Abstract
Novel boric ester-type molten salt was prepared using 1-(2-hydroxyethyl)-3-methylimidazolium chloride as a key starting material. After an ion exchange reaction of 1-(2-hydroxyethyl)-3-methylimidazolium chloride with lithium (bis-(trifluoromethanesulfonyl) imide) (LiNTf2), the resulting 1-(2-hydroxyethyl)-3-methylimidazolium NTf2 was reacted with 9-borabicyclo[3.3.1]nonane (9-BBN) to give the desired boric ester-type molten salt in a moderate yield. The structure of the boric ester-type molten salt was supported by 1H-, 13C-, 11B- and 19F-NMR spectra. In the presence of two different kinds of lithium salts, the matrices showed an ionic conductivity in the range of 1.1 × 10−4–1.6 × 10−5 S cm−1 at 51 °C. This was higher than other organoboron molten salts ever reported.
1. Introduction
In recent years, lithium ion batteries (LIB) [1,2] have taken a very important role as energy storage devices with the development and spread of high performance microelectronics and zero-emissions vehicles. However, lithium-ion batteries are not bereft of demerits. For instance, the highly flammable electrolyte has always been a subject of concern and has been a topic of continuous research. Meanwhile, ionic liquids (ILs) [3,4,5] have also been attracting immense interest as electrolytes for a variety of ionic devices, because of their high ionic conductivity, designability and non-flammable property. A few disadvantages associated with ILs have kept them away from manufacturing scale development and has kept research afresh in this topic. One such disadvantage is the migration of both anion and cation under the potential gradient, and the selective transport of target cation is not straight forward. In the search for a solution to these problem, previously, the synthesis of a few novel organoboron molten salts [6] bearing tertiary boron was proven to be an efficient electrolyte. A maximum lithium ion transference number reached as high as 0.7 for those systems. It was shown that the highly Lewis acidic alkylborane group trapped anions more efficiently than boric ester groups did. Moreover, polymer homologues of organoboron molten salts prepared by hydroboration polymerization showed tLi+ of 0.87 at 30 °C [7]. Furthermore, it was found that anion trapping in ionic liquid-based media worked more efficiently than was observed in polyether-based media. However, the ionic conductivity of such organoboron molten salts was low, ranging in the order of 10−5–10−6 S cm−1 at 50 °C.
As an alternative approach to improve the lithium transference number of electrolytes, the design of zwitterionic molten salts has been examined by H. Ohno et al. [8,9]. Organoboron zwitterionic molten salt bearing a highly dissociable pentafluorophenylborate structure [10] showed a lithium transference number of 0.69 with ionic conductivity of 3.0 × 10−5 S cm−1, which was comparable to alkylborane-type molten salt. So far, boron incorporated into lithium ion conductive electrolytes was found to be effective for any type of electrolyte, such as solid polymer electrolytes, ionic liquids (molten salts) and polymer ion–gel electrolytes.
Boron incorporation in electrolytes [11,12,13,14,15,16,17,18,19,20,21] generally leads to either: (1) promotion of lithium salt dissociation; or (2) an enhanced lithium transference number via anion trapping by the boron atom. Usually, in the case of polymer/salt hybrids [22,23] in which the anion structure was immobilized onto the polymer framework, enhancement of the lithium transference number always results in a decrease of ionic conductivity. However, an important point of organoboron electrolytes, in principle, is that both the lithium transference number and ionic conductivity can be enhanced at the same time, if the strength of the Lewis acid–anion interaction is adequately tuned.
In the search for such an attractive system, in the present work, a novel organoboron molten salt 5 was synthesized using 9-borabicyclo[3.3.1]nonane (9-BBN) and 1-(2-hydroxyethyl)-3-methyl imidazolium chloride as starting materials (Scheme 1). The synthesized organoboron molten salt was well characterized using 1H-, 13C-, 19F- and 11B-NMR. The intrinsic characteristics, such as the conductivity and thermal stability of the obtained organoboron molten salt, were evaluated.
