Therapeutic Phytogenic Compounds for Obesity and Diabetes
Abstract
:1. Introduction
1.1. History of Natural Compounds
1.2. Advantages of Natural Compounds
1.3. Disadvantages of Natural Compounds
2. Obesity and Diabetes
2.1. Obesity: Current Issues and Treatments
2.2. Diabetes: Current Issues and Treatments
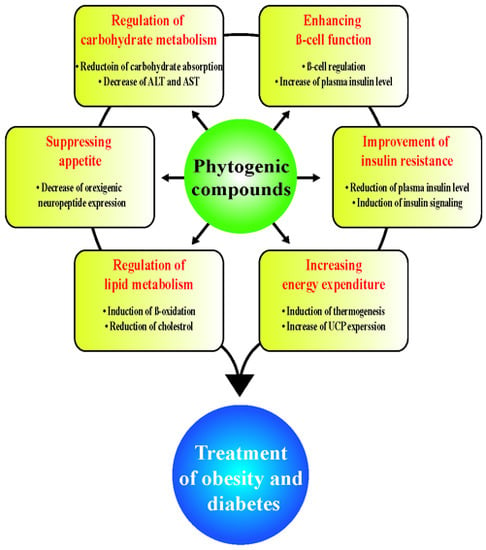
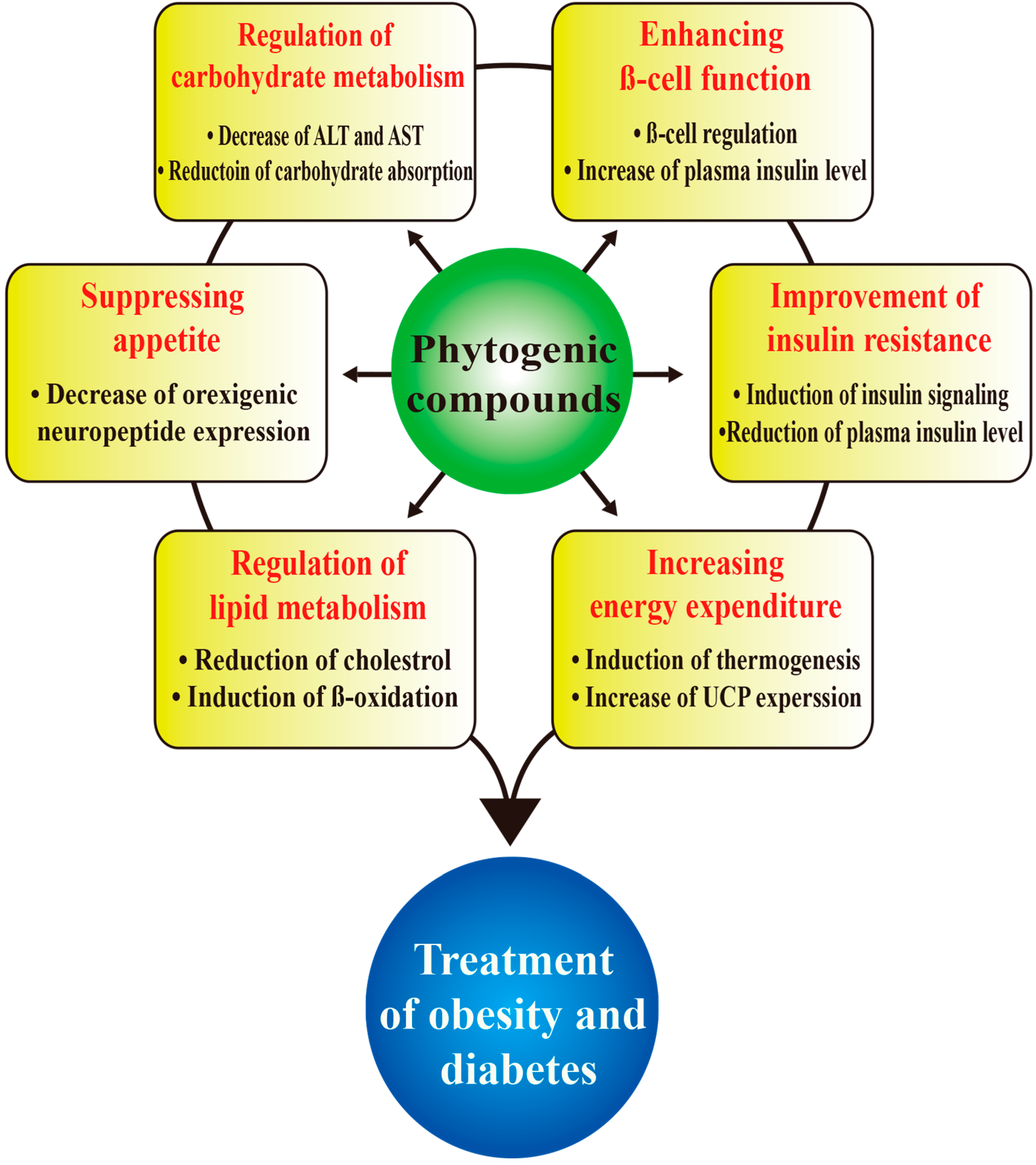
3. Phytogenic Compounds
3.1. Possible Therapeutic Compounds for Obesity
3.1.1. Compounds that Suppress Food Intake
Panax quinquefolium (American Ginseng)
Panax ginseng (Asian Ginseng)
| Scientific Name (Common Name) | Methods | Results | Function | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | Obesity | |||||||||
| Improve Insulin Resistance | Enhance β-Cell Function | Multiple Anti-Diabetic | Suppress Appetite | Stimulate Energy Expenditure | Regulate Lipid Metabolism | Regulate Carbohydrate Metabolism | ||||
| Vaccinium spp. (Blueberry) | Blueberry powder fed to HFD-induced obese mice | ↓Blood glucose level | ● | - | - | - | - | - | - | [26] |
| Oral administration of blueberry with Labrasol | ↓Blood glucose level | ● | - | - | - | - | - | - | [40] | |
| Fermented blueberry fed to KKAy mice | ↓Blood glucose level | ● | - | - | - | - | - | - | [41] | |
| Blueberry fed to Zucker rats | ↑PPAR-α and PPAR-γ activity | ● | - | - | - | - | - | - | [42] | |
| Oral administration of blueberry extract to rats | ↓Food intake and body weight gain | - | - | - | ● | - | - | - | [43] | |
| Oral administration of water containing with blueberry extract to HFD fed mice | ↓Total body fat and body fat | - | - | - | ● | - | - | - | [44] | |
| Vaccinium angustifolium (Wild blueberry) | Wild blueberry-enriched diet for obese rats | ↓Triacylglycerol, total cholesterol, SREBP-1 and fatty acid synthase ↑PPAR-α and PPAR-γ | - | - | - | - | - | ● | - | [45] |
| Vitis vinifera (Grape vine) | Resveratrol treated to C2C12 mytotube cell line. | ↑Glucose uptake and AMPK | - | - | ● | - | - | - | - | [46] |
| Polyphenolic extract treated to HepG2 cell line | ↓Glycogen phosphorylase | - | - | ● | - | - | - | - | [47] | |
| Resveratrol treated to L6 rat skeletal muscle cell line | ↑Glucose uptake and AMPK | - | - | ● | - | - | - | - | [48] | |
| Cinnamomum (Cinnamon) | Cinnamon treated to rat adipocytes | ↑PI3K | ● | - | - | - | - | - | - | [49] |
| Water and polyphenol extracts treated to 3T3-L1 adipocytes | ↑Insulin receptor and GLUT4 protein expression | ● | - | - | - | - | - | - | [50] | |
| Administration of cinnamon extract to 3T3-L1 adipocytes | ↑The expression of LPL, CD36, GLUT4, and acyl-CoA oxidase | - | - | - | - | - | ● | - | [51] | |
| Administration of cinnamon powder with water to C57BL/6J db/db mice | ↓Fasting glucose level, free fatty acid, LDL cholesterol, and AST levels | - | - | - | - | - | ● | - | [51] | |
| Trigonella foenum graecum (Fenugreek) | Oral administration of fenugreek seed to diabetic patients | ↓Blood glucose level ↑Plasma insulin level | ● | - | - | - | - | - | - | [52] |
| Oral administration of fenugreek to NIDD patients | ↓Fasting blood glucose level | ● | - | - | - | - | - | - | [53] | |
| Ervatamia microphylla (Kerr) | Oral administration of conophylline to STZ-induced diabetic rats | ↓Blood glucose level ↑β-cell proliferation | - | ● | - | - | - | - | - | [54] |
| Conophylline treatment to pancreatic stellate cells | ↓Activation of pancreatic stellate cells | - | ● | - | - | - | - | - | [55] | |
| Conophylline administration to Goto-Kakazaki rats | ↓Blood glucose level ↑Plasma insulin level | - | ● | - | - | - | - | - | [55] | |
| Anoectochilus roxburghii (Jewel orchid) | Oral administration of kinsenoside to STZ-induced rats | ↓Blood glucose level ↑Plasma insulin level | - | ● | - | - | - | - | - | [56] |
| Carica papaya (Papaya) | Papaya extract administered to STZ-induced mice | ↓Blood glucose level ↑Insulin synthesis and β-cell regeneration | - | ● | - | - | - | - | - | [57] |
