Effects of SC-560 in Combination with Cisplatin or Taxol on Angiogenesis in Human Ovarian Cancer Xenografts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Ovarian Cancer Growth
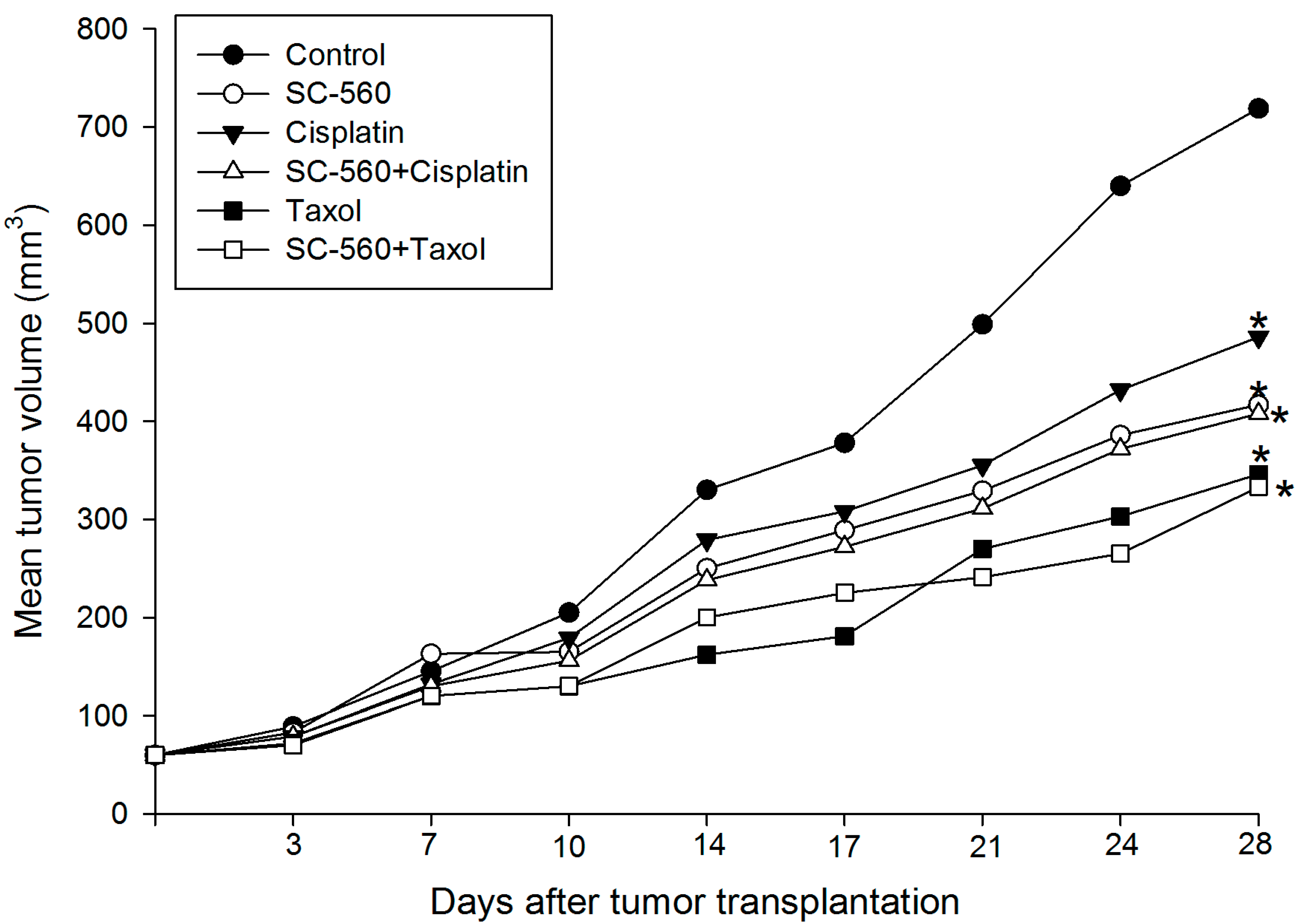
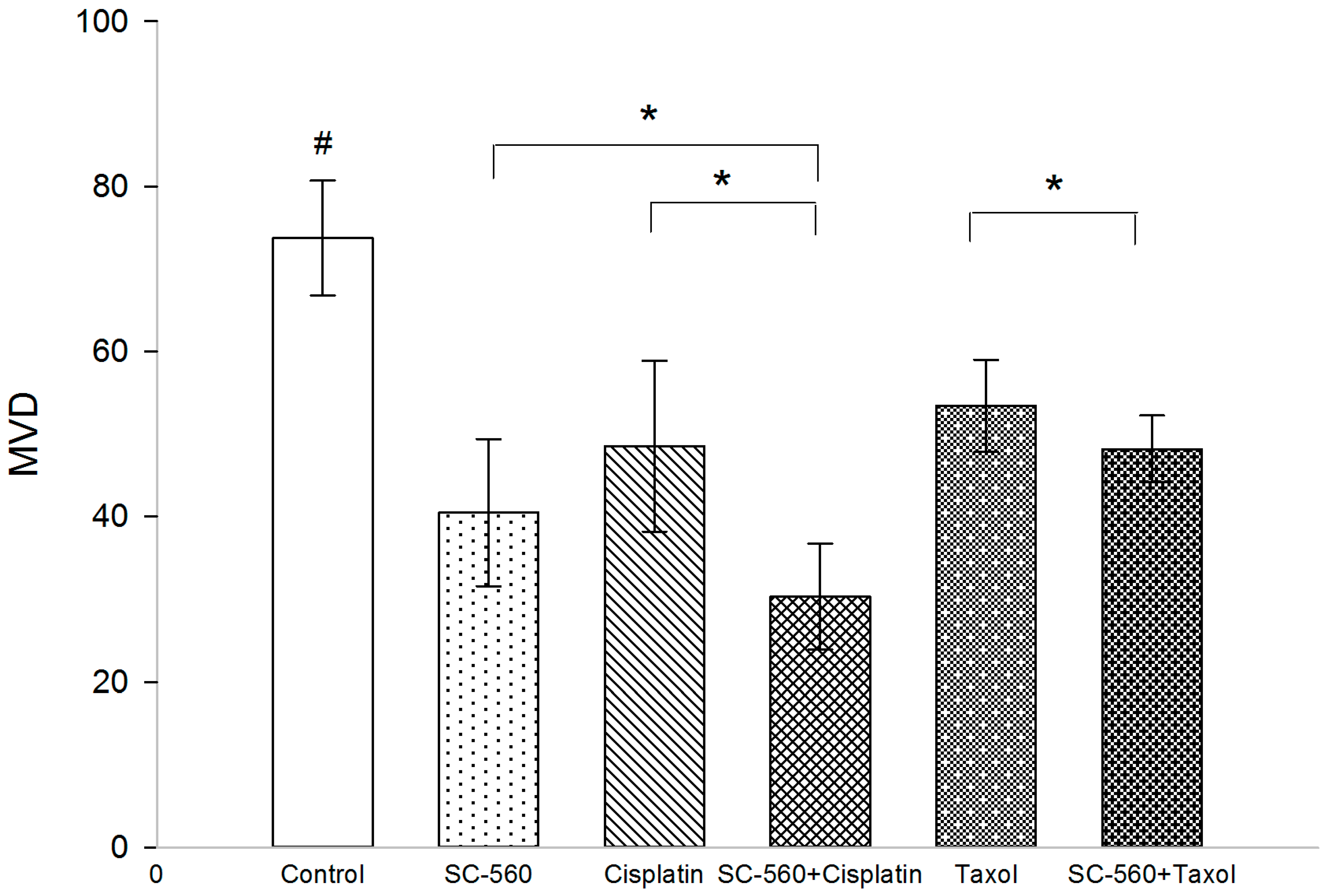
2.2. Effect on Microvessel Density (MVD)

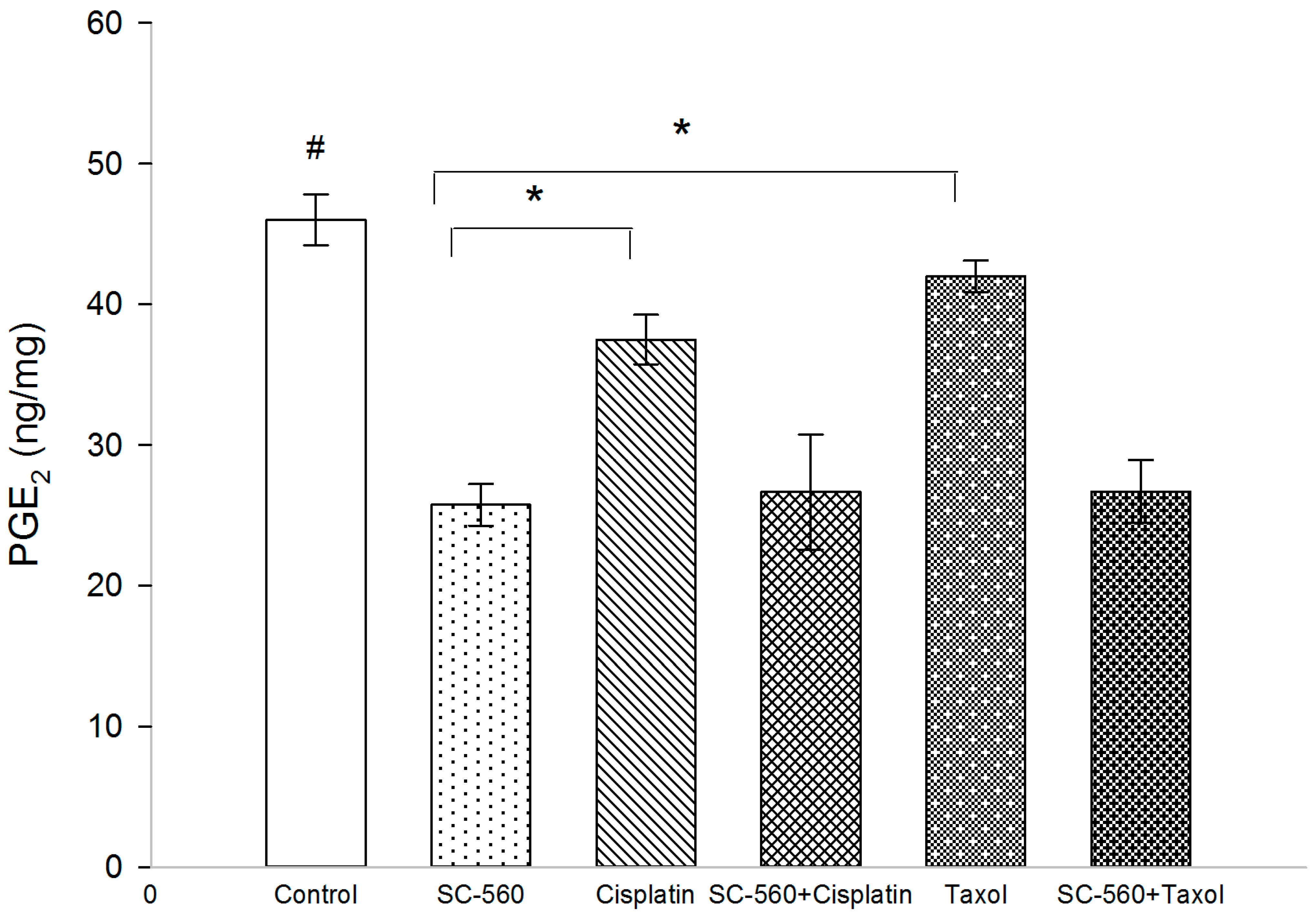
2.3. Effect on Prostaglandin E2 (PGE2) Production
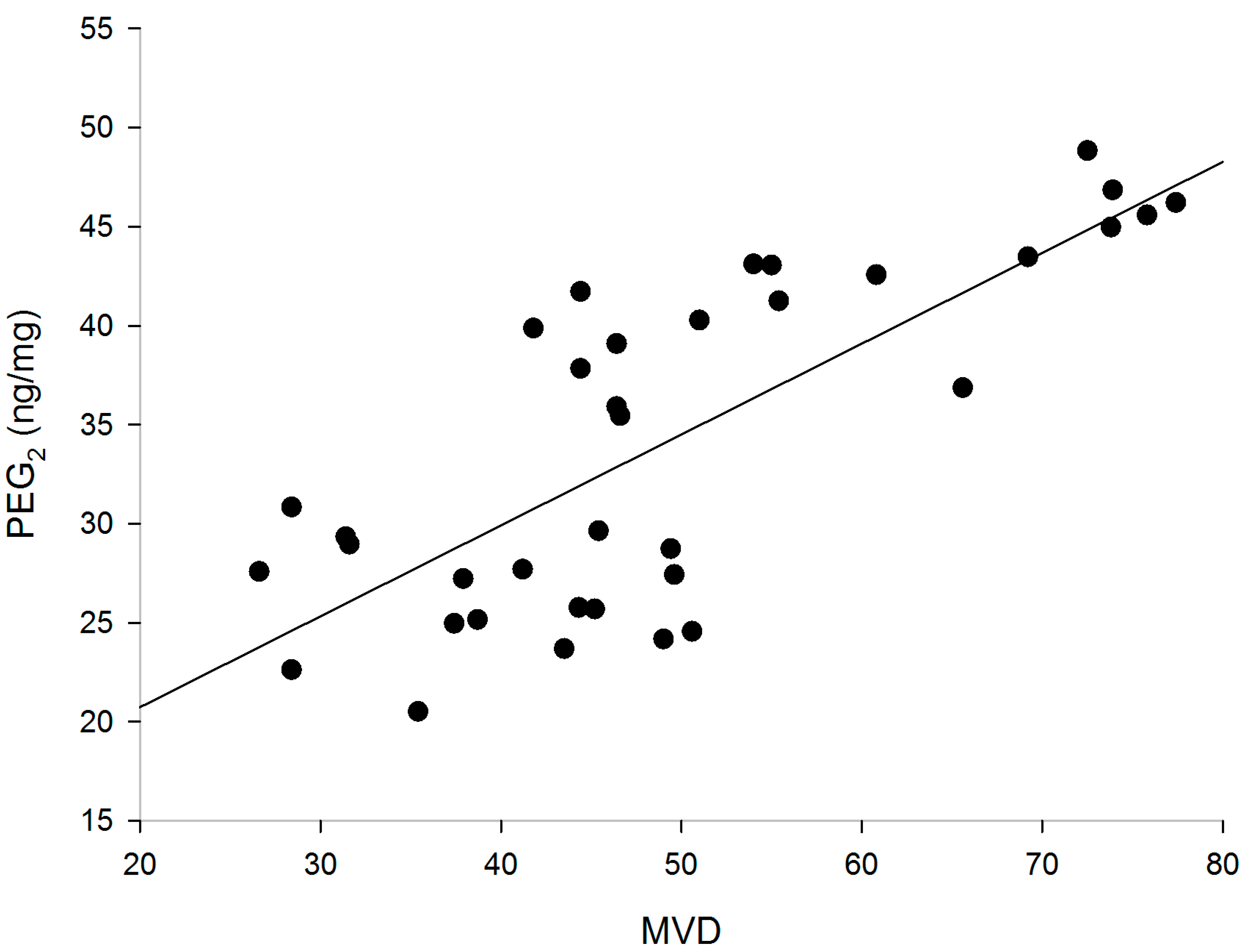
2.4. Correlation of PGE2 with MVD
2.5. Effect on Vascular Endothelial Growth Factor (VEGF) Production
| Group | VEGF 121 | VEGF 165 | VEGF 189 |
|---|---|---|---|
| Control | 6.94 ± 0.23 | 4.58 ± 0.26 | 6.34 ± 0.23 |
| SC-560 | 7.13 ± 0.30 | 5.34 ± 0.30 | 6.54 ± 0.21 |
| Cisplatin | 7.71 ± 0.38 | 5.63 ± 0.51 | 7.14 ± 0.28 |
| SC-560 + Cisplatin | 8.16 ± 0.36 | 6.83 ± 0.37 | 8.27 ± 0.42 |
| Taxol | 7.87 ± 0.32 | 6.28 ± 0.49 | 7.79 ± 0.29 |
| SC-560 + Taxol | 9.14 ± 0.33 | 7.00 ± 0.48 | 8.99 ± 0.23 |

2.6. Cyclooxygenase-1 (COX-1) Expression
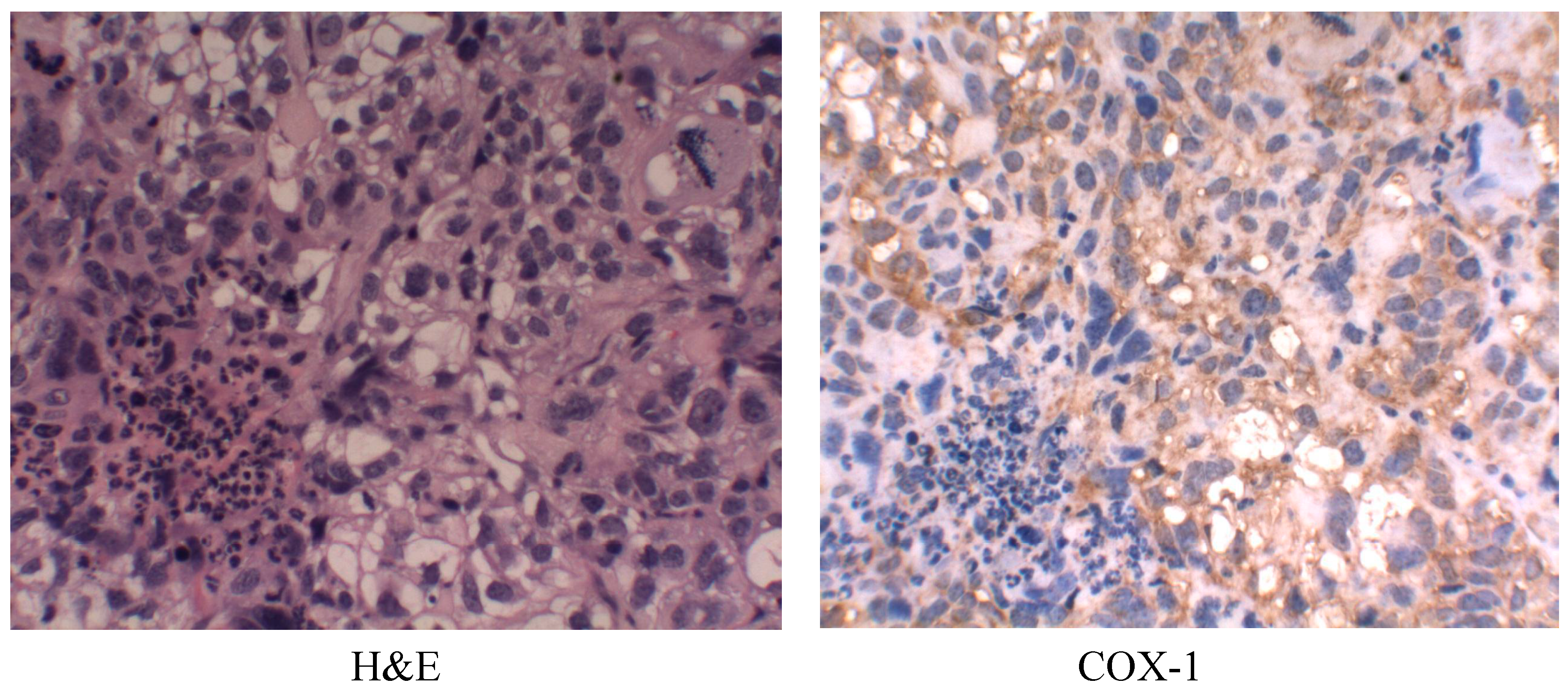
2.7. Discussion
3. Experimental Section
3.1. Human Ovarian Tumors in Nude Mice
3.2. Dose and Administration of Drugs
3.3. Measurement of Tumor Volume
3.4. Immunohistochemistry for MVD
3.5. Real-Time PCR
3.6. Determination of PGE2 Levels in Tumor Tissues
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Woopen, H.; Sehouli, J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res. 2009, 29, 3353–3359. [Google Scholar]
- Li, L.; Wang, L.M.; Zhang, W.; Tang, B.J.; Zhang, J.Q.; Song, H.L.; Yao, D.S.; Tang, Y.; Chen, X.Q.; Yang, Z.H.; et al. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res. 2004, 24, 1973–1980. [Google Scholar]