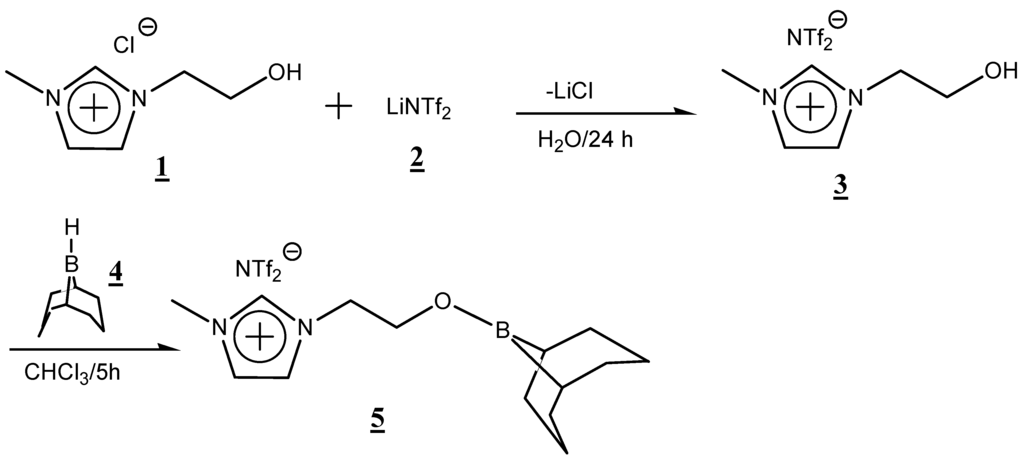
Scheme 1.
Synthesis of novel organoboron molten salt derived from 1-(2-hydroxyethyl)-3-methylimidazolium chloride.
2. Results and Discussion
After the ion exchange reaction of 1-(2-hydroxyethyl)-3-methylimidazolium chloride (1) with lithium (bis-(trifluoromethanesulfonyl) imide) (LiNTf2) (2), the resulting 1-(2-hydroxyethyl)-3-methylimidazolium NTf2 (3) was reacted with an excess amount of 9-BBN (4) to give the desired organoboron molten salt in an 85% yield. The molten salt, obtained as a white soft solid, was soluble in common organic solvents, such as chloroform, methanol, dimethyl sulfoxide (DMSO) and so forth, and the structure was supported by 1H-, 13C-, 11B- and 19F-NMR spectra.
In the 1H-NMR spectrum of organoboron molten salt measured in CDCl3 (Figure 1), the peaks due to the 9-BBN structure were observed in 1.26–2.50 ppm. Moreover, the chemical shift of the methylene proton adjacent to the hydroxyl group (4.33 ppm, NCH2CH2OH) was shifted after dehydrocoupling reaction (5.62 ppm, NCH2CH2OBR2). The integration ratios for all of the peaks were in good agreement with that of the expected structure. The 13C-NMR spectrum (Figure 2) was also in accord with the expected structure.
The 11B-NMR spectrum (Figure 3) was also measured in CDCl3 using B(OCH3)3 as the external standard. The only peak due to O–BR2 was observed at 33.3 ppm, showing that the boron atom had a single chemical environment. The 19F-NMR spectrum (Figure 4) measured using C6H5CF3 as the external standard also showed a single main peak at −80.7 ppm. This also supported the incorporation of the NTf2 anion in the ionic liquid structure.
The effect of temperature on ionic conductivity (Figure 5) was studied by AC-impedance measurements after a designated amount of lithium salt (lithium trifluoromethanesulfonate (LiCF3SO3)3 or LiNTf2) was added to the molten salts. The ionic conductivity observed was in the range of 1.1 × 10−4–1.6 × 10−5 S cm−1 at 51 °C. All of the systems showed lower ionic conductivity compared with bulk molten salt 5, which is free of lithium salt. This should be due to the increase in viscosity with the addition of lithium salt. The ion conductive matrices containing LiNTf2 showed higher ionic conductivity in comparison with that containing LiCF3SO3. It was observed that the samples containing a 5:Li ratio of 2:1 (molar ratio) exhibited higher ionic conductivity in comparison with the ratio of 5:Li = 1:1 (molar ratio).
