Reactivation of Hepatitis B Virus in Hematopoietic Stem Cell Transplant Recipients in Japan: Efficacy of Nucleos(t)ide Analogues for Prevention and Treatment
Abstract
:1. Introduction
2. Results
2.1. Hepatitis B Virus (HBV) Reactivation from Hepatitis B Surface Antigen (HBsAg)-Positive Recipients
2.2. HBV Reactivation from HBsAg-Negative Recipients
2.3. Status of the Antibody to the Hepatitis B Surface Antigen (anti-HBs) and to the Hepatitis B Core Antigen (anti-HBc) in HBsAg-Negative Recipients with HBV Reactivation
| Status of Anti-HBc/Anti-HBs | Number of Patients | Number of HBV Reactivation | Cumulative HBV Reactivation Rates |
|---|---|---|---|
| (+)/(+) | 27 (9%) | 4 (11%) | 9.1% at 2 years; 14.5% at 5 years |
| (+)/(−) | 8 (3%) | 0 (0%) | |
| (−)/(+) | 48 (17%) | 1 (2%) | 0.4% at 2 years; 1.3% at 5 years |
| (−)/(−) | 157 (54%) | 0 (0%) | |
| NA/NA | 49 (17%) | 1 (2%) |
2.4. Characteristics of HBsAg-Negative Recipients with HBV Reactivation
| Case | Age (Years) | Gender | Type of Disease | Transplant Type | HSCT Type | Year of HSCT | Period of Immunosuppression after HSCT (Months) | Outcome | Cause of Death | Period from HSCT to Outcome (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Male | AML | Allogeneic | BMT | 1988 | NA | Death | Unknown | 18 |
| 2 | 44 | Male | CML | Allogeneic | BMT | 1994 | >20 | Death | Unknown | 89 |
| 3 | 37 | Male | ALL | Allogeneic | BMT | 1995 | 8 | Death | Primary disease | 188 |
| 4 | 46 | Male | AML | Allogeneic | BMT | 1997 | 47 | Death | Renal failure | 47 |
| 5 | 40 | Female | AML | Allogeneic | PBSCT | 1999 | 48 | Death | Infection | 48 |
| 6 | 49 | Female | CML | Allogeneic | PBSCT | 2000 | 54 | Alive | 163 | |
| 7 | 49 | Male | MM | Autologous/ Allogeneic | PBSCT | 2000 | 37 | Death | Primary disease | 105 |
| 8 | 22 | Male | ALL | Allogeneic | PBSCT | 2004 | 52 | Alive | 109 | |
| 9 | 54 | Male | MDS | Allogeneic | BMT | 2008 | 65 | Death | Esophageal cancer | 66 |
| 10 | 53 | Female | NHL | Allogeneic | BMT | 2010 | 31 | Lost to follow-up | NA | 31 |
| 11 | 39 | Male | MF | Allogeneic | PBSCT | 2005 | 100 | Alive | 100 |
| Recipients (Before HSCT) | Recipients (After HSCT) | Donor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | anti-HBs | anti-HBc | Last Confirmed Time of HBsAg-Negativity after HSCT (Months) | Time of HBsAg-Positivity after HSCT (Months) | HBV DNA at the Time of HBsAg-Positive (log copies/mL) | Type of NUCs | Period from HBsAg Positive to NUCs Start (Months) | Outcome of HBV Status | Period from Treatment Start to Achievement of HBV Outcome (Months) | Treatment Period (Months) | anti-HBs | anti-HBc |
| 1 | NA | NA | 2 | 8 | (+) | ND | NA | HBsAg (−) | NA | NA | NA | NA |
| 2 | NA | NA | 1 | 18 | (+) | ND | NA | HBsAg (+) | 19 * | NA | NA | NA |
| 3 | NA | NA | 0 | 33 | 8.2 | ETV | 141 | HBsAg (+) | 12 | 12 | (−) | (−) |
