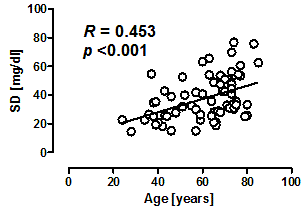Glycemic Variability and Oxidative Stress: A Link between Diabetes and Cardiovascular Disease?
Abstract
:1. Introduction
2. Assessment of Glycemic Variability
| Index | Description | Note |
|---|---|---|
| Intra-day GV (needs SMBG or CGM) | ||
| SD | Commonly reported expression of GV. | |
| CV | SD divided by mean | SD corrected for mean. |
| MAGE | Mean glucose value by summing absolute rises and falls of more than 1 SD | Smaller excursions of less than 1 SD are ignored. Determinant of glucose excursion could be subjective. |
| M value | Mean of logarithmic transformation of deviation from reference value | This formula puts greater emphasis on hypoglycemia than on hyperglycemia. |
| CONGA-n | SD of summed differences between current observation and observation n hours earlier | This calculation is more objective than MAGE. CGM data are needed for calculation. |
| Between-day GV (needs SMBG or CGM) | ||
| MODD | Mean absolute value of differences between glucose values at the same time on two consecutive days | In daily practice, differences in mealtimes influence the value. |
| Visit-to-visit GV | ||
| SD-FPG | SD of FPG over weeks to years | Reflects longer-term GV. |
| SD-HbA1c | SD of HbA1c over months to years | Reflects longer-term GV. |
| Others | ||
| 75 g OGTT | Assesses glycemic excursion after oral glucose load | Gold standard for diagnosis of glucose intolerance. |
| MTT | Reflects more physiological postprandial glycemic excursion | Needs standard meal for comparison. |
| 1,5-AG | Value is lower in the presence of glucosuria | Reflects the presence of postprandial hyperglycemia. |
| GA | Reflects average glucose level over past 1 to 2 weeks | Reflects overall hyperglycemia and glycemic excursion. |
| Ratio of GA to HbA1c | Reflects glycemic excursion. | |
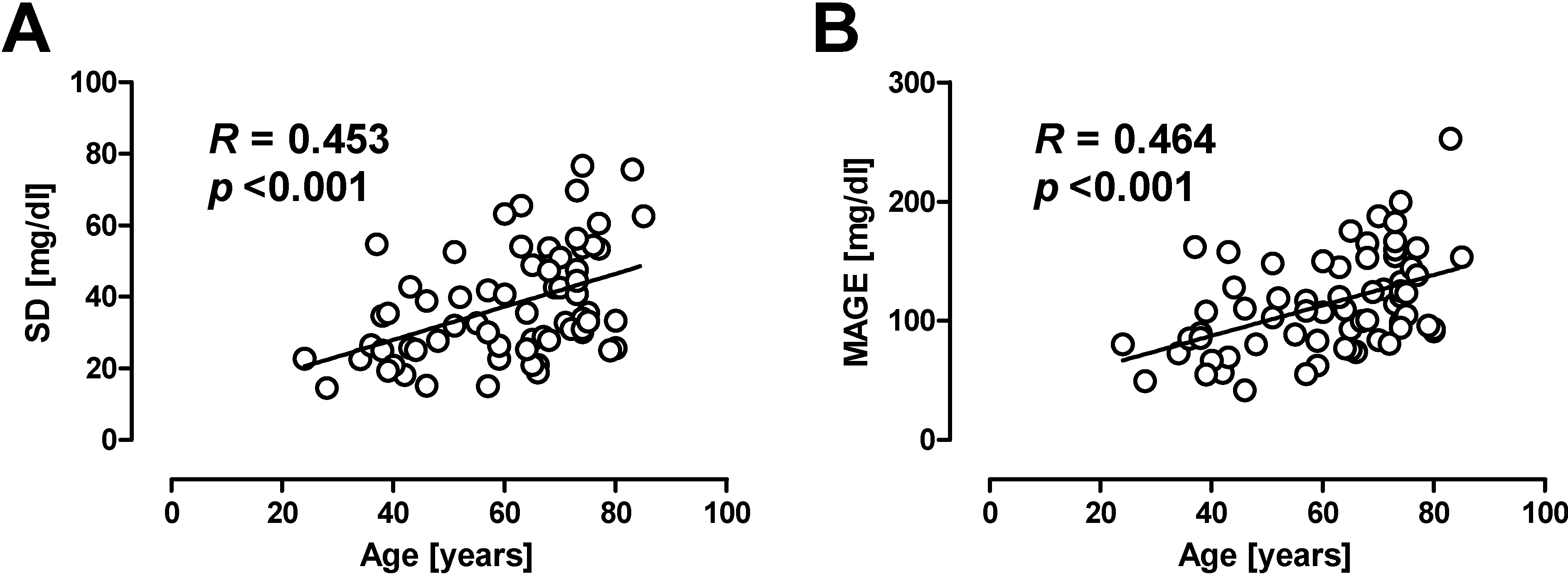
3. Glycemic Variability and Vascular Events
4. Underlying Mechanisms: Oxidative Stress
4.1. Preclinical Studies
4.2. Clinical Studies
5. Other Mechanisms
5.1. Dyslipidemia
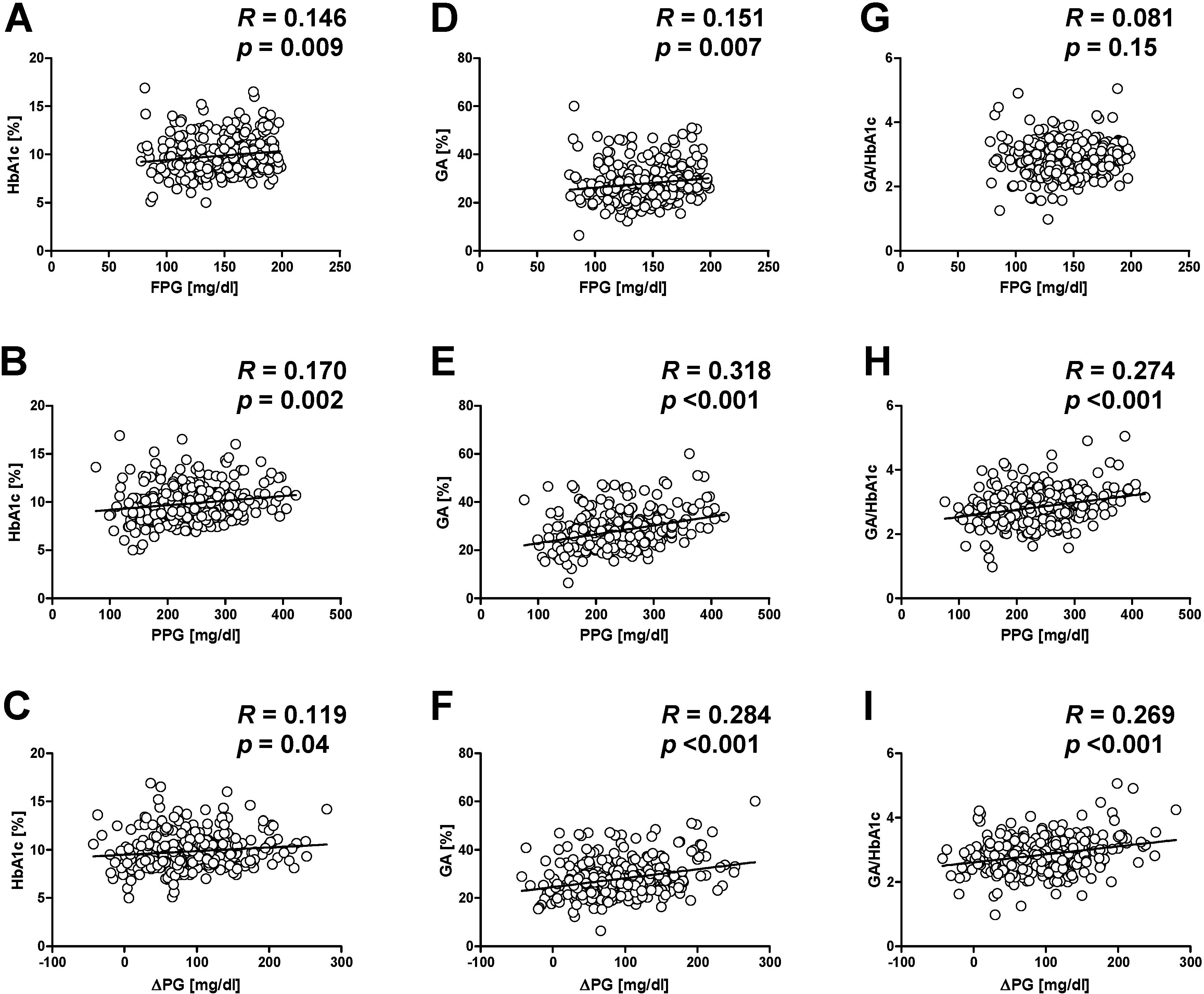
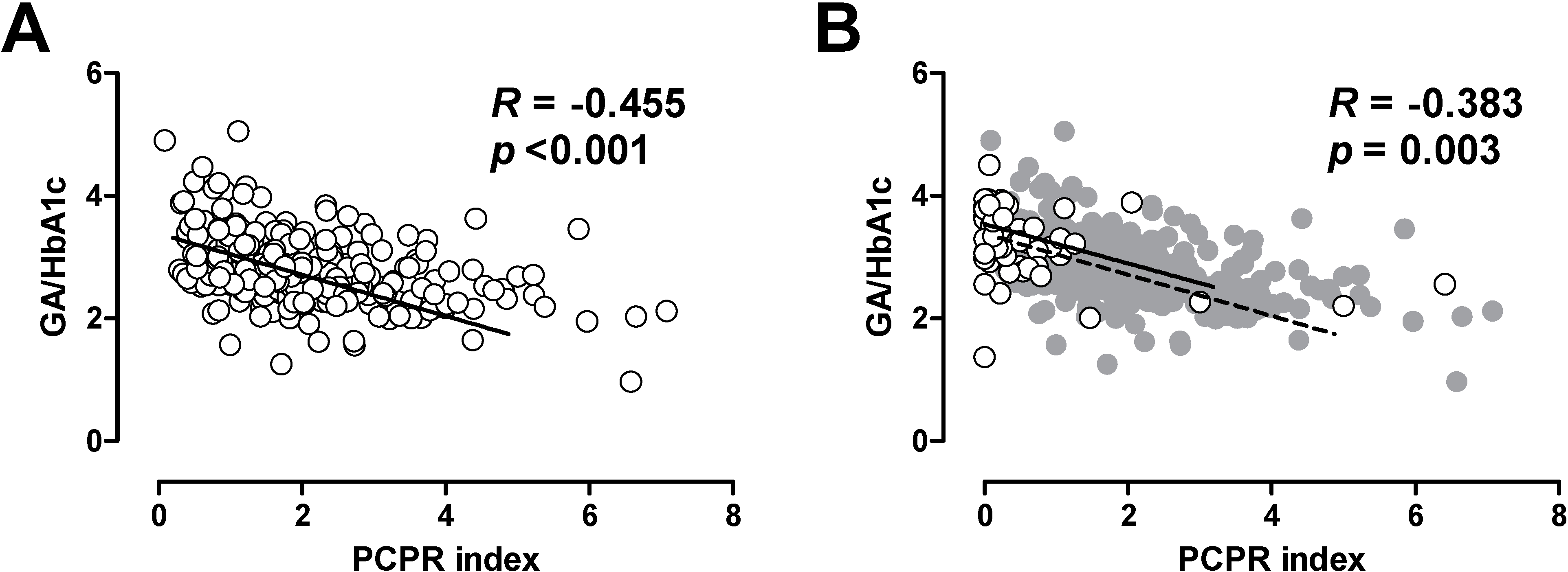
5.2. Glycated Albumin
5.3. Hypoglycemia
6. Clinical Implications
6.1. Effects of Treatment of Glycemic Variability on Oxidative Stress and Cardiovascular Outcomes
6.2. Factors Associated with Glycemic Variability
| Reduced β cell function |
| Older age |
| Liver failure |
| Renal impairment |
| Reduced lean mass |
| Autonomic neuropathy |
| Anti-diabetic medication |
| Polypharmacy |
| Cognitive impairment/dementia |
| Poor compliance with treatment |
| Intake of food with higher glycemic index and/or glycemic load |
| Amount of vegetables/fiber intake |
| Irregular timing of meals |
| Physical inactivity |
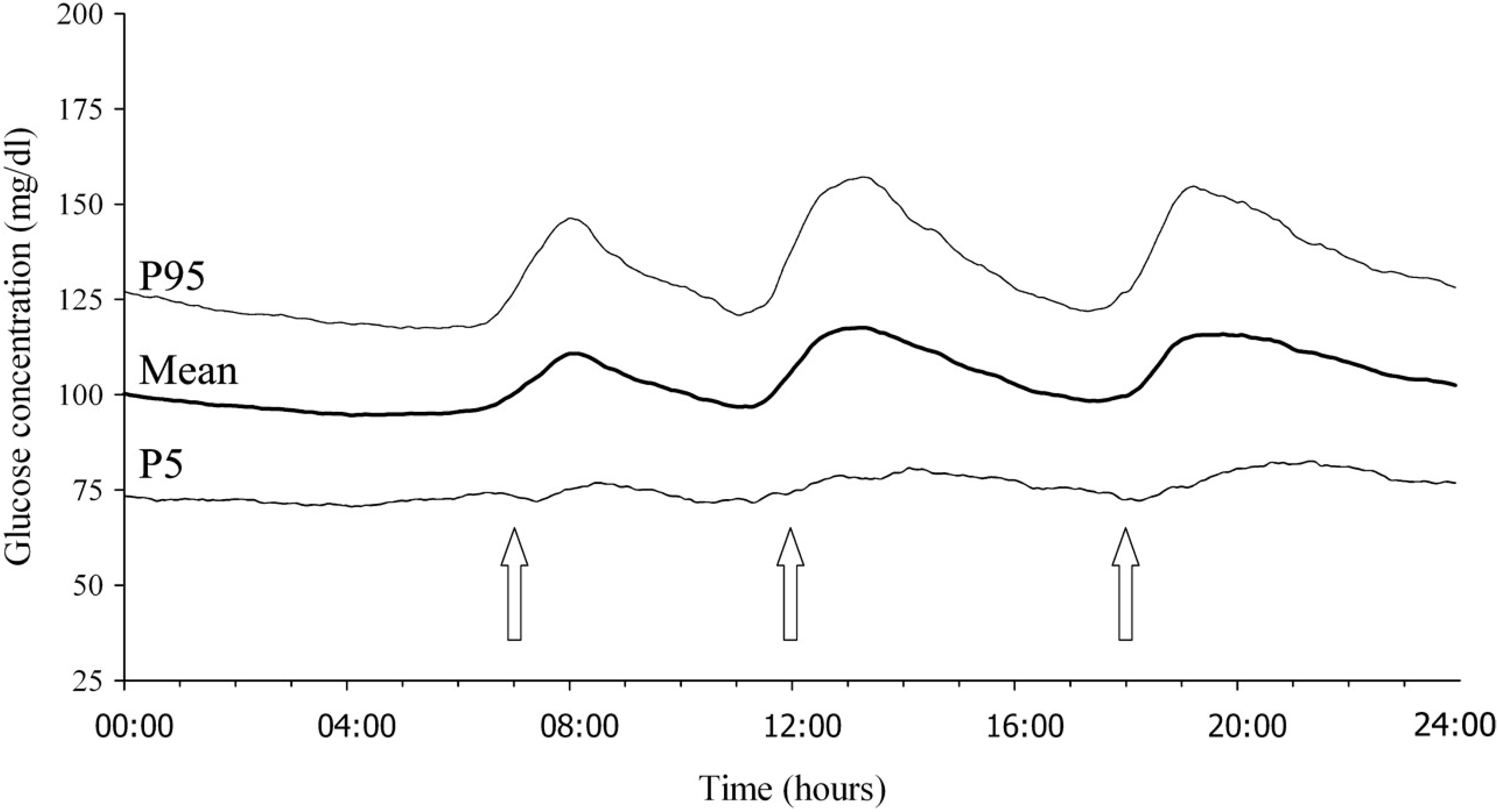
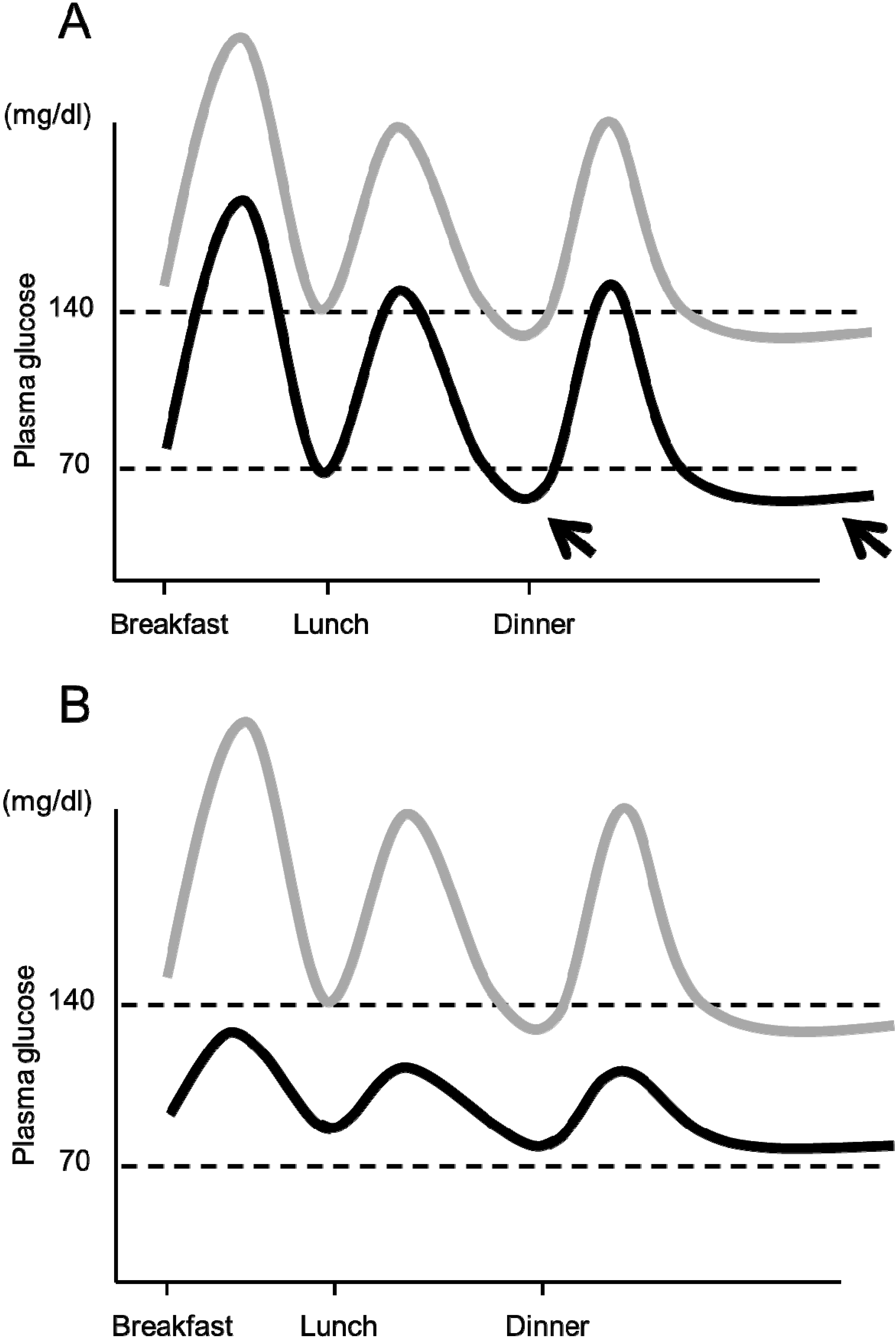
6.3. Importance of Preventing Hypoglycemia
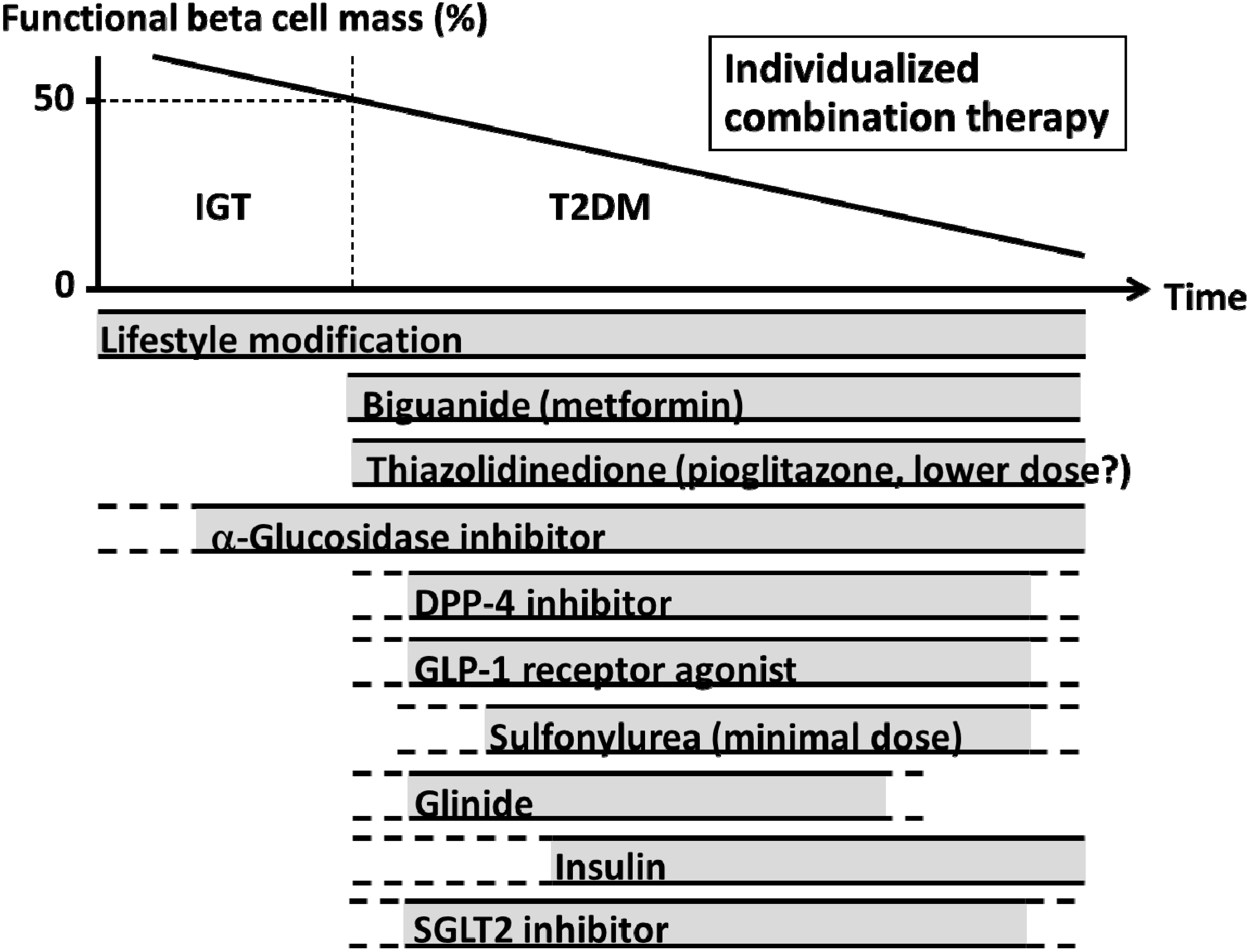
6.4. Treatment Strategy to Minimize Glycemic Variability
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seshasai, S.R.; Kaptoge, S.; Thompson, A.; di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar] [CrossRef]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.Y.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef]
- Currie, C.J.; Peters, J.R.; Tynan, A.; Evans, M.; Heine, R.J.; Bracco, O.L.; Zagar, T.; Poole, C.D. Survival as a function of HbA(1c) in people with type 2 diabetes: A retrospective cohort study. Lancet 2010, 375, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, H.; Ran, X.; Yang, W.; Li, Q.; Peng, Y.; Li, Y.; Gao, X.; Luan, X.; Wang, W.; et al. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care 2009, 32, 1188–1193. [Google Scholar] [CrossRef]