- Wu, H.; Wang, K.; Liu, W.; Hao, Q. PTEN over-expression improves cisplatin-resistance of human ovarian cancer cells through up-regulating KRT10 expression. Biochem. Biophys. Res. Commun. 2014, 444, 141–146. [Google Scholar]
- Stordal, B.; Pavlakis, N.; Davey, R. A systematic review of platinum and taxane resistance from bench to clinic: An inverse relationship. Cancer Treat. Rev. 2007, 33, 688–703. [Google Scholar]
- Go, R.S.; Adjei, A.A. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J. Clin. Oncol. 1999, 17, 409–422. [Google Scholar]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 2005, 3, 277–285. [Google Scholar]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar]
- Lawson, E.; Irada, I.; Hamdi, J.; Sevtap, S.; Mehran, M.; Kathleen, I.P.; Hilmi, O. Bioinformatic analyses identifies novel proteincoding pharmacogenomic markers associated with paclitaxel sensitivity in NCI60 cancer cell lines. BMC Med. Genomics 2011, 4, 18. [Google Scholar]
- Kohler, D.R.; Goldspiel, B.R. Evaluation of new drugs: Paclitaxel (taxol). Pharmacotherapy 1994, 14, 3–34. [Google Scholar]
- Wang, Y.; Qu, Y.; Niu, X.L.; Sun, W.J.; Zhang, X.L.; Li, L.Z. Autocrine production of interleukin-8 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cytokine 2011, 56, 365–375. [Google Scholar]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar]
- Radi, Z.A.; Khan, N.K. Effect of cyclooxygenase inhibition on the gastrointestinal tract. Exp. Toxicol. Pathol. 2006, 58, 163–173. [Google Scholar]