| 4 | NA | NA | 0 | 10 | (+) | ND | NA | NA | NA | NA | (−) | (−) |
| 5 | NA | NA | 0 | 19 | 7.4 | LAM | 24 | HBsAg (+) | 1 | 1 | NA | NA |
| 6 | (+) | (+) | 10 | 28 | 4.5 | LAM | 0 | anti-HBs (+) | 8 | >8 | NA | NA |
| 7 | (+) | NA | 0 | 31 | 7.3 | LAM | 0 | anti-HBs (+) | 48 | 9 | NA | NA |
| 8 | (+) | (−) | 0 | 4 | 4 | LAM | 0 | anti-HBs (+) | 11 | >11 | (−) | (−) |
| 9 | (+) | (+) | 5 | 14 | 8.9 | ETV | 41 | HBsAg (+) | 10 | >10 | (−) | (−) |
| 10 | (+) | (+) | 3 | 20 | >9 | ETV | 4 | HBsAg (+) | 6 | >6 | (−) | (−) |
| 11 | (+) | (+) | 74 | 91 | 8.6 | ETV | 0 | HBsAg (−) | 15 | >15 | (−) | (−) |
3. Discussion
4. Patients and Methods
4.1. Ethics
4.2. Patients
| Characteristics | Total (n = 413) | 1986–1999 (n = 120) | 2000–2013 (n = 293) |
|---|---|---|---|
| Gender | |||
| Male, n (%) | 250 (60.5) | 70 (58.3) | 180 (61.4) |
| Female, n (%) | 163 (39.5) | 50 (41.7) | 113 (38.6) |
| Median age, years (range) | 42 (15–69) | 32 (15–57) | 47 (16–69) |
| Type of disease, n (%) | |||
| AML | 115 (27.8) | 36 (30.0) | 79 (26.7) |
| ALL | 61 (14.8) | 28 (23.3) | 31 (10.6) |
| CML | 38 (9.2) | 20 (16.7) | 18 (6.1) |
| MDS | 31 (7.5) | 9 (7.5) | 22 (7.5) |
| Lymphoma | 84 (20.3) | 18 (15.0) | 68 (23.2) |
| Plasma cell dyscrasia *1 | 61 (14.8) | 0 (0) | 61 (20.8) |
| Aplastic anemia | 16 (3.9) | 9 (7.5) | 7 (2.4) |
| Others *2 | 7 (1.7) | 0 (0) | 7 (2.4) |
| Transplant type, n (%) | |||
| Autologous | 114 (27.6) | 15 (12.5) | 99 (33.8) |
| Allogeneic | 299 (72.4) | 105 (87.5) | 194 (66.2) |
| For Allogeneic-SCT | |||
| Donor source, n (%) | |||
| Related | 126 (42.1%) | 59 (56.2%) | 67 (34.5%) |
| Unrelated | 135 (45.2%) | 46 (43.8%) | 89 (45.9%) |
| Unrelated cord blood | 38 (12.7%) | 0 (0%) | 38 (19.6%) |
4.3. Serological Examination
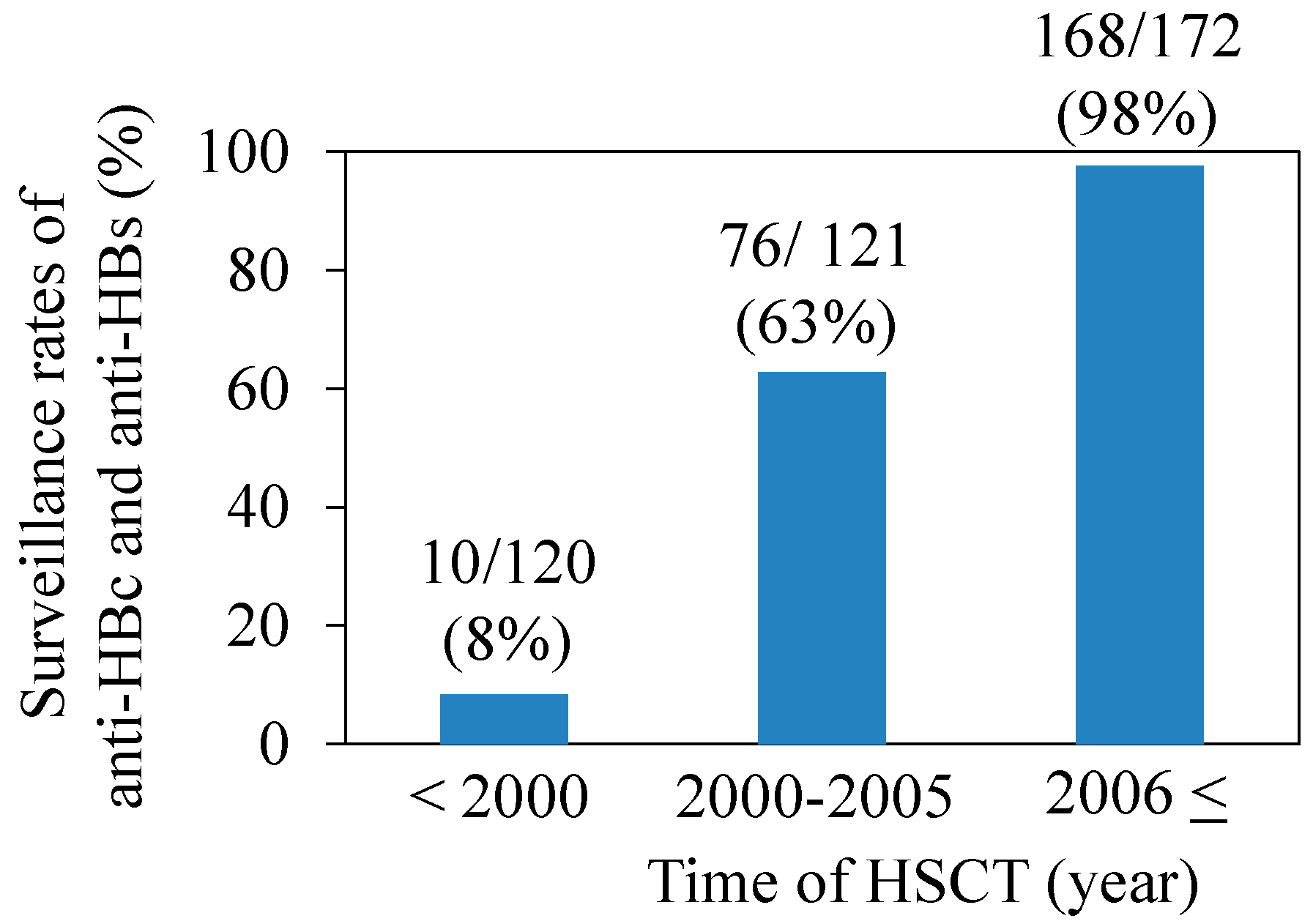
4.4. Definition of HBV Infection Status and HBV Reactivation
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di Bisceglie, A.M. Hepatitis B and hepatocellular carcinoma. Hepatology 2009, 49, S56–S60. [Google Scholar]
- Lok, A.S.; McMahon, B.J. Chronic hepatitis B. Hepatology 2007, 45, 507–539. [Google Scholar] [CrossRef]
- Tanaka, J.; Koyama, T.; Mizui, M.; Uchida, S.; Katayama, K.; Matsuo, J.; Akita, T.; Nakashima, A.; Miyakawa, Y.; Yoshizawa, H. Total numbers of undiagnosed carriers of hepatitis C and B viruses in Japan estimated by age- and area-specific prevalence on the national scale. Intervirology 2011, 54, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ramimondo, G.; Pollicino, T.; Romano, L.; Zanetti, A.R. A 2010 update on occult hepatitis B infection. Pathol. Biol. 2010, 58, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, S.; Sugiyama, K.; Marusawa, H.; Inomata, Y.; Asonuma, K.; Egawa, H.; Kiuchi, T.; Miyake, Y.; Tanaka, K.; Chiba, T. Transmission of hepatitis B virus