- Service, F.J.; Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970, 19, 644–655. [Google Scholar] [PubMed]
- Schlichtkrull, J.; Munck, O.; Jersild, M. The M-valve, an index of blood-sugar control in diabetics. Acta Med. Scand. 1965, 177, 95–102. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.M.; Donath, S.M.; Vidmar, S.I.; Werther, G.A.; Cameron, F.J. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol. Ther. 2005, 7, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Service, F.J.; Nelson, R.L. Characteristics of glycemic stability. Diabetes Care 1980, 3, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Owens, D.R. Glycemic variability: The third component of the dysglycemia in diabetes. Is it important? How to measure it? J. Diabetes Sci. Technol. 2008, 2, 1094–1100. [Google Scholar]
- Rodbard, D. The challenges of measuring glycemic variability. J. Diabetes Sci. Technol. 2012, 6, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Buse, J.B.; Largay, J.; Kelly, M.M.; Button, E.A.; Kato, S.; Wittlin, S. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care 2006, 29, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Sakane, N.; Yoshida, T.; Kogure, A.; Kondo, M.; Yoshioka, K. Different effects of acarbose and voglibose on serum 1,5-anhydroglucitol concentrations/response. Diabetes Care 1998, 21, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Uchino, H.; Ohmura, C.; Tanaka, Y.; Onuma, T.; Kawamori, R. Different effects of two alpha-glucosidase inhibitors, acarbose and voglibose, on serum 1,5-anhydroglucitol (1,5AG) level. J. Diabetes Complicat. 2004, 18, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Kasayama, S. Clinical impact of glycated albumin as another glycemic control marker. Endocr. J. 2010, 57, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Yoshiuchi, K.; Matsuhisa, M.; Katakami, N.; Nakatani, Y.; Sakamoto, K.; Matsuoka, T.; Umayahara, Y.; Kosugi, K.; Kaneto, H.; Yamasaki, Y.; et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr. J. 2008, 55, 503–507. [Google Scholar] [CrossRef]
- Saisho, Y.; Tanaka, K.; Abe, T.; Shimada, A.; Kawai, T.; Itoh, H. Glycated albumin to glycated hemoglobin ratio reflects postprandial glucose excursion and relates to beta cell function in both type 1 and type 2 diabetes. Diabetol. Int. 2011, 2, 146–153. [Google Scholar] [CrossRef]
- The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995, 44, 968–983. [Google Scholar]
- Lachin, J.M.; Genuth, S.; Nathan, D.M.; Zinman, B.; Rutledge, B.N. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—Revisited. Diabetes 2008, 57, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care 2009, 32, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Siegelaar, S.E.; Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L.; Hoekstra, J.B.; Devries, J.H. Glucose variability does not contribute to the development of peripheral and autonomic neuropathy in type 1 diabetes: Data from the DCCT. Diabetologia 2009, 52, 2229–2232. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. Mean blood glucose compared with HbA1c in the prediction of cardiovascular disease in patients with type 1 diabetes. Diabetologia 2008, 51, 365–371. [Google Scholar] [CrossRef] [PubMed]
- The DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar]