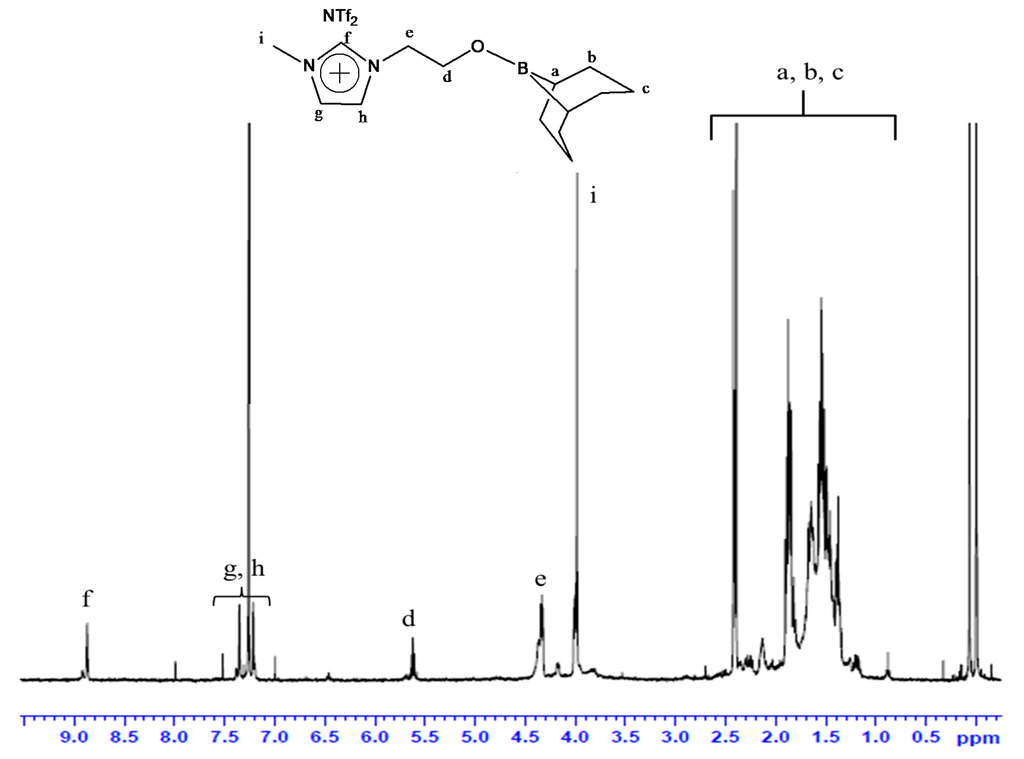
Figure 1.
1H-NMR of 5 (solvent: CDCl3; 10%; r.t.; TMS; 400 MHz).
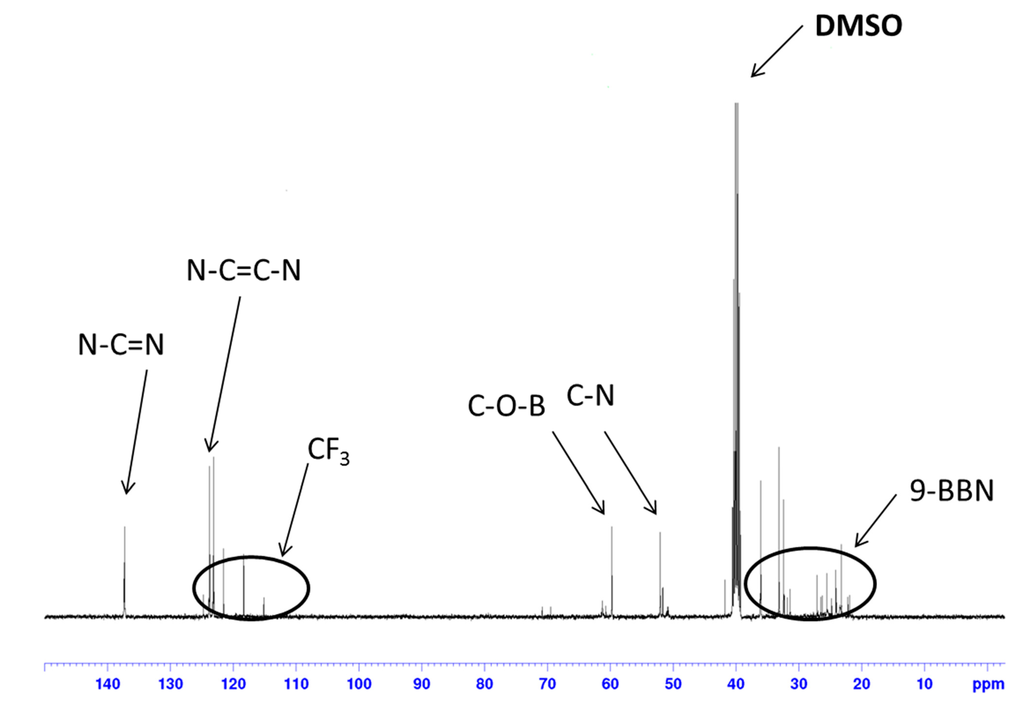
Figure 2.
13C-NMR of 5 (solvent: DMSO; r.t.; 100 MHz). 9-BBN, 9-borabicyclo[3.3.1]nonane.