| Momordica charantia (Bitter melon) | Intraperitoneal injection of bitter melon aqueous to STZ-induced mice | ↓Blood glucose level | - | - | ● | - | - | - | - | [58] |
| Oral administration of bitter melon juice to maturity onset diabetes patients | ↓Blood glucose level | - | - | ● | - | - | - | - | [59] | |
| Oral administration of bitter melon powder to HFD fed mice | ↓Body weight gain, hyperlipidemia, and hyperglycemia | - | - | - | - | - | ● | - | [60] | |
| Oral administration of bitter melon powder to HFD-induced obese rats | ↓The number of large adipocytes, adipose tissue mass, TAG content, FAS, ACC-1, LPL and adipocyte fatty acid-binding protein (aP2) | - | - | - | - | - | ● | - | [61] | |
| Capsicum (Chili pepper) | Capsaicin administered to ZFD rats | ↓Blood glucose level and Plasma insulin level | - | - | ● | - | - | - | - | [62] |
| Oral administration of chili pepper powder to HFD-induced diabetic rats | ↑Plasma insulin level | - | - | ● | - | - | - | - | [63] | |
| Administration of capsaicin to 3T3-L1 adipocytes | ↑Hormone sensitive lipase, CPT-1a and UCP2 | - | - | - | - | ● | - | - | [64] | |
| Oral administration of capsinoids to men and give a cold exposure to them | ↑BAT activity | - | - | - | - | ● | - | - | [65] | |
| Calorie restricted diet with capsaicin or without capsaicin fed to human | ↑Resting energy expenditure and diet-induced thermogenesis (capsaicin containing group) | - | - | - | - | ● | - | - | [66] | |
| Glycyrrhiza (Liquorice) | Synthetic amorfrutins administered to HFD-induced obese mice | ↓Blood glucose level, Plasma insulin level and body weight | ● | - | - | - | - | - | - | [67] |
| Oral administration of ethanol extract to KKAy mice | ↓Blood glucose, weight and intra-abdominal adipose tissue | ● | - | - | - | - | - | - | [68] | |
| Oral administration of licorice flavonoid oil to HFD-induced obese mice | ↓Genes related to acetyl-CoA synthesis and lipid biosynthesis, weight of abdominal white adipose tissues, and body weight gain ↑Genes related to β-oxidation and acetyl-CoA degradation. | - | - | - | - | - | ● | - | [69] | |
| Dioscoreaceae (Dioscorea) | Dioscorea polysaccharide treated to TNF-α-induced insulin resistant mouse liver cell line | ↑Glucose uptake and activate insulin signaling | ● | - | - | - | - | - | - | [70] |
| Oral administration of dioscorea extract to fructose-induced insulin resistant Wistar rats | ↓Blood glucose level and Plasma insulin level | ● | - | - | - | - | - | - | [71] | |
| Nymphaea stellata (Egyptian lotus) | Oral administration of lotus extract to STZ-induced diabetic rats | ↓Blood glucose ↑Plasma insulin level and number of β-cell mass | - | ● | - | - | - | - | - | [72] |
| Nelumbo nucifera (Indian lotus) | Oral administration of lotus leaves extract to HFD-induced obesity mice and rats | ↓Pancreatic lipase, α-amylase, α-glucosidase, total cholesterol, triglycerides, and LDL cholesterol ↑HDL cholesterol | - | - | - | - | - | ● | - | [73] |
| Oral administration of lotus seeds extract to HFD-induced obesity mice | ↓α-amylase, α-lipase, body weight gain and triglycerol ↑Expression of UCP3 mRNA in C2C12 myotubes | - | - | - | - | ● | - | - | [74] | |
| Silybum marianum (Milk thistle) | Oral administration of silymarin to alloxan-induced diabetic rats | ↓Blood glucose, ↑Pancreatic SOD, GSHPx and CAT | - | ● | - | - | - | - | - | [75] |
| Oral administration of silymarin seed extract to T2DM patients | ↓Fasting blood glucose and glycosylated hemoglobin level. | - | ● | - | - | - | - | - | [76] | |
| Oral administration of silymarin as adjuncts to glibenclamide | ↓Postprandial and fasting blood glucose level, and glycosylated hemoglobin | - | ● | - | - | - | - | - | [77] | |
| Panax quinquefolium (American ginseng) | Intraperitoneal injection of Rb1 to HFD-induced obese rats | ↓Body weight, total food intake, fat contents, serum leptin, serum nitric oxide, and NPY ↑CCK | - | - | - | ● | - | - | - | [31] |
