values for predicting gastric acid secretion status. Tohoku J. Exp. Med. 2014, 232, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Fevang, J.; Ovrebø, K.; Myking, O.; Grong, K.; Svanes, K. Role of endothelin in the circulatory changes associated with small bowel strangulation obstruction in pigs: Effects of the endothelin receptor antagonist bosentan. J. Surg. Res. 2001, 96, 224–232. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul. Pept. 2014, 188, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Moriya, R.; Fujikawa, T.; Ito, J.; Shirakura, T.; Hirose, H.; Suzuki, J.; Fukuroda, T.; Macneil, D.J.; Kanatani, A. Pancreatic polypeptide enhances colonic muscle contraction and fecal output through neuropeptide YY4 receptor in mice. Eur. J. Pharmacol. 2010, 627, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yik, Y.I.; Farmer, P.J.; King, S.K.; Chow, C.W.; Hutson, J.M.; Southwell, B.R. Gender differences in reduced substance P (SP) in children with slow-transit constipation. Pediatr. Surg. Int. 2011, 27, 699–704. [Google Scholar] [CrossRef] [PubMed]
- King, S.K.; Sutcliffe, J.R.; Ong, S.Y.; Lee, M.; Koh, T.L.; Wong, S.Q.; Farmer, P.J.; Peck, C.J.; Stanton, M.P.; Keck, J.; et al. Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol. Motil. 2010, 22, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genomics 2005, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Wu, W.; Li, Y.; Hoppe, D.; Stannek, P.; Glinka, A.; Niehrs, C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 2001, 411, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, G. Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil. 2008, 20, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Brading, A.F.; McCloskey, K.D. Mechanisms of disease: Specialized interstitial cells of the urinary tract—An assessment of current knowledge. Nat. Clin. Pract. Urol. 2005, 2, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Geppetti, P.; Trevisani, M. Activation and sensitisation of the vanilloid receptor: Role in gastrointestinal inflammation and function. Br. J. Pharmacol. 2004, 141, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.P.; Fan, Y.H.; Lv, B. Advances in understanding the role of neurotrophins in physiological and pathological processes in the intestinal tract. World J. Gastroenterol. 2010, 18, 2884–2888. [Google Scholar]
- Tomita, R.; Igarashi, S.; Fujisaki, S.; Tanjoh, K. The effects of neurotensin in the colon of patients with slow transit constipation. Hepatogastroenterology 2007, 54, 1662–1666. [Google Scholar] [PubMed]
- Zhao, W.J.; Lv, J.L.; Ma, L.Y. Analysis of the surface hydrophobicity of Lactobacillus acidophilus. China Dairy Ind. 2011, 39, 8–11. [Google Scholar]
- Qian, Y.; Zhao, X.; Kan, J. Preventive effect of resistant starch on activated carbon-induced constipation in mice. Exp. Ther. Med. 2013, 6, 228–232. [Google Scholar] [PubMed]
- Chen, L.H.; Song, J.L.; Qian, Y.; Zhao, X.; Suo, H.Y.; Li, J. Increased preventive effect on colon carcinogenesis by use of resistant starch (RS3) as the carrier for polysaccharide of Larimichthys crocea swimming bladder. Int. J. Mol. Sci. 2013, 15, 817–829. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suo, H.; Zhao, X.; Qian, Y.; Li, G.; Liu, Z.; Xie, J.; Li, J. Therapeutic Effect of Activated Carbon-Induced Constipation Mice with Lactobacillus fermentum Suo on Treatment. Int. J. Mol. Sci. 2014, 15, 21875-21895. https://doi.org/10.3390/ijms151221875
Suo H, Zhao X, Qian Y, Li G, Liu Z, Xie J, Li J. Therapeutic Effect of Activated Carbon-Induced Constipation Mice with Lactobacillus fermentum Suo on Treatment. International Journal of Molecular Sciences. 2014; 15(12):21875-21895. https://doi.org/10.3390/ijms151221875
Chicago/Turabian StyleSuo, Huayi, Xin Zhao, Yu Qian, Guijie Li, Zhenhu Liu, Jie Xie, and Jian Li. 2014. "Therapeutic Effect of Activated Carbon-Induced Constipation Mice with Lactobacillus fermentum Suo on Treatment" International Journal of Molecular Sciences 15, no. 12: 21875-21895. https://doi.org/10.3390/ijms151221875
APA StyleSuo, H., Zhao, X., Qian, Y., Li, G., Liu, Z., Xie, J., & Li, J. (2014). Therapeutic Effect of Activated Carbon-Induced Constipation Mice with Lactobacillus fermentum Suo on Treatment. International Journal of Molecular Sciences, 15(12), 21875-21895. https://doi.org/10.3390/ijms151221875




