Circulating microRNA-19a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Clinical Characteristics of the Study Population
| Index | AMI | Ctl | p |
|---|---|---|---|
| Ages (years) | 60.13 | 58.64 | >0.05 |
| Sex (M/F) | 125/55 | 106/63 | >0.05 |
| MYO | 116.6 | 59.64 | <0.001 |
| hs-TnI | 1.04 | 0.15 | <0.001 |
| BNP | 917.12 | 934.57 | <0.001 |
| CK | 375.64 | 95.36 | <0.001 |
| CK-MB | 42.40 | 17.96 | <0.001 |
2.2. Circulating miR-19a Levels in AMI Patients and Their Correlation with Biochemical Indicators
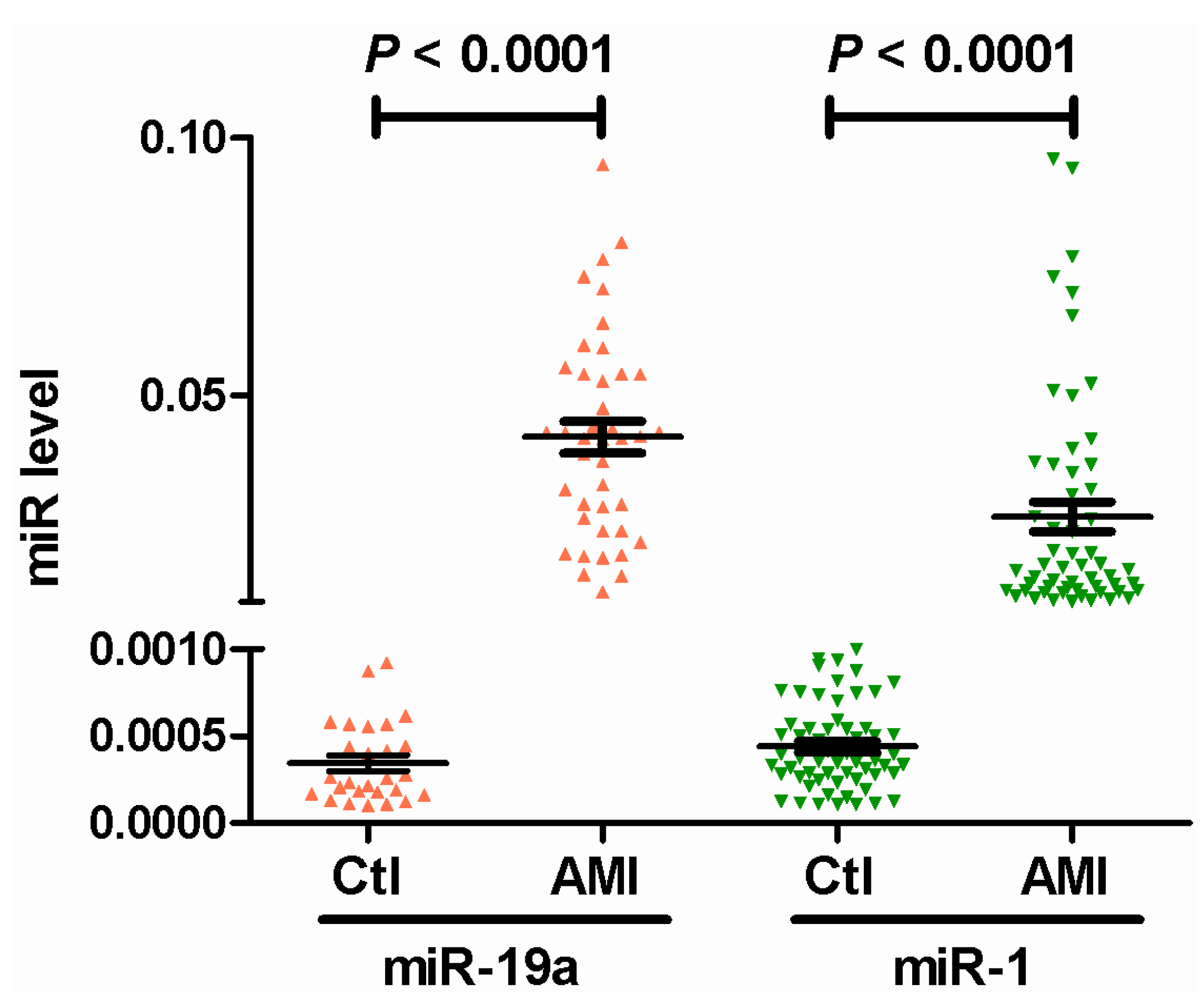
| Group Indicators | Ctl | AMI | p | OR (95% CI) |
|---|---|---|---|---|
| LPa (mg/L) | 146.44 ± 21.24 | 189.36 ± 34.19 | 0.066 | 1.007 (1.000, 1.015) |
| BUN (mmol/L) | 5.34 ± 0.24 | 7.95 ± 2.04 | 0.605 | 0.794 (0.332,1.901) |
| Scr (μmol/L) | 68.45 ± 4.93 | 97.75 ± 4.01 | 0.313 | 1.030 (0.972, 1.092) |
| SUA (μmol/L) | 307.74 ± 22.05 | 355.61 ± 18.98 | 0.120 | 1.014 (0.996, 1.031) |
| Glu (mmol/L) | 5.88 ± 0.52 | 5.94 ± 0.35 | 0.130 | 1.370 (0.911, 2.059) |
| Chol (mmol/L) | 4.93 ± 0.15 | 4.10 ± 0.17 | 0.087 | 0.268 (0.059, 1.213) |
| TG (mmol/L) | 1.29 ± 0.16 | 1.42 ± 0.13 | 0.272 | 2.743 (0.453, 16.619) |
| HDL (μmol/L) | 1.62 ± 0.08 | 1.33 ± 0.09 | 0.750 | 0.516 (0.009, 30.205) |
| LDL (μmol/L) | 2.78 ± 0.16 | 2.39 ± 0.13 | 0.214 | 0.349 (0.066, 1.835) |
| ApoA1 (g/L) | 1.39 ± 0.08 | 1.26 ± 0.06 | 0.033 | 0.008 (0.000, 0.678) |
| ApoB (g/L) | 0.86 ± 0.03 | 1.34 ± 0.49 | 0.057 | 7.594 × 103 (0.778, 7.417 × 107) |

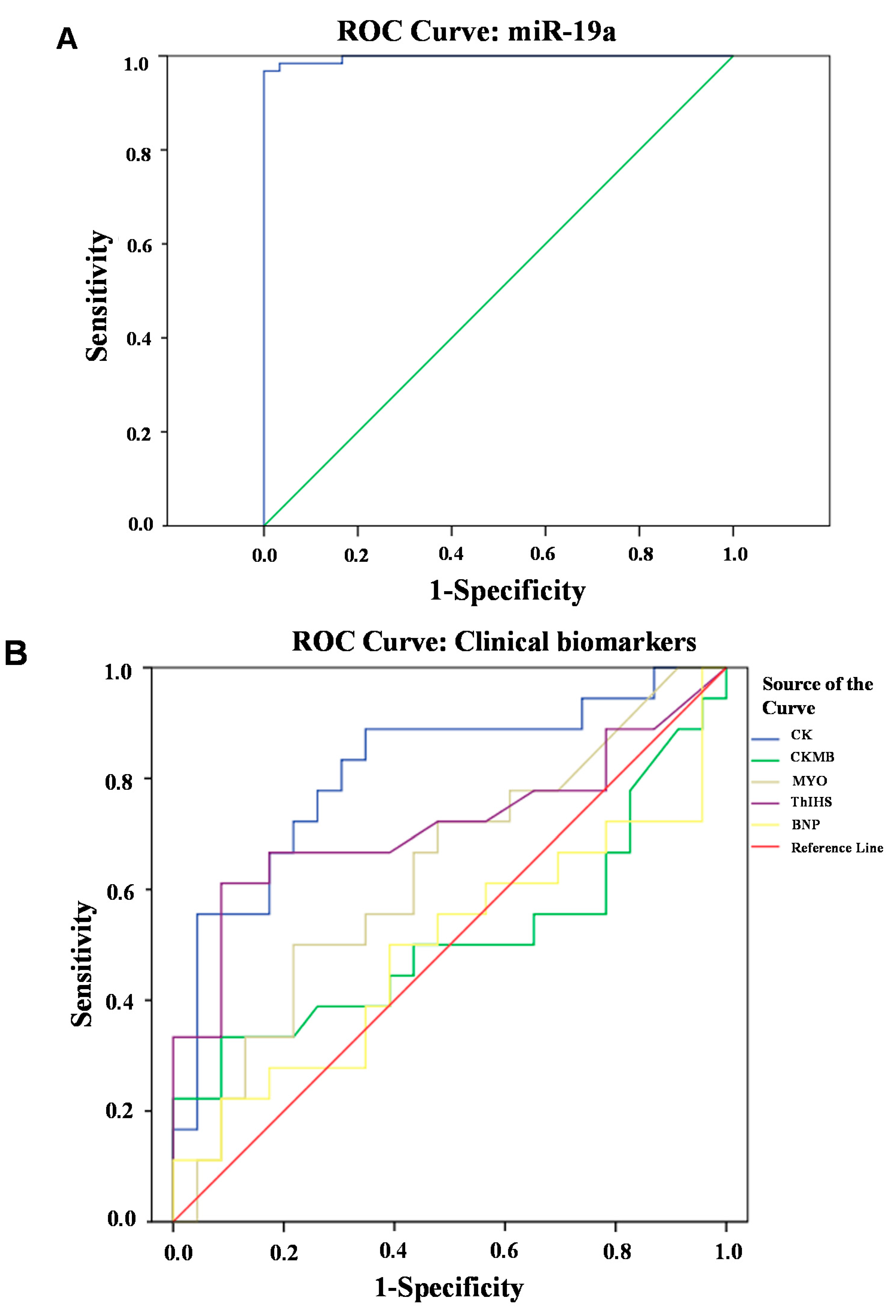
3. Materials and Methods
3.1. Study Population
3.2. Biochemical Assays and Blood Collection
3.3. Isolation of RNA and Real-Time PCR
3.4. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Q. Molecular genetics of coronary artery disease. Curr. Opin. Cardiol. 2005, 20, 182–188. [Google Scholar]