from hepatitis B core antibody-positive donors in living donors in living related liver transplants. Transplantation 1998, 65, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Marusawa, H.; Uemoto, S.; Hijikata, M.; Ueda, Y.; Tanaka, K.; Shimotohno, K.; Chiba, T. Latent hepatitis B infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology 2000, 31, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Palmore, T.N.; Shah, N.L.; Loomba, R.; Borg, B.B.; Lopatin, U.; Feld, J.J.; Khokhar, F.; Lutchman, G.; Kleiner, D.E.; Young, N.S.; et al. Reactivation of hepatitis B with reappearance of hepatitis B surface antigen after chemotherapy and immunosuppression. Clin. Gastroenterol. Hepatol. 2009, 7, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Ustün, C.; Koç, H.; Karayalcin, S.; Akyol, G.; Gürman, G.; Ilhan, O.; Akan, H.; Ozcan, M.; Arslan, O.; Konuk, N.; et al. Hepatitis B virus infection in allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997, 20, 289–296. [Google Scholar] [CrossRef]
- Ma, S.Y.; Lau, G.K.; Cheng, V.C.; Liang, R. Hepatitis B reactivation in patients positive for hepatitis B surface antigen undergoing autologous hematopoietic cell transplantation. Leuk. Lymphoma 2003, 44, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Caselitz, M.; Link, H.; Hein, R.; Maschek, H.; Böker, K.; Poliwoda, H.; Manns, M.P. Hepatitis B associated liver failure following bone marrow transplantation. J. Hepatol. 1997, 27, 572–577. [Google Scholar] [CrossRef]
- Naparstek, E. The role of rituximab in autologous and allogeneic hematopoietic stem cell transplantation for non-Hodgkin’s lymphoma. Curr. Hematol. Malig. Rep. 2006, 1, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Nicolini, L.; Signori, A.; Rivoli, G.; del Bono, V.; Raiola, A.M.; di Grazia, C.; Dominietto, A.; Varaldo, R.; Ghiso, A.; et al. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive allogeneic haematopoietic stem cell transplant recipients: Risk factors and outcome. Clin. Microbiol. Infect. 2014. [Google Scholar] [CrossRef]
- Endo, T.; Sakai, T.; Fujimoto, K.; Yamamoto, S.; Takashima, H.; Haseyama, Y.; Nishio, M.; Koizumi, K.; Koike, T.; Sawada, K. A possible role for lamivudine as prophylaxis against hepatitis B reactivation in carriers of hepatitis B who undergo chemotherapy and autologous peripheral blood stem cell transplantation for non-Hodgkin's lymphoma. Bone Marrow Transplant. 2001, 27, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Henkes, M.; Martin, S.; Einsele, H.; Aulitzky, W.E. Successful antiviral treatment for fulminant reactivated hepatitis B after autologous stem cell transplantation and prophylaxis during subsequent allogeneic stem cell transplantation. Ann. Hematol. 