- Nakagami, T. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004, 47, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kokubo, Y.; Higashiyama, A.; Ono, Y.; Miyamoto, Y.; Okamura, T. Serum 1,5-anhydro-d-glucitol levels predict first-ever cardiovascular disease: An 11-year population-based cohort study in Japan, the Suita study. Atherosclerosis 2011, 216, 477–483. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Carleo, D.; Schisano, B.; Sardelli, L.; di Tommaso, D.; Misso, L.; Saccomanno, F.; Ceriello, A.; Giugliano, D. Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F.; Pagliarino, A.; Valle, M.; di Martino, L.; Bonomo, K.; Massucco, P.; Anfossi, G.; Trovati, M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011, 34, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, K.; Okada, Y.; Mori, H.; Tanaka, Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, K.; Okada, Y.; Mori, H.; Tanaka, Y. Low levels of 1,5-anhydro-d-glucitol are associated with vascular endothelial dysfunction in type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Borg, R.; Kuenen, J.C.; Carstensen, B.; Zheng, H.; Nathan, D.M.; Heine, R.J.; Nerup, J.; Borch-Johnsen, K.; Witte, D.R. HbA(1)(c) and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: The A1C-Derived Average Glucose (ADAG) study. Diabetologia 2011, 54, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sartore, G.; Chilelli, N.C.; Burlina, S.; Lapolla, A. Association between glucose variability as assessed by continuous glucose monitoring (CGM) and diabetic retinopathy in type 1 and type 2 diabetes. Acta Diabetol. 2013, 50, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Orna, J.A.; Castro-Alonso, F.J.; Boned-Juliani, B.; Lou-Arnal, L.M. Fasting plasma glucose variability as a risk factor of retinopathy in type 2 diabetic patients. J. Diabetes Complicat. 2003, 17, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Takao, T.; Ide, T.; Yanagisawa, H.; Kikuchi, M.; Kawazu, S.; Matsuyama, Y. The effect of fasting plasma glucose variability on the risk of retinopathy in type 2 diabetic patients: Retrospective long-term follow-up. Diabetes Res. Clin. Pract. 2010, 89, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Chang, H.Y.; Huang, M.C.; Hwang, S.J.; Yang, Y.C.; Lee, Y.S.; Shin, S.J.; Tai, T.Y. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: A 7-year prospective cohort study. Diabetologia 2012, 55, 3163–3172. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Kawai, K.; Motohashi, S.; Saito, K.; Kodama, S.; Yachi, Y.; Hirasawa, R.; Shimano, H.; Yamazaki, K.; Sone, H. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia 2012, 55, 2128–2131. [Google Scholar] [CrossRef] [PubMed]
- Waden, J.; Forsblom, C.; Thorn, L.M.; Gordin, D.; Saraheimo, M.; Groop, P.H. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 2009, 58, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Marcovecchio, M.L.; Dalton, R.N.; Chiarelli, F.; Dunger, D.B. A1C variability as an independent risk factor for microalbuminuria in young people with type 1 diabetes. Diabetes Care 2011, 34, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Arima, H.; Zoungas, S.; Ninomiya, T.; Cooper, M.; Hamet, P.; Mancia, G.; Poulter, N.; Harrap, S.; Woodward, M.; et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: The ADVANCE trial. Diabetes Care 2014, 37, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Risso, A.; Mercuri, F.; Quagliaro, L.; Damante, G.; Ceriello, A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E924–E930. [Google Scholar] [PubMed]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef]