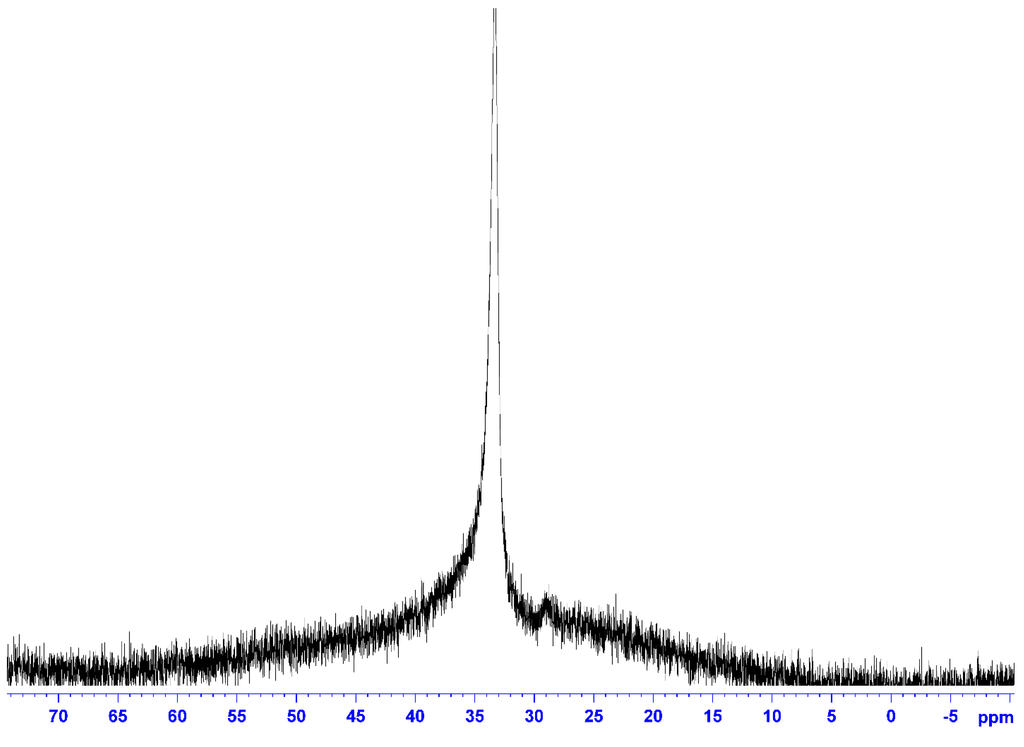
Figure 3.
11B-NMR of 5 (solvent: CDCl3; r.t.; external standard: B(OCH3); 128 MHz).
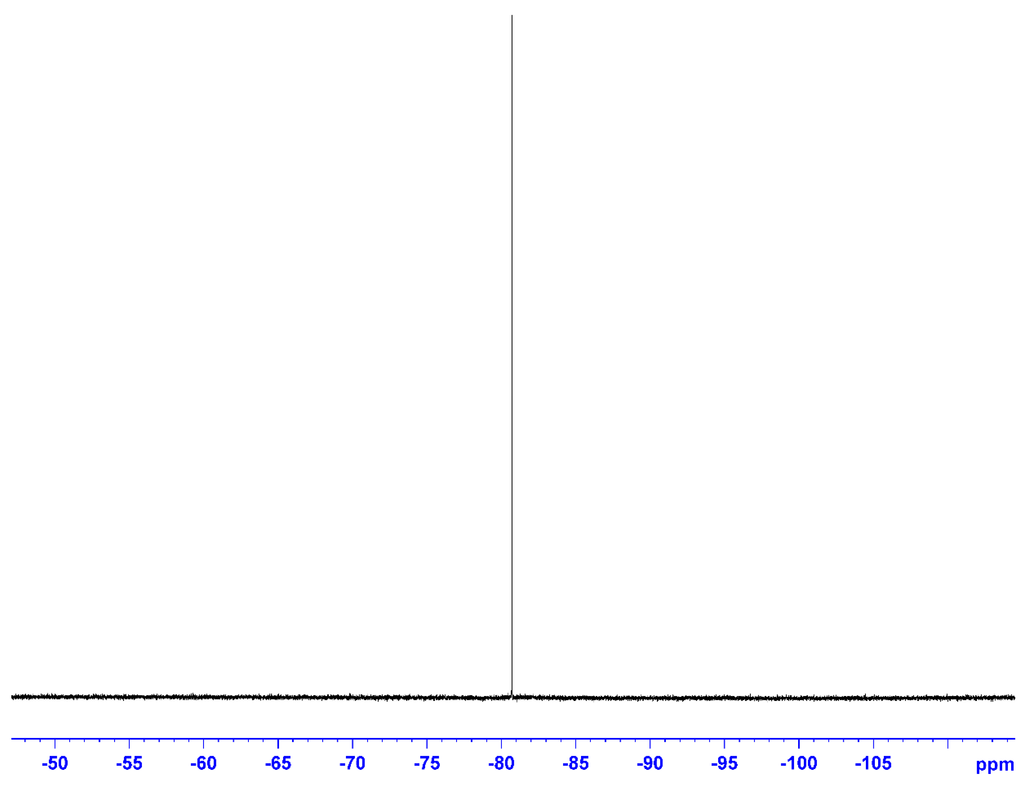
Figure 4.