| Panax ginseng (Asian ginseng) | Intraperitoneal injection of Ginseng berry extract to ob/ob mice | ↓Food intake and body weight ↑Body temperature and energy expenditure | - | - | - | ● | ● | - | - | [33] |
| Intraperitoneal injection of Rb1 to HFD-induced obese rats | ↓Food intake, body weight and body fat ↑Energy expenditure | - | - | - | - | ● | - | - | [78] | |
| Intraperitoneal administration of Rb1 to HFD-induced obese rats | ↓Liver weight, hepatic triglyceride content, and ACC ↑CPT-1 and AMPK | - | - | - | - | - | ● | - | [79] | |
| Camellia sinensis (Green tea) | Oral administration of EGCG to HFD fed mice | ↓Body weight gain, body fat percentage, and visceral fat weight | - | - | - | - | - | ● | - | [80] |
| Oral administration of EGCG to HFD fed rats | ↓Total cholesterol and LDL cholesterol | - | - | - | - | - | ● | - | [81] | |
| EGCG mixed with caffeine, orally administration to human | ↓Body weight and body weight gain | - | - | - | - | ● | ● | - | [82] | |
| Camellia sinensis (Black, green, and mulberry tea) | Oral administration of the extract of black, green, and mulberry tea to human | ↑Breath-hydrogen concentration | - | - | - | - | - | - | ● | [83] |
| Glycine max Merr (Soybeans) | Oral administration of soybean isoflavone chow to obese rats | ↓Plasma glucose, AST, and ALT | - | - | - | - | - | - | ● | [84] |
| Hoodia gordonii (Hoodia) | Intracerebroventricular injection of the purified P57AS3 to rats | ↓Food intake ↑ATP content in hypothalamic neurons | - | - | - | ● | ● | - | - | [35] |
| Oral administration of glycosides 1 and 2 to rats | ↓Food intake and body mass | - | - | - | ● | - | - | - | [36] | |
| Oral administration of organic solvent extract to rats | ↓NPY ↑CPT-1, T3, and T4 | - | - | - | ● | ● | - | ● | [37] | |
Hoodia gordonii (Hoodia)
Vaccinium spp. (Blueberry)
3.1.2. Compounds that Stimulate Energy Expenditure
Nelumbo nucifera (Indian lotus)
Capsicum (Chili pepper)
3.1.3. Compounds that Regulate Lipid Metabolism
Panax ginseng (Asian Ginseng)
Camellia sinensis (Green Tea)
Nelumbo nucifera (Indian Lotus)
Vaccinium angustifolium (Wild Blueberry)
Cinnamomum (Cinnamon)
Glycyrrhiza (Liquorice)
Momordica charantia (Bitter Melon)
3.1.4. Possible Therapeutic Compounds that Regulate Carbohydrate Metabolism
Camellia sinensis (Teas)
Glycine max Merr (Soybean)
3.2. Possible Therapeutic Compounds for Diabetes
3.2.1. Possible Therapeutic Compounds that Regulate Insulin Resistance
Trigonella foenum graecum (Fenugreek)
Cinnamomum (Cinnamon)
Dioscoreaceae (Dioscorea)
Vaccinium spp. (Blueberry)
Glycyrrhiza (Liquorice)
3.2.2. Possible Therapeutic Compounds that Regulate β-cell Function
Ervatamia microphylla (Kerr)
Anoectochilus roxburghii (Jewel Orchid)
Carica papaya (Papaya)
Silybum marianum (Milk Thistle)
Nymphaea stellata (Egyptian Lotus)
3.2.3. Compounds with Multiple Anti-Diabetic Activities
Capsicum (Chili Pepper)
Momordica charantia (Bitter Melon)
Vitis vinifera (Grape Vine)
3.3. Possible Therapeutic Compounds for both Obesity and Diabetes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17. [Google Scholar] [CrossRef]
- Lam, K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Demain, A.L. Natural products-Drug Discovery and Therapeutical Medicine; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Schmitz, R. Wilhelm serturner and the discovery of morphine. Pharm. Hist. 1985, 27, 61–74. [Google Scholar] [PubMed]
- Harvey, A.L. Natural products as a screening resource. Curr. Opin. Chem. Biol. 2007, 11, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Exarchou, V.; Krucker, M.; van Beek, T.A.; Vervoort, J.; Gerothanassis, I.P.; Albert, K. LC-NMR coupling technology: Recent advancements and applications in natural products analysis. Magn. Reson. Chem. 2005, 43, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G. Full absolute stereostructures of natural products directly from crude extracts: The HPLC-MS/MS-NMR-CD “Triad”. In Sponges (Porifera); Müller, W.G., Ed.; Springer: Berlin, Heidelberg, Germanly, 2003; Volume 37, pp. 89–116. [Google Scholar]