- Yusuf, S.; Reddy, S.; Ounpuu, S.; Anand, S. Global burden of cardiovascular diseases: part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001, 3, 2746–2753. [Google Scholar]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2013, 3, 2095–2128. [Google Scholar]
- Chen, C.; Lei, W.; Chen, W.J.; Zhong, J.; Gao, X.X.; Li, B.; Wang, H.; Huang, C.X. Serum TGF-β1 and SMAD3 levels are closely associated with coronary artery disease. BMC Cardiovasc. Disord. 2014, 14, 18. [Google Scholar]
- Pharithi, R.B.; Meela, M.; Kropmans, T.; Ward, F.; Conway, M.; Newell, M. Magnetic resonance myocardial perfusion imaging in the diagnosis of functionally significant obstructive coronary artery disease: A systematic review protocol. Syst. Rev. 2014, 3, 53. [Google Scholar]
- Tousoulis, D.; Kampoli, A.M.; Papageorgiou, N.; Androulakis, E.; Antoniades, C.; Toutouzas, K.; Stefanadis, C. Pathophysiology of atherosclerosis: the role of inflammation. Curr. Pharm. Des. 2011, 17, 4089–4110. [Google Scholar]
- Kathiresan, S.; Srivastava, D. Genetics of human cardiovascular disease. Cell 2012, 148, 1242–1257. [Google Scholar]
- Tayebi, N.; Ke, T.; Foo, J.N.; Friedlander, Y.; Liu, J.; Heng, C.K. Association of single nucleotide polymorphism rs6903956 on chromosome 6p24.1 with coronary artery disease and lipid levels in different ethnic groups of the Singaporean population. Clin. Biochem. 2013, 46, 755–759. [Google Scholar]
- Zhao, Y.; Samal, E.; Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005, 436, 214–220. [Google Scholar]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of miRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar]