19F-NMR of 5 (solvent: CDCl3; r.t.; external standard: C6H5CF3; 376 MHz).
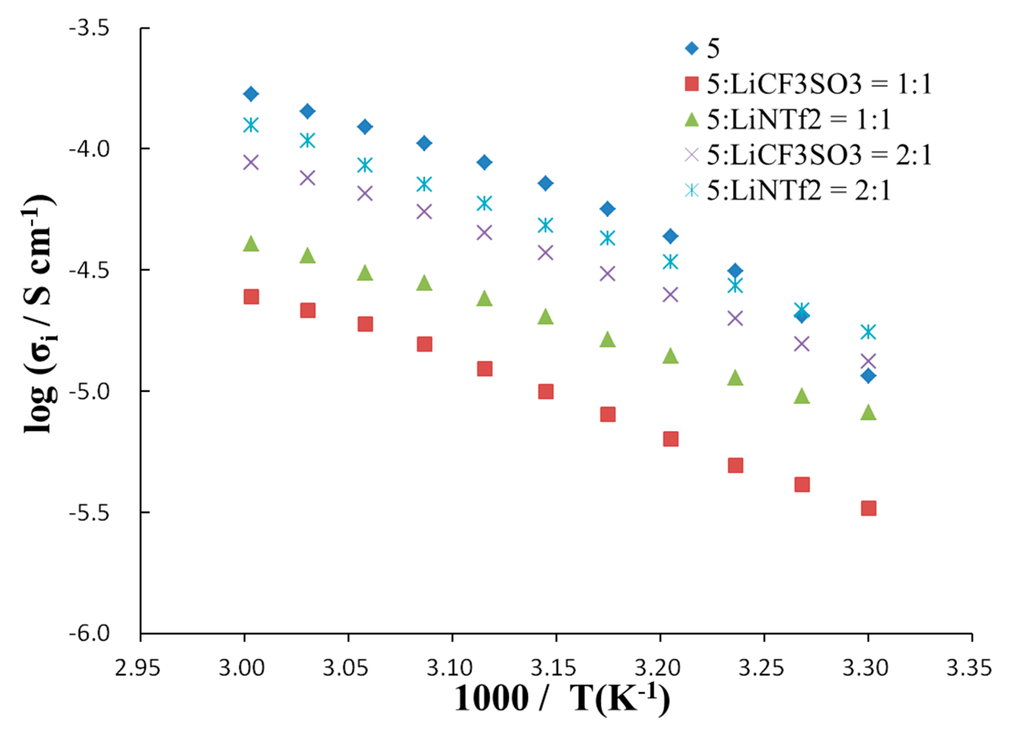
Figure 5.
Temperature dependence of ionic conductivity for novel organoboron molten salt in the presence or absence of lithium salt.
In order to obtain further information on the ion conductive behavior, the temperature dependence of ionic conductivity was fitted with the VFT (Vogel–Fulcher–Tammann) equation (Equation (1), Figure 6) [24,25,26]. The VFT parameters calculated are listed in Table 1. The VFT parameter B, corresponding to the activation energy of ion transport for matrices, was lower in the presence of LiNTf2. This indicates that the plasticizing effect of NTf2 anion was responsible for higher ionic conductivity for these systems. Under higher lithium ion concentration, the parameter B for LiCF3SO3-based systems increased. This should be due to the higher viscosity under the higher LiCF3SO3 concentration. On the contrary, the LiNTf2-based system did not show an increase in the parameter B under a higher lithium salt concentration, possibly because of the plasticizing effect of the NTf2 anion. However, both systems showed a decrease in ionic conductivity with increasing lithium salt concentrations, due to the decreased number of carrier ions (decreased dissociation degree of lithium salt) under the significantly high ionic strength of the matrices. The values of B were in accordance with the viscosity of the Compound 5 as a soft solid.