- Piconi, L.; Quagliaro, L.; Assaloni, R.; da Ros, R.; Maier, A.; Zuodar, G.; Ceriello, A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab. Res. Rev. 2006, 22, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.M.; Benko, R.; Kiss, L.; Muranyi, M.; Pek, T.; Fekete, K.; Barany, T.; Somlai, A.; Csordas, A.; Szabo, C. Rapid “glycaemic swings” induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia 2009, 52, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Wentholt, I.M.; Kulik, W.; Michels, R.P.; Hoekstra, J.B.; DeVries, J.H. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia 2008, 51, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Siegelaar, S.E.; Barwari, T.; Kulik, W.; Hoekstra, J.B.; DeVries, J.H. No relevant relationship between glucose variability and oxidative stress in well-regulated type 2 diabetes patients. J. Diabetes Sci. Technol. 2011, 5, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Di Flaviani, A.; Picconi, F.; di Stefano, P.; Giordani, I.; Malandrucco, I.; Maggio, P.; Palazzo, P.; Sgreccia, F.; Peraldo, C.; Farina, F.; et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care 2011, 34, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Shepherd, J.; Wakil, A.; Kilpatrick, E.S. A comparison of methods for the measurement of 8-isoPGF(2α): A marker of oxidative stress. Ann. Clin. Biochem. 2011, 48, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.; Ooi, T.C. Postprandial lipaemia and vascular disease. Curr. Opin. Cardiol. 2013, 28, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Baraka-Vidot, J.; Guerin-Dubourg, A.; Dubois, F.; Payet, B.; Bourdon, E.; Rondeau, P. New insights into deleterious impacts of in vivo glycation on albumin antioxidant activities. Biochim. Biophys. Acta 2013, 1830, 3532–3541. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.J.; Lu, L.; Xu, X.W.; Zhang, R.Y.; Zhang, Q.; Zhang, J.S.; Hu, J.; Yang, Z.K.; Ding, F.H.; Chen, Q.J.; et al. Value of serum glycated albumin and high-sensitivity C-reactive protein levels in the prediction of presence of coronary artery disease in patients with type 2 diabetes. Cardiovasc. Diabetol. 2006, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Pu, L.J.; Zhang, Q.; Wang, L.J.; Kang, S.; Zhang, R.Y.; Chen, Q.J.; Wang, J.G.; de Caterina, R.; Shen, W.F. Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis 2009, 206, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Song, S.O.; Kim, K.J.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Lee, H.C. Serum glycated albumin predicts the progression of carotid arterial atherosclerosis. Atherosclerosis 2012, 225, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nagao, M.; Asai, A.; Nakajima, Y.; Takaya, M.; Takeichi, N.; Takemitsu, S.; Sudo, M.; Kano-Wakakuri, T.; Ishizaki, A.; et al. Association of glycated albumin with the presence of carotid plaque in patients with type 2 diabetes. J. Diabetes Investig. 2013, 4, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; McGee, P.; Steffes, M.W.; Lachin, J.M. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014, 63, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Rawlings, A.M.; Grams, M.; Klein, R.; Sharrett, A.R.; Steffes, M.; Coresh, J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: A prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014, 2, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Kovatchev, B.P.; Julian, D.M.; Gonder-Frederick, L.A.; Polonsky, W.H.; Schlundt, D.G.; Clarke, W.L. Frequency of severe hypoglycemia in insulin-dependent diabetes mellitus can be predicted from self-monitoring blood glucose data. J. Clin. Endocrinol. Metab. 1994, 79, 1659–1662. [Google Scholar] [PubMed]
- Murata, G.H.; Hoffman, R.M.; Shah, J.H.; Wendel, C.S.; Duckworth, W.C. A probabilistic model for predicting hypoglycemia in type 2 diabetes mellitus: The Diabetes Outcomes in Veterans Study (DOVES). Arch. Intern. Med. 2004, 164, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.S.; Rigby, A.S.; Goode, K.; Atkin, S.L. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 2007, 50, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Bonds, D.E.; Miller, M.E.; Bergenstal, R.M.; Buse, J.B.; Byington, R.P.; Cutler, J.A.; Dudl, R.J.; Ismail-Beigi, F.; Kimel, A.R.; Hoogwerf, B.; et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: Retrospective epidemiological analysis of the ACCORD study. BMJ 2010, 340, b4909. [Google Scholar] [CrossRef] [PubMed]