- Kingston, D.G. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [PubMed]
- Leistner, E. Die biologie der taxane: Arzneimittel aus der natur. Pharm. Unserer Zeit 2005, 34, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world-A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Jebb, S. Obesity: Causes and consequences. Womens Health Med. 2004, 1, 38–41. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [PubMed]
- Melville, C.A.; Cooper, S.A.; McGrother, C.W.; Thorp, C.F.; Collacott, R. Obesity in adults with down syndrome: A case-control study. J. Intellect. Disabil. Res. 2005, 49, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Obesity: Causes and control of excess body fat. Nature 2009, 459, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, M.; Barzon, L.; Fallo, F.; Sonino, N. Cushing’s syndrome. Lancet 2001, 357, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, M.A.; Vagenakis, A.G.; Leonardou, A.S.; Argentou, M.N.; Habeos, I.G.; Makri, M.G.; Psyrogiannis, A.I.; Kalfarentzos, F.E.; Kyriazopoulou, V.E. Thyroid function in humans with morbid obesity. Thyroid 2006, 16, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Naslund, E.; Hellstrom, P.M. Appetite signaling: From gut peptides and enteric nerves to brain. Physiol. Behav. 2007, 92, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Wynne, K.; Stanley, S.; McGowan, B.; Bloom, S. Appetite control. J. Endocrinol. 2005, 184, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.S.; Majumdar, S.R. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 2007, 369, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.; van Gaal, L. Medication of the month. Rimonabant (Acomplia): First CB1 receptor antagonist of the endocannabinoid system. Rev. Med. Liege 2008, 63, 50–55. [Google Scholar] [PubMed]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.-Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W., 2nd; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Ripsin, C.M.; Kang, H.; Urban, R.J. Management of blood glucose in type 2 diabetes mellitus. Am. Fam. Physician 2009, 79, 29–36. [Google Scholar] [PubMed]
- UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar]
- Knowler, W.C.; Barret-Connor, E.; Fowler, S.E.; Hamman, R.E.; Lachin, J.M.; Walker, E.A; Nathan, D. M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Joung, H.Y.; Kang, S.A.; Pyun, K.H.; Shim, I. Ginsenoside RB1 as a suppressor in central modulation of feeding in the rat. Appetite 2007, 49, 303. [Google Scholar] [CrossRef]
- Xie, J.T.; Zhou, Y.P.; Dey, L.; Attele, A.S.; Wu, J.A.; Gu, M.; Polonsky, K.S.; Yuan, C.S. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine 2002, 9, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Zhou, Y.P.; Xie, J.T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Pappe, K.W.L. Silva Capensis; or, a Description of South African Forest-Trees and Arborescent Shrubs Used for Technical and Oeconomical Purposes by the Colonists of the Cape of Good Hope; Harvard University Press: Cambridge, MA, USA, 1862. [Google Scholar]
- MacLean, D.B.; Luo, L.G. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: Studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004, 1020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, F.R.; Marthinus Horak, R.; Maharaj, V.J.; Vleggaar, R.; Senabe, J.V.; Gunning, P.J. An appetite suppressant from Hoodia species. Phytochemistry 2007, 68, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Singh, S. Metabolic effect of short term administration of Hoodia gordonii, an herbal appetite suppressant. South. Afr. J. Bot. 2013, 86, 51–55. [Google Scholar] [CrossRef]
- Pucci, E.; Chiovato, L.; Pinchera, A. Thyroid and lipid metabolism. Int. J. Obes. Relat. Metab. Disord. 2000, 24, S109–S112. [Google Scholar] [CrossRef] [PubMed]