From the TGA measurements, the organoboron molten salt was found to be stable, even at temperatures higher than 100 °C. T50% was observed to be around 430 °C for all the samples.
3. Experimental Section
3.1. Instruments and Materials
1-(2-Hydroxyethyl)-3-methylimidazolium chloride was purchased from Kanto Chemical Co. Ltd. (Tokyo, Japan) and used without further purification. Lithium (bis-(trifluoromethanesulfonyl) imide) (LiNTf2) was also purchased from Kanto Chemical Co. Ltd. and used as received. Lithium trifluoromethanesulfonate (LiCF3SO3) was purchased from Wako Co. Ltd. (Osaka, Japan) and used as received. Lithium sheets were purchased from Honjo Chemical Co. Ltd. (Osaka, Japan). The 0.5 M THF solution of 9-borabicyclo[3.3.1]nonane was purchased from Across Co. Ltd. (Geel, Belgium). Dehydrated organic solvents (diethyl ether, chloroform, n-hexane, tetrahydrofuran) were purchased either from Kanto Chemical Co. Ltd. or Wako Co. Ltd. and used as received. All of the reactions were carried out under a nitrogen atmosphere.
1H- and 11B-NMR spectra were recorded on a Bruker Avance III 400 (Bruker, Billerica, MA, USA). After adding the appropriate amount (molar ratio) of LiNTf2 or lithium trifluoromethanesulfonate (LiCF3SO3, Kanto Chemical Co. Ltd.), the ionic conductivity of the ion conductive matrices was measured with a complex-impedance gain-phase analyzer (Solartron model 1260; Schlumberger, Houston, TX, USA) under the frequency range from 1 Hz to 1 MHz. IR spectra were measured on a JASCO FT/IR-4100 spectrometer (Tokyo, Japan). Thermogravimetric analysis was made on a PerkinElmer TGA7 (PerkinElmer, Waltham, MA, USA).
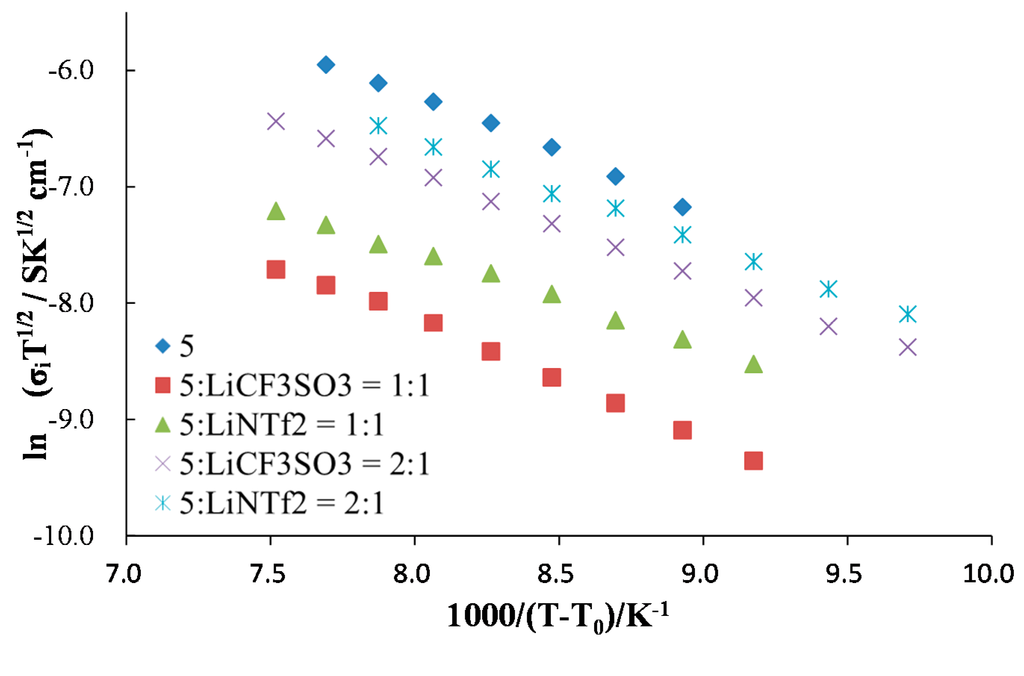
Figure 6.
VFT (Vogel–Fulcher–Tammann) plots for ion transport matrices.

Table 1.