- Wang, K.; Zhang, S.; Marzolf, B.; Troisch, P.; Brightman, A.; Hu, Z.; Hood, L.E.; Galas, D.J. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc. Natl. Acad. Sci. USA 2009, 106, 4402–4407. [Google Scholar]
- Ai, J.; Zhang, R.; Li, Y.; Pu, J.; Lu, Y.; Jiao, J.; Li, K.; Yu, B.; Li, Z.; Wang, R.; et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2010, 391, 73–77. [Google Scholar]
- Qin, X.; Wang, X.; Wang, Y.; Tang, Z.; Cui, Q.; Xi, J.; Li, Y.S.; Chien, S.; Wang, N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3240–3244. [Google Scholar]
- Zhou, P.Z.; Liu, F.B.; Luo, Q.; Sun, Y.; Ding, F.Y.; Chen, B. Effect of Baitouweng Decoction on intestinal miR-19a expression in mice with ulcerative colitis. J. South. Med. Univ. 2012, 32, 1597–1599. [Google Scholar]
- Feng, Y.; Liu, J.; Kang, Y.; He, Y.; Liang, B.; Yang, P.; Yu, Z. miR-19a acts as an oncogenic microRNA and is up-regulated in bladder cancer. J. Exp. Clin. Cancer Res. 2014, 33, 67. [Google Scholar]
- Anfossi, S.; Giordano, A.; Gao, H.; Cohen, E.N.; Tin, S.; Wu, Q.; Garza, R.J.; Debeb, B.G.; Alvarez, R.H.; Valero, V.; et al. High serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+ inflammatory breast cancer. PLoS One 2014, 9, e83113. [Google Scholar]
- Wu, T.Y.; Zhang, T.H.; Qu, L.M.; Feng, J.P.; Tian, L.L.; Zhang, B.H.; Li, D.D.; Sun, Y.N.; Liu, M. MiR-19a is correlated with prognosis and apoptosis of laryngeal squamous cell carcinoma by regulating TIMP-2 expression. Int. J. Clin. Exp. Pathol. 2013, 7, 56–63. [Google Scholar]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS One 2008, 3, e3148. [Google Scholar]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell. Res. 2008, 18, 997–1006. [Google Scholar]
- Fichtlscherer, S.; Zeiher, A.M.; Dimmeler, S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2383–2390. [Google Scholar]