- Daikoku, T.; Wang, D.Z.; Tranguch, S.; Morrow, J.D.; Orsulic, S.; Dubois, R.N.; Dey, S.K. Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer. Cancer Res. 2005, 65, 3735–3744. [Google Scholar]
- Dore, M.; Cote, L.C.; Mitchell, A.; Sirois, J. Expression of prostaglandin G/H synthase type 1, but not type 2, in human ovarian adenocarcinomas. J. Histochem. Cytochem. 1998, 46, 77–84. [Google Scholar]
- Sales, K.J.; Katz, A.A; Howard, B.; Soeters, R.P.; Millar, R.P.; Jabbour, H.N. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, prostaglandine receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002, 62, 424–432. [Google Scholar]
- Erovic, B.M.; Woegerbauer, M.; Pammer, J.; Selzer, E.; Grasl, M.Ch.; Thurnher, D. Strong evidence for up-regulation of cyclooxygenase-1 in head and neck cancer. Eur. J. Clin. Investig. 2008, 38, 61–66. [Google Scholar]
- Gupta, R.A.; Tejada, L.V.; Tong, B.J.; Das, S.K.; Morrow, J.D.; Dey, S.K.; Dubois, R.N. Cyclooxygenase-1 is over-expressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003, 63, 906–911. [Google Scholar]
- Urick, M.E.; Johnson, P.A. Cyclooxygenase 1 and 2 mRNA and protein expression in the Gallus domesticus model of ovarian cancer. Gynecol. Oncol. 2006, 103, 673–678. [Google Scholar]
- Daikoku, T.; Tranguch, S.; Trofimova, I.N.; Dinulescu, D.M.; Jacks, T.; Nikitin, A.Y.; Connolly, D.C.; Dey, S.K. Cyclooxygenase-1 is over-expressed in multiple genetically engineered mouse models of epithelial ovarian cancer. Cancer Res. 2006, 66, 2527–2531. [Google Scholar]
- Ferrandina, G.; Lauriola, L.; Zannoni, G.F.; Faqotti, A.; Fanfani, F.; Maqqiano, N.; Gessi, M.; Mancuso, S.; Ranelletti, F.O.; Scambia, G. Increased cyclooxygenase-2 (COX-2) expression is associated with chemotherapy resistance and outcome in ovarian cancer patients. Ann. Oncol. 2002, 13, 1205–1211. [Google Scholar]