- Zoungas, S.; Patel, A.; Chalmers, J.; de Galan, B.E.; Li, Q.; Billot, L.; Woodward, M.; Ninomiya, T.; Neal, B.; MacMahon, S.; et al. Severe hypoglycemia and risks of vascular events and death. N. Engl. J. Med. 2010, 363, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Mellbin, L.G.; Ryden, L.; Riddle, M.C.; Probstfield, J.; Rosenstock, J.; Diaz, R.; Yusuf, S.; Gerstein, H.C. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur. Heart J. 2013, 34, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, M.L.; Tulppo, M.P.; Kiviniemi, A.M.; Kallio, M.A.; Perkiomaki, J.S.; Salmela, P.I.; Airaksinen, K.E.; Huikuri, H.V. Autonomic cardiac regulation during spontaneous nocturnal hypoglycemia in patients with type 1 diabetes. Diabetes Care 2012, 35, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; McKeon, K.; Comment, N.; Henderson, J.; Swanson, S.; Plunkett, C.; Nelson, P.; Pop-Busui, R. Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 2014, 37, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.V.; Woodward, A.; Casson, I.F.; Weston, P.J. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes—The “dead in bed” syndrome revisited. Diabetologia 2009, 52, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Bernjak, A.; Williams, S.; Fawdry, R.A.; Hibbert, S.; Freeman, J.; Sheridan, P.J.; Heller, S.R. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014, 63, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Stahn, A.; Pistrosch, F.; Ganz, X.; Teige, M.; Koehler, C.; Bornstein, S.; Hanefeld, M. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: Silent hypoglycemias and silent arrhythmias. Diabetes Care 2014, 37, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; la Sala, L.; Pujadas, G.; Testa, R.; Bonfigli, A.R.; Esposito, K.; Giugliano, D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 2012, 61, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Raz, I.; Wilson, P.W.; Strojek, K.; Kowalska, I.; Bozikov, V.; Gitt, A.K.; Jermendy, G.; Campaigne, B.N.; Kerr, L.; Milicevic, Z.; et al. Effects of prandial vs. fasting glycemia on cardiovascular outcomes in type 2 diabetes: The HEART2D trial. Diabetes Care 2009, 32, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Raz, I.; Ceriello, A.; Wilson, P.W.; Battioui, C.; Su, E.W.; Kerr, L.; Jones, C.A.; Milicevic, Z.; Jacober, S.J. Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial vs. fasting/premeal glycemia. Diabetes Care 2011, 34, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Siegelaar, S.E.; Kulik, W.; van Lenthe, H.; Mukherjee, R.; Hoekstra, J.B.; Devries, J.H. A randomized clinical trial comparing the effect of basal insulin and inhaled mealtime insulin on glucose variability and oxidative stress. Diabetes Obes. Metab. 2009, 11, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Mas, E.; Michel, F.; Cristol, J.P.; Boegner, C.; Owens, D.R. Regulation of oxidative stress by glycaemic control: Evidence for an independent inhibitory effect of insulin therapy. Diabetologia 2010, 53, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003, 290, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Cagatay, M.; Petrowitsch, T.; Neuser, D.; Petzinna, D.; Rupp, M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: Meta-analysis of seven long-term studies. Eur. Heart J. 2004, 25, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D.; Nappo, F.; Marfella, R. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004, 110, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Watada, H.; Shimizu, T.; Tamura, Y.; Sato, F.; Watanabe, T.; Choi, J.B.; Hirose, T.; Tanaka, Y.; Kawamori, R. Nateglinide reduces carotid intima-media thickening in type 2 diabetic patients under good glycemic control. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Haffner, S.M.; McMurray, J.J.; Bethel, M.A.; Holzhauer, B.; Hua, T.A.; Belenkov, Y.; Boolell, M.; Buse, J.B.; Buckley, B.M.; et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 2010, 362, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Shiotani, H.; Terashita, D.; Nagasawa, Y.; Kim, S.S.; Koide, M.; Yokoyama, M. Comparison of effects of α-Glucosidase inhibitors and glinide drugs on endothelial dysfunction in diabetic patients with coronary artery disease. Circ. J. 2013, 78, 248–255. [Google Scholar] [PubMed]
- Kodani, N.; Saisho, Y.; Tanaka, K.; Kawai, T.; Itoh, H. Effects of mitiglinide, a short-acting insulin secretagogue, on daily glycemic variability and oxidative stress markers in Japanese patients with type 2 diabetes mellitus. Clin. Drug Investig. 2013, 33, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, L.; Zhang, L.; Zhou, Y.; He, Q.; Zhang, Z.; Wang, M. Effects of glucose load and nateglinide intervention on endothelial function and oxidative stress. J. Diabetes Res. 2013, 2013, 849295. [Google Scholar] [PubMed]
- Marfella, R.; Barbieri, M.; Grella, R.; Rizzo, M.R.; Nicoletti, G.F.; Paolisso, G. Effects of vildagliptin twice daily vs. sitagliptin once daily on 24-hour acute glucose fluctuations. J. Diabetes Complicat. 2010, 24, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Nishimura, R.; Irako, T.; Tsujino, D.; Ando, K.; Utsunomiya, K. Comparison of vildagliptin twice daily vs. sitagliptin once daily using continuous glucose monitoring (CGM): Crossover pilot study (J-VICTORIA study). Cardiovasc. Diabetol. 2012, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Barbieri, M.; Marfella, R.; Paolisso, G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: Role of dipeptidyl peptidase-IV inhibition. Diabetes Care 2012, 35, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Barbieri, M.; Ruggiero, R.; Rizzo, M.R.; Grella, R.; Mozzillo, A.L.; Docimo, L.; Paolisso, G. Bariatric surgery reduces oxidative stress by blunting 24-h acute glucose fluctuations in type 2 diabetic obese patients. Diabetes Care 2010, 33, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; la Sala, L.; Pujadas, G.; Esposito, K.; Giugliano, D.; Genovese, S. Glucagon-like Peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013, 36, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; la Sala, L.; Pujadas, G.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care 2013, 36, 4104–4108. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Canivell, S.; la Sala, L.; Pujadas, G.; Esposito, K.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, antiinflammatory, and antioxidant action in type 2 diabetes. Diabetes Care 2014, 37, 1938–1943. [Google Scholar] [CrossRef]
- Kelly, A.S.; Bergenstal, R.M.; Gonzalez-Campoy, J.M.; Katz, H.; Bank, A.J. Effects of exenatide vs. metformin on endothelial function in obese patients with pre-diabetes: A randomized trial. Cardiovasc. Diabetol. 2012, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 3, Cd007176. [Google Scholar] [PubMed]
- Saisho, Y. Obesity, type 2 diabetes and beta cell failure: An Asian perspective. J. Mol. Genet. Med. 2014, S1, 8. [Google Scholar]
- Saisho, Y. Importance of beta cell function for the treatment of type 2 diabetes. J. Clin. Med. 2014, 3, 923–943. [Google Scholar] [CrossRef]