VFT parameters of ion transport matrices.
| Sample | A (S cm−1 K1/2) | B (K) | T0 (K) | R2 |
|---|---|---|---|---|
 | 5.27 | 985 | 200 | 0.996 |
 | 0.954 | 1,013 | 200 | 0.998 |
 | 0.302 | 796 | 200 | 0.997 |
 | 1.47 | 907 | 200 | 0.999 |
 | 1.54 | 879 | 200 | 0.999 |
3.2. Synthesis of Organoboron Molten Salt
3.2.1. Ion Exchange Reaction of 1-(2-Hydroxyethyl)-3-methylimidazolium Chloride
To an aqueous solution of 1-(2-hydroxyethyl)-3-methylimidazolium chloride (2.51 g, 15.3 mmol), 5.32 g (18.4 mmol) of LiNTf2 was added, and the resulting mixture was stirred at room temperature for 24 h. An Oily liquid was spontaneously separated from the aqueous layer. After removing the aqueous layer by decantation, the oily product was purified by reprecipitation into diethyl ether. After removing the solvent under a reduced pressure, the desired compound, 1-(2-hydroxyethyl)-3-methylimidazolium NTf2, was obtained as a colorless soft solid (5.29 g, 13.0 mmol, 84% yield).
1H-NMR (CDCl3, δ, ppm): 3.82 (1H, CH2CH2OH), 3.99 (3H, CH3), 4.19 (2H, –CH2CH2OH), 4.33 (2H, –CH2CH2OH), 7.21, 7.35 (2H, –NCHCHN–), 8.88 (1H, –NCHN–).
3.2.2. Dehydrocoupling Reaction of 1-(2-Hydroxyethyl)-3-methylimidazolium NTf2
To a chloroform (15 mL) solution of 1-(2-hydroxyethyl)-3-methylimidazolium NTf2 (4.10 g, 10.1 mmol), 38 mL of 0.5 M THF solution of 9-borabicyclo[3.3.1]nonane (9-BBN) (19.1 mmol) were added at 0 °C, and the resulting mixture was stirred for 5 h. After removing the solvent under a reduced pressure, excess 9-BBN was removed by washing with n-hexane several times. The product was thoroughly dried under a reduced pressure before use. The desired compound was obtained as a soft white solid (4.52 g, 8.57 mmol, 85% yield). 1H-NMR (CDCl3, δ, ppm) 1.26–2.50 (20H, 9-BBN), 3.98 (3H, CH3), 4.33 (2H, –NCH2CH2O), 5.62 (2H, –NCH2CH2OB–), 7.21, 7.35 (2H, –NCHCHN–), 8.87 (1H, –NCHN–). 13C-NMR (DMSO-d6, δ, ppm) 23.3–33.2 (9-BBN), 36.1, 52.1, 59.8, 119.4 (q, CF3), 123.1–123.8 (N–C=C–N), 137.3 (N–C=N). 11B-NMR (CDCl3, δδ, ppm) 33.3.
4. Conclusions
Novel organoboron molten salt using 1-(2-hydroxyethyl)-3-methylimidazolium chloride as a starting material was synthesized in an 85% yield. The structure was supported by 1H-, 13C-, 11B- and 19F-NMR spectra. A maximum ionic conductivity of 1.1 × 10−4 S cm−1 was observed for the sample with LiNTf2 salt. This system showed higher ionic conductivity than any other organoboron molten salt reported so far. All of the samples showed good thermal stability with T50% around 430 °C. The high thermal stability and the high ionic conductivity render the new organoboron-IL with desirable characteristics as a lithium ion transport material.