- Subbaramaiah, K.; Hart, J.C.; Norton, L.; Dannenberq, A.J. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2: Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J. Biol. Chem. 2000, 275, 14838–14845. [Google Scholar]
- Munkarah, A.R.; Ali-Fehmi, R.; Jiang, J.Z.; Elhammady, E.; Malone, J.M., Jr.; Saed, G.M. The effects of combining docetaxel and cyclooxygenase-2 inhibitors on proliferation and apoptosis in epithelial ovarian cancer. Anticancer Drugs 2007, 18, 889–896. [Google Scholar]
- Bijman, M.N.; Hermelink, C.A.; van Berkel, M.P.; Laan, A.C.; Janmaat, M.L.; Peters, G.J.; Boven, E. Interaction between celecoxib and docetaxel or cisplatin in human cell lines of ovarian cancer and colon cancer is independent of COX-2 expression levels. Biochem. Pharmacol. 2008, 75, 427–437. [Google Scholar]
- Mutter, R.; Lu, B.; Carbone, D.P.; Csiki, I.; Moretti, L.; Johnson, D.H.; Morrow, J.D.; Sandler, A.B.; Shyr, Y.; Ye, F.; et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin. Cancer Res. 2009, 15, 2158–2165. [Google Scholar]
- Bhatt, R.S.; Merchan, J.; Parker, R.; Wu, H.K.; Zhang, L.; Seery, V.; Heymach, J.V.; Atkins, M.B.; Mcdermott, D.; Sukhatme, V.P. A phase 2 pilot trial of low-dose, continuous infusion, or “metronomic” paclitaxel and oral celecoxib in patients with metastatic melanoma. Cancer 2010, 116, 1751–1756. [Google Scholar]