- Li, C.; Pei, F.; Zhu, X.; Duan, D.D.; Zeng, C. Circulating microRNAs as novel and sensitive biomarkers of acute myocardial Infarction. Clin. Biochem. 2012, 45, 727–732. [Google Scholar]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar]
- Zethelius, B.; Johnston, N.; Venge, P. Troponin I as a predictor of coronary heartdisease and mortality in 70-year-old men: A community-based cohort study. Circulation 2006, 113, 1071–1078. [Google Scholar]
- Jaffe, A.S.; Babuin, L.; Apple, F.S. Biomarkers in acute cardiac disease: The present and the future. J. Am. Coll. Cardiol. 2006, 48, 1–11. [Google Scholar]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010, 50, 298–301. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; He, Y.; Chen, W.; Shui, X.; Chen, C.; Lei, W. Circulating microRNA-19a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction. Int. J. Mol. Sci. 2014, 15, 20355-20364. https://doi.org/10.3390/ijms151120355
Zhong J, He Y, Chen W, Shui X, Chen C, Lei W. Circulating microRNA-19a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction. International Journal of Molecular Sciences. 2014; 15(11):20355-20364. https://doi.org/10.3390/ijms151120355
Chicago/Turabian StyleZhong, Jianfeng, Yuan He, Wenjiang Chen, Xiaorong Shui, Can Chen, and Wei Lei. 2014. "Circulating microRNA-19a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction" International Journal of Molecular Sciences 15, no. 11: 20355-20364. https://doi.org/10.3390/ijms151120355
APA StyleZhong, J., He, Y., Chen, W., Shui, X., Chen, C., & Lei, W. (2014). Circulating microRNA-19a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction. International Journal of Molecular Sciences, 15(11), 20355-20364. https://doi.org/10.3390/ijms151120355