Author Contributioins
Noriyoshi Matsumi conceived of the research and drafted the manuscript. Yoshiyuki Toyota prepared the molten salt and measured the ion conductive properties. Puhup Puneet and Prerna Joshi prepared the molten salt and measured NMR spectra. Raman Vedarajan and Toshihiro Takekawa helped ion conductive measurements and VFT analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar]
- Ohno, H. Electrochemical Aspects of Ionic Liquids; Wiley Interscience: Hoboken, NJ: USA, 2005. [Google Scholar]
- Ohno, H. Functional design of ionic liquid. Bull. Chem. Soc. Jpn. 2006, 79, 1665–1680. [Google Scholar]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Pringle, J.M.; Sun, J.; Annat, G.; Neil, W.; Izgorodina, E.I. Ionic liquids in electrochemical devices and processes: Managing interfacial chemistry. Acc. Chem. Res. 2007, 40, 1165–1173. [Google Scholar]
- Matsumi, N.; Miyake, M.; Ohno, H. Molten salts bearing anion receptor. Chem. Commun. 1994, 2852–2853. [Google Scholar]
- Matsumi, N.; Sugai, K.; Miyake, M.; Ohno, H. Polymerized ionic liquids via hydroboration polymerization as single ion conductive polymer electrolytes. Macromolecules 2006, 39, 6924–6927. [Google Scholar]
- Yoshizawa, M.; Hirao, M.; Ito-Akita, K.; Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 2001, 11, 1057–1062. [Google Scholar]
- Yoshizawa, M.; Narita, A.; Ohno, H. Design of ionic liquids for electrochemical applications. Aust. J. Chem. 2004, 57, 139–144. [Google Scholar]
- Narita, A.; Shibayama, W.; Sakamoto, K.; Mizumo, T.; Matsumi, N.; Ohno, H. Lithium ion conduction in an organoborate zwitterion-LiTFSI mixture. Chem. Commun. 2006, 1926–1928. [Google Scholar]
- Mehta, M.A.; Fujinami, T. Li+ transference number enhancement in polymer electrolytes by incorporation of anion trapping boroxine rings into the polymer host. Chem. Lett. 1997, 9, 915–916. [Google Scholar]
- Hirakimoto, T.; Nishiura, M.; Watanabe, M. Effects of addition of a boric acid ester monomer to electrolyte solutions and gel electrolytes on their ionic transport properties. Electrochim. Acta 2001, 46, 1609–1614. [Google Scholar]
- Matsumi, N.; Sugai, K.; Ohno, H. Ion conductive characteristics of alkylborane type and boric ester type polymer electrolytes derived from mesitylborane. Macromolecules 2003, 36, 2321–2326. [Google Scholar]
- Matsumi, N.; Sugai, K.; Ohno, H. Selective ion transport in organoboron polymer electrolytes bearing a mesitylborane unit. Macromolecules 2002, 35, 5731–5733. [Google Scholar]
- Matsumi, N.; Mizumo, T.; Ohno, H. Single ion conductive characteristics of poly(organoboron halide)-imidazole complex. Polym. Bull. 2004, 51, 389–394. [Google Scholar]
- Matsumi, N.; Sugai, K.; Sakamoto, K.; Mizumo, T.; Ohno, H. Direct synthesis of poly(lithium organoborate)s and their ion conductive properties. Macromolecules 2005, 38, 4951–4954. [Google Scholar]
- Matsumi, N.; Nakashiba, M.; Mizumo, T.; Ohno, H. Novel polymer/salt hybrids composed of comb like organoboron polymer electrolyte and boron stabilized imido anion. Macromolecules 2005, 38, 2040–2042. [Google Scholar]
- Matsumi, N.; Kagata, A.; Aoi, K. Synthesis of supramolecular solid polymer electrolytes via self-assembly of diborylated ionic liquid. J. Power Sources 2010, 195, 6182–6186. [Google Scholar]
- Matsumi, N.; Yoshioka, N.; Aoi, K. Synthesis of boric ester type ion-gels by dehydrocoupling of cellulose with hydroboranes in ionic liquid. Solid State Ionics 2012, 226, 37–40. [Google Scholar]
- Matsumi, N.; Nakamura, Y.; Aoi, K.; Watanabe, T.; Mizumo, T.; Ohno, H. Enhanced ionic conduction in organoboron ion gels facilely designed via condensation of cellulose with boric acids in ionic liquids. Polym. J. 2009, 41, 437–441. [Google Scholar]
- Mizumo, T.; Watanabe, T.; Matsumi, N.; Ohno, H. Preparation of ion conductive inorganic-organic composite systems by in situ sol-gel reaction of polymerizable ionic liquids. Polym. Adv. Technol. 2008, 19, 1445–1450. [Google Scholar]
- Hardy, L.C.; Shriver, D.F. Preparation and electrical response of solid polymer electrolytes with only one mobile species. J. Am. Chem. Soc. 1985, 107, 3823–3828. [Google Scholar]
- Tsuchida, E.; Kobayashi, N.; Ohno, H. Single ion conduction in poly(oligo(oxyethylene)methacrylate)-co-(alkali-metalmethacrylates). Macromolecules 1988, 21, 96–100. [Google Scholar]
- Vogel, H. The temperature dependence law of the viscosity of fluids. Phys. Z. 1921, 22, 645–646. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339. [Google Scholar]
- Tamman, G.; Hesse, W. Die abhangigkeit der viskositat von der temperature bei unterkuhltenflussigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. (In German) [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).