- Altorki, N.K.; Christos, P.; Port, J.L.; Lee, P.C.; Mirza, F.; Spinelli, C.; Keresztes, R.; Beneck, D.; Paul, S.; Stiles, B.M.; et al. Preoperative taxanebased chemotherapy and celecoxib for carcinoma of the esophagus and gastroesophageal junction: Results of a phase 2 trial. J. Thorac. Oncol. 2011, 6, 1121–1127. [Google Scholar]
- Li, W.; Xu, R.J.; Lin, Z.Y.; Zhuo, G.C.; Zhang, H.H. Effects of a cyclooxygenase-1-selective inhibitor in a mouse model of ovarian cancer, administered alone or in combination with ibuprofen, a nonselective cyclooxygenase inhibitor. Med. Oncol. 2009, 26, 170–177. [Google Scholar]
- Moysich, K.B.; Mettlin, C.; Piver, M.S.; Natarajan, N.; Menezes, R.J.; Swede, H. Regular use of analgesic drugs and ovarian cancer risk. Cancer Epidemiol. Biomark. Prev. 2001, 10, 903–906. [Google Scholar]
- Li, W.; Cai, J.H.; Zhang, J.; Tang, Y.X.; Wan, L. Effects of cyclooxygenase inhibitors in combination with taxol on expression of cyclin d1 and ki-67 in a xenograft model of ovarian carcinoma. Int. J. Mol. Sci. 2012, 13, 9741–9753. [Google Scholar]
- Zivkovic, V.; Djuric, D.; Turjacanin-Pantelic, D.; Marinkovic, Z.; Stefanovic, D.; Srejovic, I.; Jakovljevic, V. The effects of cyclooxygenase and nitric oxide synthase inhibition on cardiodynamic parameters and coronary flow in isolated rat hearts. Exp. Clin. Cardiol. 2013, 18, e102–e110. [Google Scholar]
- Hong, T.T.; Huang, J.; Barrett, T.D.; Lucchesi, B.R. Effects of cyclooxygenase inhibition on canine coronary artery blood flow and thrombosis. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H145–H155. [Google Scholar]
- Gamarra-Luques, C.D.; Hapon, M.B.; Goyeneche, A.A.; Telleria, C.M. Resistance to cisplatin and paclitaxel does not affect the sensitivity of human ovarian cancer cells to antiprogestin-induced cytotoxicity. J. Ovarian Res. 2014, 7, 45. [Google Scholar]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar]