- Fukuda, M.; Tanaka, A.; Tahara, Y.; Ikegami, H.; Yamamoto, Y.; Kumahara, Y.; Shima, K. Correlation between minimal secretory capacity of pancreatic β-cells and stability of diabetic control. Diabetes 1988, 37, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kobayashi, T.; Inoko, H.; Tsuji, K.; Murase, T.; Kosaka, K. Residual β-cell function and HLA-A24 in IDDM. Markers of glycemic control and subsequent development of diabetic retinopathy. Diabetes 1995, 44, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Sassa, M.; Yamada, Y.; Hosokawa, M.; Fukuda, K.; Fujimoto, S.; Toyoda, K.; Tsukiyama, K.; Seino, Y.; Inagaki, N. Glycemic instability in type 1 diabetic patients: Possible role of ketosis or ketoacidosis at onset of diabetes. Diabetes Res. Clin. Pract. 2008, 81, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Murai, J.; Saito, H.; Kasayama, S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care 2010, 33, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Hayashi, A.; Kishihara, E.; Yoshino, S.; Takeuchi, A.; Shichiri, M. New indices for predicting glycaemic variability. PLoS One 2012, 7, e46517. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Tanaka, K.; Abe, T.; Kawai, T.; Itoh, H. Lower β cell function relates to sustained higher glycated albumin to glycated hemoglobin ratio in Japanese patients with type 2 diabetes. Endocr. J. 2014, 61, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, K.J.; Huh, J.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Lee, H.C. The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clin. Endocrinol. 2012, 77, 679–683. [Google Scholar] [CrossRef]
- Kramer, C.K.; Choi, H.; Zinman, B.; Retnakaran, R. Glycemic variability in patients with early type 2 diabetes: The impact of improvement in β-cell function. Diabetes Care 2014, 37, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Munshi, M.N.; Pandya, N.; Umpierrez, G.E.; DiGenio, A.; Zhou, R.; Riddle, M.C. Contributions of basal and prandial hyperglycemia to total hyperglycemia in older and younger adults with type 2 diabetes mellitus. J. Am. Geriatr. Soc. 2013, 61, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Marfella, R.; Barbieri, M.; Boccardi, V.; Vestini, F.; Lettieri, B.; Canonico, S.; Paolisso, G. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 2010, 33, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.E.; Williamson, J.D.; Gerstein, H.C.; Byington, R.P.; Cushman, W.C.; Ginsberg, H.N.; Ambrosius, W.T.; Lovato, L.; Applegate, W.B. Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older vs. younger adults in the ACCORD trial. Diabetes Care 2014, 37, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Lipska, K.J.; Ross, J.S.; Wang, Y.; Inzucchi, S.E.; Minges, K.; Karter, A.J.; Huang, E.S.; Desai, M.M.; Gill, T.M.; Krumholz, H.M. National Trends in US Hospital Admissions for Hyperglycemia and Hypoglycemia Among Medicare Beneficiaries, 1999 to 2011. JAMA Intern. Med. 2014, 174, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.; Saisho, Y.; Tanaka, K.; Kou, K.; Tanaka, M.; Meguro, S.; Irie, J.; Jo, R.; Kawai, T.; Itoh, H. Factors associated with glycemic variability in Japanese patients with diabetes. Diabetol. Int. 2014, 5, 36–42. [Google Scholar] [CrossRef]