- Chidakel, A.; Mentuccia, D.; Celi, F. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid 2005, 15, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.; Benhaddou-Andaloussi, A.; Brault, A.; Harbilas, D.; Martineau, L.C.; Vallerand, D.; Ramassamy, C.; Matar, C.; Haddad, P.S. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKAy mice. Int. J. Obes. 2009, 33, 1166–1173. [Google Scholar] [CrossRef]
- Seymour, E.M.; Tanone, II; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.; Lila, M.; Mawson, J. Satiety in rats following blueberry extract consumption induced by appetite-suppressing mechanisms unrelated to in vitro or in vivo antioxidant capacity. Food Chem. 2008, 107, 1039–1044. [Google Scholar] [CrossRef]
- Prior, R.L.; E Wilkes, S.; R Rogers, T.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese zucker rats. Br. J. Nutr. 2014, 111, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Kim, M.; Lee, J.H.; Min, B.; Bae, H.; Choe, W.; Kim, S.; Ha, J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 2007, 39, 222. [Google Scholar] [CrossRef] [PubMed]
- Kantsadi, A.L.; Apostolou, A.; Theofanous, S.; Stravodimos, G.A.; Kyriakis, E.; Gorgogietas, V.A.; Chatzileontiadou, D.S.; Pegiou, K.; Skamnaki, V.T.; Stagos, D.; et al. Biochemical and biological assessment of the inhibitory potency of extracts from vinification byproducts of Vitis vinifera extracts against glycogen phosphorylase. Food Chem. Toxicol. 2014, 67, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.M.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and ampk. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Imparl-Radosevich, J.; Deas, S.; Polansky, M.M.; Baedke, D.A.; Ingebritsen, T.S.; Anderson, R.A.; Graves, D.J. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: Implications for cinnamon regulation of insulin signalling. Horm. Res. 1998, 50, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Polansky, M.M.; Anderson, R.A. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2007, 459, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, Y.; Gong, Z.; Huang, C.; Zang, Y.Q. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008, 581348. [Google Scholar] [CrossRef]
- Sharma, R.D. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. Nutr. Res. 1986, 6, 1353–1364. [Google Scholar] [CrossRef]
- Sharma, R.; Sarkar, A.; Hazara, D.; Mishra, B.; Singh, J.; Sharma, S.; Maheshwari, B.; Maheshwari, P. Use of fenuqreek seed powder in the management of non-insulin dependent diabetes mellitus. Nutr. Res. 1996, 16, 1331–1339. [Google Scholar] [CrossRef]
- Fujii, M.; Takei, I.; Umezawa, K. Antidiabetic effect of orally administered conophylline-containing plant extract on streptozotocin-treated and Goto-Kakizaki rats. Biomed. Pharmacother. 2009, 63, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Yamada, S.; Yamamoto, Y.; Kodera, T.; Hara, A.; Tanaka, Y.; Kimura, F.; Takei, I.; Umezawa, K.; Kojima, I. Conophylline suppresses pancreatic stellate cells and improves islet fibrosis in Goto-Kakizaki rats. Endocrinology 2012, 153, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Ruan, H.; Pi, H.; Wu, J. Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J. Ethnopharmacol. 2007, 114, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Sumathi, V.; Jegathambigai, N.R.; Latha, L.Y. Antihyperglycaemic effects of ethanol extracts of Carica papaya and Pandanus amaryfollius leaf in streptozotocin-induced diabetic mice. Nat. Prod. Res. 2011, 25, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Vats, V.; Rathi, S.S.; Dawar, R. Traditional indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J. Ethnopharmacol. 2001, 76, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Welihinda, J.; Karunanayake, E.H.; Sheriff, M.H.R.; Jayasinghe, K.S.A. Effect of momordica charantia on the glucose tolerance in maturity onset diabetes. J. Ethnopharmacol. 1986, 17, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, K.; Li, Y.; Zou, X.; Chen, C.; Szeto, I.M.