- Ratnasinghe, D.; Daschner, P.J.; Anver, M.R.; Kasprzak, B.H.; Taylor, P.R.; Yeh, G.C.; Tangrea, J.A. Cyclooxygenase-2, P-glycoprotein-170 and drug resistance; is chemoprevention against multidrug resistance possible? Anticancer Res. 2001, 21, 2141–2147. [Google Scholar]
- Okada, T.; Takigawa, N.; Kishino, D.; Katayama, H.; Kuyama, S.; Sato, K.; Mimoto, J.; Ueoka, H.; Tanimoto, M.; Kiura, K. Selective cyclooxygenase-2 inhibitor prevents cisplatin-induced tumorigenesis in A/J mice. Acta. Med. Okayama 2012, 66, 245–251. [Google Scholar]
- Harlozinska, A.; Sedlaczek, P.; Kulpa, J.; Grybos, M.; Wójcik, E.; van Dalen, A.; Einarsson, R. Vascular endothelial growth factor (VEGF) concentration in sera and tumor effusions from patients with ovarian carcinoma. Anticancer Res. 2004, 24, 1149–1157. [Google Scholar]
- Prager, G.W.; Poettler, M. Angiogenesis in cancer. Basic mechanisms and therapeutic advances. Hamostaseologie 2011, 32, 105–114. [Google Scholar]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar]
- Hazelton, D.A.; Hamilton, T.C. Vascular endothelial growth factor on ovarian cancer. Curr. Oncol. Rep. 1999, 1, 59–63. [Google Scholar]
- Von Rahden, B.H.; Stein, H.J.; Pühringer, F.; Koch, I.; Langer, R.; Piontek, G.; Siewert, J.R.; Höler, H.; Sarbia, M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005, 65, 5038–5044. [Google Scholar]