2002, 81, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G. Prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HbsAg carriers with hemato-oncological neoplasias treated with chemotherapy. Leuk. Lymphoma 2003, 44, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, L.; Corbellino, M.; Foschi, A.; Micheli, V.; Dodero, A.; Mazzocchi, A.; Montefusco, V.; Zehender, G.; Antinori, S. Late onset of hepatitis B virus reactivation following hematopoietic stem cell transplantation: successful treatment with combined entecavir plus tenofovir therapy. Transpl. Infect. Dis. 2012, 14, 95–98. [Google Scholar] [PubMed]
- Lin, P.C.; Poh, S.B.; Lee, M.Y.; Hsiao, L.T.; Chen, P.M.; Chiou, T.J. Fatal fulminant hepatitis B after withdrawal of prophylactic lamivudine in hematopoietic stem cell transplantation patients. Int. J. Hematol. 2005, 81, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, L.T.; Chiou, T.J.; Liu, J.H.; Chu, C.J.; Lin, Y.C.; Chao, T.C.; Wang, W.S.; Yen, C.C.; Yang, M.H.; Tzeng, C.H.; et al. Extended lamivudine therapy against hepatitis B virus infection in hematopoietic stem cell transplant recipients. Biol. Blood Marrow Transplant. 2006, 12, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Goyama, S.; Kanda, Y.; Nannya, Y.; Kawazu, M.; Takeshita, M.; Niino, M.; Komeno, Y.; Nakamoto, T.; Kurokawa, M.; Tsujino, S.; et al. Reverse seroconversion of hepatitis B virus after hematopoietic stem cell transplantation. Leuk. Lymphoma 2002, 43, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, M.; Hashino, S.; Izumiyama, K.; Kahata, K.; Chuma, M.; Mori, A.; Kondo, T.; Toyoshima, N.; Ota, S.; Kobayashi, S.; et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation 2005, 79, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J.; Baden, L.; Boeckh, M.J.; Chakrabarti, S.; Einsele, H.; Ljungman, P.; McDonald, G.B.; Hirsch, H.; Center for International Blood and Marrow Transplant Research; National Marrow Donor Program; et al. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009, 44, 471–482. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.A.; Boeckh, M.A. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: A global perspective. Bone Marrow Transplant. 2009, 44, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.M.; Savani, B.N. Hepatitis B reactivation in patients with hematological malignancies and stem cell transplantation. J. Blood Lymph 2013, 4, 114. [Google Scholar]
- Beasley, R.P.; Hwang, L.Y.; Lee, G.C.; Lan, C.C.; Roan, C.H.; Huang, F.Y.; Chen, C.L. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983, 2, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kanda, T.; Wu, S.; Imazeki, F.; Yokosuka, O. Hepatitis A, B, C and E virus markers in Chinese residing in Tokyo, Japan. Hepatol. Res. 2012, 42, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Idilman, R.; Ustün, C.; Karayalçin, S.; Aktemel, A.; Turkyilmaz, A.R.; Ozcan, M.; Arslan, O.; Bozdayi, A.M.; van Thiel, D.H.; Akan, H. Hepatitis B virus vaccination of recipients and donors of allogeneic peripheral blood stem cell transplantation. Clin. Transplant. 2003, 17, 438–433. [Google Scholar] [CrossRef]