-Y.; Dong, Z.; Zhao, Y.; Shi, Y.; Wang, J. Bitter gourd inhibits the development of obesity-associated fatty liver in C57BL/6 mice fed a high-fat diet. J. Nutr. 2014, 144, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Hong, Y.W.; Wong, Y.H.; Chen, Y.N.; Chyuan, J.H.; Huang, C.J.; Chao, P.M. Bitter melon (Momordica charantia L.) inhibits adipocyte hypertrophy and down regulates lipogenic gene expression in adipose tissue of diet-induced obese rats. Br. J. Nutr. 2008, 99, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Ahren, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Choi, H. Dietary red chilli (Capsicum frutescens L.) is insulinotropic rather than hypoglycemic in type 2 diabetes model of rats. Phytother. Res. 2008, 22, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, C.T.; Kim, I.H.; Kim, Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother. Res. 2011, 25, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Kawai, Y.; Iwanaga, T.; Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 2012, 95, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Hursel, R.; Martens, E.A.; Westerterp-Plantenga, M.S. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS One 2013, 8, e67786. [Google Scholar] [CrossRef] [PubMed]
- Weidner, C.; de Groot, J.C.; Prasad, A.; Freiwald, A.; Quedenau, C.; Kliem, M.; Witzke, A.; Kodelja, V.; Han, C.T.; Giegold, S.; et al. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. USA 2012, 109, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- Mae, T.; Kishida, H.; Nishiyama, T.; Tsukagawa, M.; Konishi, E.; Kuroda, M.; Mimaki, Y.; Sashida, Y.; Takahashi, K.; Kawada, T. A licorice ethanolic extract with peroxisome proliferator-activated receptor-γ ligand-binding activity affects diabetes in KK-Ay mice, abdominal obesity in diet-induced obese C57BL mice and hypertension in spontaneously hypertensive rats. J. Nutr. 2003, 133, 3369–3377. [Google Scholar] [PubMed]
- Aoki, F.; Honda, S.; Kishida, H.; Kitano, M.; Arai, N.; Tanaka, H.; Yokota, S.; Nakagawa, K.; Asakura, T.; Nakai, Y.; et al. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci. Biotechnol. Biochem. 2014, 71, 206–214. [Google Scholar] [CrossRef]
- Lee, B.H.; Hsu, W.H.; Pan, T.M. Inhibitory effects of dioscorea polysaccharide on TNF-α-induced insulin resistance in mouse FL83B cells. J. Agric. Food Chem. 2011, 59, 5279–5285. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.; Wu, Y.C.; Liu, I.M.; Cheng, J.T. Dioscorea as the principal herb of Die-Huang-Wan, a widely used herbal mixture in china, for improvement of insulin resistance in fructose-rich chow-fed rats. J. Ethnopharmacol. 2007, 112, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Subash-Babu, P.; Ignacimuthu, S.; Agastian, P.; Varghese, B. Partial regeneration of β-cells in the islets of Langerhans by Nymphayol a sterol isolated from Nymphaea stellata (Willd.) flowers. Bioorg. Med. Chem. 2009, 17, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, alpha-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Recoba, R.; Barron, H.; Alvarez, C.; Favari, L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp. Biochem. Physiol. C 2003, 136, 205–212. [Google Scholar] [CrossRef]
- Huseini, H.F.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.R. Silymarin as an adjunct to gilbenclamide therapy improves long-term and postprandial glycemic contril and body mass index in type 2 diabetes. J. Med. Food 2007, 10, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shen, L.; Liu, K.J.; Tso, P.; Xiong, Y.; Wang, G.; Woods, S.C.; Liu, M. Antiobesity and antihyperglycemic effects of ginsenoside rb1 in rats. Diabetes 2010, 59, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xiong, Y.; Wang, D.Q.; Howles, P.; Basford, J.E.; Wang, J.; Xiong, Y.Q.; Hui, D.Y.; Woods, S.C.; Liu, M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J. Lipid Res. 2013, 54, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [PubMed]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Dulloo, A.G.; Tremblay, A.; Tappy, L.; Rumpler, W.; Westerterp-Plantenga, M.S. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: A meta-analysis. Obes. Rev. 2011, 12, e573–e581. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Furne, J.K.; Levitt, M.D. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am J. Clin. Nutr. 2006, 84, 551–555. [Google Scholar] [PubMed]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J. Nutr. Biochem. 2005, 16, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Lyons, T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: Clinical perspectives. J. Agric. Food Chem. 2012, 60, 5687–5692. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hanger, T.J.; Hanger, A.; Howard, L.R. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef] [PubMed]
- Azzu, V.; Jastroch, M.; Divakaruni, A.S.; Brand, M.D. The regulation and turnover of mitochondrial uncoupling proteins. Biochim. Biophys. Acta 2010, 1797, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.R.; Bhat, R. Lotus—A potential nutraceutical source. J. Agric. Technol. 2007, 3, 143–155. [Google Scholar]
- Boss, O.; Samec, S.; Paoloni-Giacobino, A.; Rossier, C.; Dulloo, A.; Seydoux, J.; Muzzin, P.; Giacobino, J.-P. Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997, 408, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, E.S.; Lee, C.; Kim, S.; Cho, S.H.; Hwang, B.Y.; Lee, M.K. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg. Med. Chem. 2013, 23, 3604–3608. [Google Scholar] [CrossRef]
- Rousset, S.; Alves-Guerra, M.-C.; Mozo, J.; Miroux, B.; Cassard-Doulcier, A.-M.; Bouillaud, F.; Ricquier, D. The biology of mitochondrial uncoupling proteins. Diabetes 2004, 53, S131–S135. [Google Scholar] [CrossRef]
- Ohnuki, K.; Niwa, S.; Maeda, S.; Inoue, N.; Yazawa, S.; Fushiki, T. CH-19 sweet, a non-pungent cultivar of red pepper, increased body temperature and oxygen consumption in humans. Biosci. Biotechnol. Biochem. 2001, 65, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, Y.; Nagao, K.; Kai, S.; Tsuge, K.; Yoshimura, T.; Koganemaru, K.; Yanagita, T. Polyphenolic extract of lotus root (edible rhizome of Nelumbo nucifera) alleviates hepatic steatosis in obese diabetic db/db mice. Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Lee, Y.J.; Kim, K.S.; Kim, S.H.; Chang, K.J. Anti-obesity and hypolipidaemic effects of Nelumbo nucifera seed ethanol extract in human pre-adipocytes and rats fed a high-fat diet. J. Sci. Food Agric. 2014, 94, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Sartorius, T.; Peter, A.; Schulz, N.; Drescher, A.; Bergheim, I.; Machann, J.; Schick, F.; Siegel-Axel, D.; Schurmann, A.; Weigert, C.; et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS One 2014, 9, e92358. [Google Scholar] [CrossRef] [PubMed]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; RAnderson, R.A. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J. Int. Soc. Sports Nutr. 2006, 3, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kamisoyama, H.; Honda, K.; Tominaga, Y.; Yokota, S.; Hasegawa, S. Investigation of the anti-obesity action of licorice flavonoid oil in diet-induced obese rats. Biosci. Biotechnol. Biochem. 2008, 72, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, Y.; Nakagawa, K.; Mae, T.; Kitano, M.; Yokota, S.; Arai, T.; Ikematsu, H.; Inoue, S. Licorice flavonoid oil reduces total body fat and visceral fat in overweight subjects: A randomized, double-blind, placebo-controlled study. Obes. Res. Clin. Pract. 2009, 3, 169–178. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Chao, C.-Y.; Huang, C.-J. Bitter gourd (Momordica charantia) extract activates peroxisome proliferator-activated receptors and upregulates the expression of the acyl CoA oxidase gene in H4IIEC3 hepatoma cells. J. Biomed. Sci. 2003, 10, 782–791. [Google Scholar] [PubMed]