- Lee, J.P.; Hahn, H.S.; Hwang, S.J.; Choi, J.Y.; Park, J.S.; Lee, I.H.; Kim, T.J. Selective cyclooxygenase inhibitors increase paclitaxel sensitivity in taxane-resistant ovarian cancer by suppressing P-glycoprotein expression. J. Gynecol. Oncol. 2013, 24, 273–279. [Google Scholar]
- Joarder, F.; Abouissa, H.; Robertson, F.; Parrett, M.; Alshafie, G.; Harris, R. Growth arrest of DMBA-induced mammary carcinogenesis with ibuprofen treatment in female Sprague–Dawley rats. Oncol. Rep. 1997, 4, 1271–1273. [Google Scholar]
- Spinella, F.; Rosano, L.; di Castro, V.; Nicotra, M.R.; Natali, P.G.; Bagnato, A. Inhibition of cyclooxygenase-1 and -2 expression by targeting the endothelin a receptor in human ovarian carcinoma cells. Clin. Cancer Res. 2004, 10, 4670–4679. [Google Scholar]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic seich during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar]
- Munkarah, A.R.; Genhai, Z.; Morris, R.; Baker, W.; Ceppe, G.; Diamond, M.P.; Saed, G.M. Inhibition of paclitaxel-induced apoptosis by the specific COX-2 inhibitor, NS398, in epithelial ovarian cancer cells. Gynecol. Oncol. 2003, 88, 429–433. [Google Scholar]
- Devalapally, H.; Duan, Z.F.; Seiden, M.V.; Amiji, M.M. Modulation of drug resistance in ovarian adenocarcinoma by enhangcing intracellular ceramide using tamoxifen-loaded bioderadable polymeric nanoparticles. Clin. Cancer Res. 2008, 14, 3193–3203. [Google Scholar]
- Reese, J.; Zhao, X.; Ma, W.G.; Brown, N.; Maziasz, T.J.; Dey, S.K. Comparative analysis of pharmacologic and/or genetic disruption of cyclooxygenase-1 and cyclooxygenase-2 function in female reproduction in mice. Endocrinology 2001, 142, 3198–3206. [Google Scholar]