- Hui, C.K.; Lie, A.; Au, W.Y.; Leung, Y.H.; Ma, S.Y.; Cheung, W.W.; Zhang, H.Y.; Chim, C.S.; Kwong, Y.L.; Liang, R.; et al. A long-term follow-up study on hepatitis B surface antigen-positive patients undergoing allogeneic hematopoietic stem cell transplantation. Blood 2005, 106, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Oketani, M.; Ido, A.; Uto, H.; Tsubouchi, H. Prevention of hepatitis B virus reactivation in patients receiving immunosuppressive therapy or chemotherapy. Hepatol. Res. 2012, 42, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Kamezaki, H.; Kanda, T.; Wu, S.; Nakamoto, S.; Arai, M.; Maruyama, H.; Fujiwara, K.; Imazeki, F.; Yokosuka, O. Emergence of entecavir-resistant mutations in nucleos(t)ide-naive Japanese patients infected with hepatitis B virus: Virological breakthrough is also dependent on adherence to medication. Scand. J. Gastroenterol. 2011, 46, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamoto, S.; Kanda, T.; Nakaseko, C.; Sakaida, E.; Ohwada, C.; Takeuchi, M.; Takeda, Y.; Mimura, N.; Iseki, T.; Wu, S.; et al. Reactivation of Hepatitis B Virus in Hematopoietic Stem Cell Transplant Recipients in Japan: Efficacy of Nucleos(t)ide Analogues for Prevention and Treatment. Int. J. Mol. Sci. 2014, 15, 21455-21467. https://doi.org/10.3390/ijms151121455
Nakamoto S, Kanda T, Nakaseko C, Sakaida E, Ohwada C, Takeuchi M, Takeda Y, Mimura N, Iseki T, Wu S, et al. Reactivation of Hepatitis B Virus in Hematopoietic Stem Cell Transplant Recipients in Japan: Efficacy of Nucleos(t)ide Analogues for Prevention and Treatment. International Journal of Molecular Sciences. 2014; 15(11):21455-21467. https://doi.org/10.3390/ijms151121455
Chicago/Turabian StyleNakamoto, Shingo, Tatsuo Kanda, Chiaki Nakaseko, Emiko Sakaida, Chikako Ohwada, Masahiro Takeuchi, Yusuke Takeda, Naoya Mimura, Tohru Iseki, Shuang Wu, and et al. 2014. "Reactivation of Hepatitis B Virus in Hematopoietic Stem Cell Transplant Recipients in Japan: Efficacy of Nucleos(t)ide Analogues for Prevention and Treatment" International Journal of Molecular Sciences 15, no. 11: 21455-21467. https://doi.org/10.3390/ijms151121455
APA StyleNakamoto, S., Kanda, T., Nakaseko, C., Sakaida, E., Ohwada, C., Takeuchi, M., Takeda, Y., Mimura, N., Iseki, T., Wu, S., Arai, M., Imazeki, F., Saito, K., Shirasawa, H., & Yokosuka, O. (2014). Reactivation of Hepatitis B Virus in Hematopoietic Stem Cell Transplant Recipients in Japan: Efficacy of Nucleos(t)ide Analogues for Prevention and Treatment. International Journal of Molecular Sciences, 15(11), 21455-21467. https://doi.org/10.3390/ijms151121455