- U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: A progressive disease. Diabetes 1995, 44, 1249–1258. [Google Scholar]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Tanaka, K.; Abe, T.; Shimada, A.; Kawai, T.; Itoh, H. Effect of obesity on declining β cell function after diagnosis of type 2 diabetes: A possible link suggested by cross-sectional analysis. Endocr. J. 2012, 59, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Shin, J.A.; Lee, S.H.; Kim, E.S.; Cho, J.H.; Son, H.Y.; Yoon, K.H. A comparative study of the effects of a dipeptidyl peptidase-IV inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metformin. Diabetes Technol. Ther. 2013, 15, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Irace, C.; Fiorentino, R.; Carallo, C.; Scavelli, F.; Gnasso, A. Exenatide improves glycemic variability assessed by continuous glucose monitoring in subjects with type 2 diabetes. Diabetes Technol. Ther. 2011, 13, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, S.F.; Terada, T.; Chahal, B.S.; Boule, N.G. Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: A meta-analysis of studies using continuous glucose monitoring. Diabetes Metab. Res. Rev. 2013, 29, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Bergenstal, R.M.; Glass, L.C.; Heilmann, C.R.; Lewis, M.S.; Kwan, A.Y.; Hoogwerf, B.J.; Rosenstock, J. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: A randomized, controlled trial. Ann. Intern. Med. 2011, 154, 103–112. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Polidori, D.; Sha, S.; Mudaliar, S.; Ciaraldi, T.P.; Ghosh, A.; Vaccaro, N.; Farrell, K.; Rothenberg, P.; Henry, R.R. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: Results of a randomized, placebo-controlled study. Diabetes Care 2013, 36, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Farmer, A.J.; Davies, M.J.; Levy, J.C.; Darbyshire, J.L.; Keenan, J.F.; Paul, S.K. Three-year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 2009, 361, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Komiya, N.; Hirose, H. Effect of valsartan, an angiotensin II receptor blocker, on markers of oxidation and glycation in Japanese type 2 diabetic subjects: Blood pressure-independent effect of valsartan. Diabetes Res. Clin. Pract. 2006, 74, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Mori, T.; Nako, K.; Kato, T.; Takeuchi, K.; Ito, S. Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension 2006, 47, 699–705. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Cipollone, F.; Filardo, F.P.; Zimarino, M.; Bernini, W.; Lazzerini, G.; Bucciarelli, T.; Falco, A.; Marchesani, P.; Muraro, R.; et al. Low-density lipoprotein level reduction by the 3-hydroxy-3-methylglutaryl coenzyme-A inhibitor simvastatin is accompanied by a related reduction of F2-isoprostane formation in hypercholesterolemic subjects: No further effect of vitamin E. Circulation 2002, 106, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Loffredo, L.; Carnevale, R.; Perri, L.; Patrizi, M.P.; Sanguigni, V.; Pignatelli, P.; Violi, F. Early decrease of oxidative stress by atorvastatin in hypercholesterolaemic patients: Effect on circulating vitamin E. Eur. Heart J. 2008, 29, 54–62. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saisho, Y. Glycemic Variability and Oxidative Stress: A Link between Diabetes and Cardiovascular Disease? Int. J. Mol. Sci. 2014, 15, 18381-18406. https://doi.org/10.3390/ijms151018381
Saisho Y. Glycemic Variability and Oxidative Stress: A Link between Diabetes and Cardiovascular Disease? International Journal of Molecular Sciences. 2014; 15(10):18381-18406. https://doi.org/10.3390/ijms151018381
Chicago/Turabian StyleSaisho, Yoshifumi. 2014. "Glycemic Variability and Oxidative Stress: A Link between Diabetes and Cardiovascular Disease?" International Journal of Molecular Sciences 15, no. 10: 18381-18406. https://doi.org/10.3390/ijms151018381
APA StyleSaisho, Y. (2014). Glycemic Variability and Oxidative Stress: A Link between Diabetes and Cardiovascular Disease? International Journal of Molecular Sciences, 15(10), 18381-18406. https://doi.org/10.3390/ijms151018381