- Williams, C.S.; Watson, A.J.M.; Sheng, H.; Helou, R.; Shao, J.; DuBois, R.N. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000, 60, 6045–6051. [Google Scholar]
- Weidner, N.; Folkman, J.; Pozza, F.; Bevilacqua, P.; Allred, E.N.; Moore, D.H.; Meli, S.; Gasparini, G. Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. J. Natl. Cancer Inst. 1992, 84, 1875–1887. [Google Scholar]
- Trifan, O.C.; Durham, W.F.; Salazar, V.S.; Horton, J.; Levine, B.D.; Zweifel, B.S.; Davis, T.W.; Masferer, J.L. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 2002, 62, 5778–5784. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wan, L.; Zhai, L.-Y.; Wang, J. Effects of SC-560 in Combination with Cisplatin or Taxol on Angiogenesis in Human Ovarian Cancer Xenografts. Int. J. Mol. Sci. 2014, 15, 19265-19280. https://doi.org/10.3390/ijms151019265
Li W, Wan L, Zhai L-Y, Wang J. Effects of SC-560 in Combination with Cisplatin or Taxol on Angiogenesis in Human Ovarian Cancer Xenografts. International Journal of Molecular Sciences. 2014; 15(10):19265-19280. https://doi.org/10.3390/ijms151019265
Chicago/Turabian StyleLi, Wei, Liang Wan, Ling-Yun Zhai, and Jane Wang. 2014. "Effects of SC-560 in Combination with Cisplatin or Taxol on Angiogenesis in Human Ovarian Cancer Xenografts" International Journal of Molecular Sciences 15, no. 10: 19265-19280. https://doi.org/10.3390/ijms151019265
APA StyleLi, W., Wan, L., Zhai, L.-Y., & Wang, J. (2014). Effects of SC-560 in Combination with Cisplatin or Taxol on Angiogenesis in Human Ovarian Cancer Xenografts. International Journal of Molecular Sciences, 15(10), 19265-19280. https://doi.org/10.3390/ijms151019265