- Nerurkar, P.V.; Lee, Y.K.; Nerurkar, V.R. Momordica charantia (bitter melon) inhibits primary human adipocyte differentiation by modulating adipogenic genes. BMC complement. Altern. Med. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kashket, S. Inhibition of salivary amylase by black and green teas and their effects on the intraoral hydrolysis of starch. Caries Res. 1998, 32, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef] [PubMed]
- Kreydiyyeh, S.I.; Baydoun, E.A.-H.; Churukian, Z.M. Tea extract inhibits intestinal absorption of glucose and sodium in rats. Comp. Biochem. Physiol. C 1994, 108, 359–365. [Google Scholar] [PubMed]
- Shimizu, M.; Kobayashi, Y.; Suzuki, M.; Satsu, H.; Miyamoto, Y. Regulation of intestinal glucose transport by tea catechins. Biofactors 2000, 13, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Juhel, C.; Armand, M.; Pafumi, Y.; Rosier, C.; Vandermander, J.; Lairon, D. Green tea extract (AR25®) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J. Nutr. Biochem. 2000, 11, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bhathena, S.J.; Velasquez, M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [PubMed]
- Purkins, L.; Love, E.R.; Eve, M.D.; Wooldridge, C.L.; Cowan, C.; Smart, T.S.; Johnson, P.J.; Rapeport, W.G. The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a Phase I unit. Br. J. Clin. Pharmacol. 2003, 57, 199–208. [Google Scholar] [CrossRef]
- Sharma, R.D.a.R.T.C. Hypoglycemic effect of fenugreek seeds in non-insulin dependent diabetic subjects. Nutr. Res. 1990, 10, 731–739. [Google Scholar] [CrossRef]
- Rafehi, H.V., K.; Karagiannis, T.C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes Obes. Metab. 2012, 14, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Panickar, K.S.; Anderson, R.A. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome and type 2 diabetes. J. Diabetes Sci. Technol. 2010, 4, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Noda, T.; Kim, S.J.; Sarker, M.Z.; Yamauchi, H.; Takigawa, S.; Matsuura-Endo, C.; Suzuki, T.; Han, K.H.; Fukushima, M. Yam contributes to improvement of glucose metabolism in rats. Plant Foods Hum. Nutr. 2009, 64, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Surmi, B.K.; Hasty, A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Mills, C. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Al-Majali, K.; Betteridge, D.J. PPARS, insulin resistance and type 2 diabetes. J. Cardiovasc. Risk. 2001, 8, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Hirayama, A.; Tsuchiya, K.; Ohgawara, H.; Nakamura, M.; Umezawa, K. Promotion of β-cell differentiation by the alkaloid conophylline in porcine pancreatic endocrine cells. Biomed. Pharmacother. 2010, 64, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R. The production of resveratrol by Vitis vinifera and other members of the vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Sims, E.; Danforth, E., Jr.; Horton, E.S.; Bray, G.A.; Glennon, J.; Salans, L. Endocrine and metabolic effects of experimental obesity in man. Recent Prog. Horm. Res. 1973, 29, 457–496. [Google Scholar] [PubMed]
- Saito, M.; Yoneshiro, T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr. Opin. Lipidol. 2013, 24, 71–77. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.S.; Lim, Y.; Kim, E.-K. Therapeutic Phytogenic Compounds for Obesity and Diabetes. Int. J. Mol. Sci. 2014, 15, 21505-21537. https://doi.org/10.3390/ijms151121505
Jung HS, Lim Y, Kim E-K. Therapeutic Phytogenic Compounds for Obesity and Diabetes. International Journal of Molecular Sciences. 2014; 15(11):21505-21537. https://doi.org/10.3390/ijms151121505
Chicago/Turabian StyleJung, Hee Soong, Yun Lim, and Eun-Kyoung Kim. 2014. "Therapeutic Phytogenic Compounds for Obesity and Diabetes" International Journal of Molecular Sciences 15, no. 11: 21505-21537. https://doi.org/10.3390/ijms151121505
APA StyleJung, H. S., Lim, Y., & Kim, E.-K. (2014). Therapeutic Phytogenic Compounds for Obesity and Diabetes. International Journal of Molecular Sciences, 15(11), 21505-21537. https://doi.org/10.3390/ijms151121505