Sildenafil Attenuates Inflammation and Oxidative Stress in Pelvic Ganglia Neurons after Bilateral Cavernosal Nerve Damage
Abstract
:1. Introduction
2. Results
2.1. Bilateral Cavernosal Nerve Resection (BCNR) and Sildenafil Treatment Promotes Changes in the Gene Expression of Pro-Inflammatory and Anti-Inflammatory Cytokines
| Symbol | Description | Sham vs. BCNR | BCNR vs. BCNR + SIL |
|---|---|---|---|
| CD40 | CD40 molecule, TNF receptor superfamily member 5 | 3.68 | −1.41 |
| GMFγ | Glia maturation factor, γ | 2.44 | −1.16 |
| IL-10 | Interleukin 10 | 2.18 | 2.08 |
| IL10Rα | Interleukin 10 receptor, α | 4.03 | −1.87 |
| IL-1β | Interleukin 1 β | 5.81 | −1.18 |
| IL-6 | Interleukin 6 | 3.06 | 2.14 |
| TGFβ1 | Transforming growth factor, β 1 | 2.86 | −1.35 |
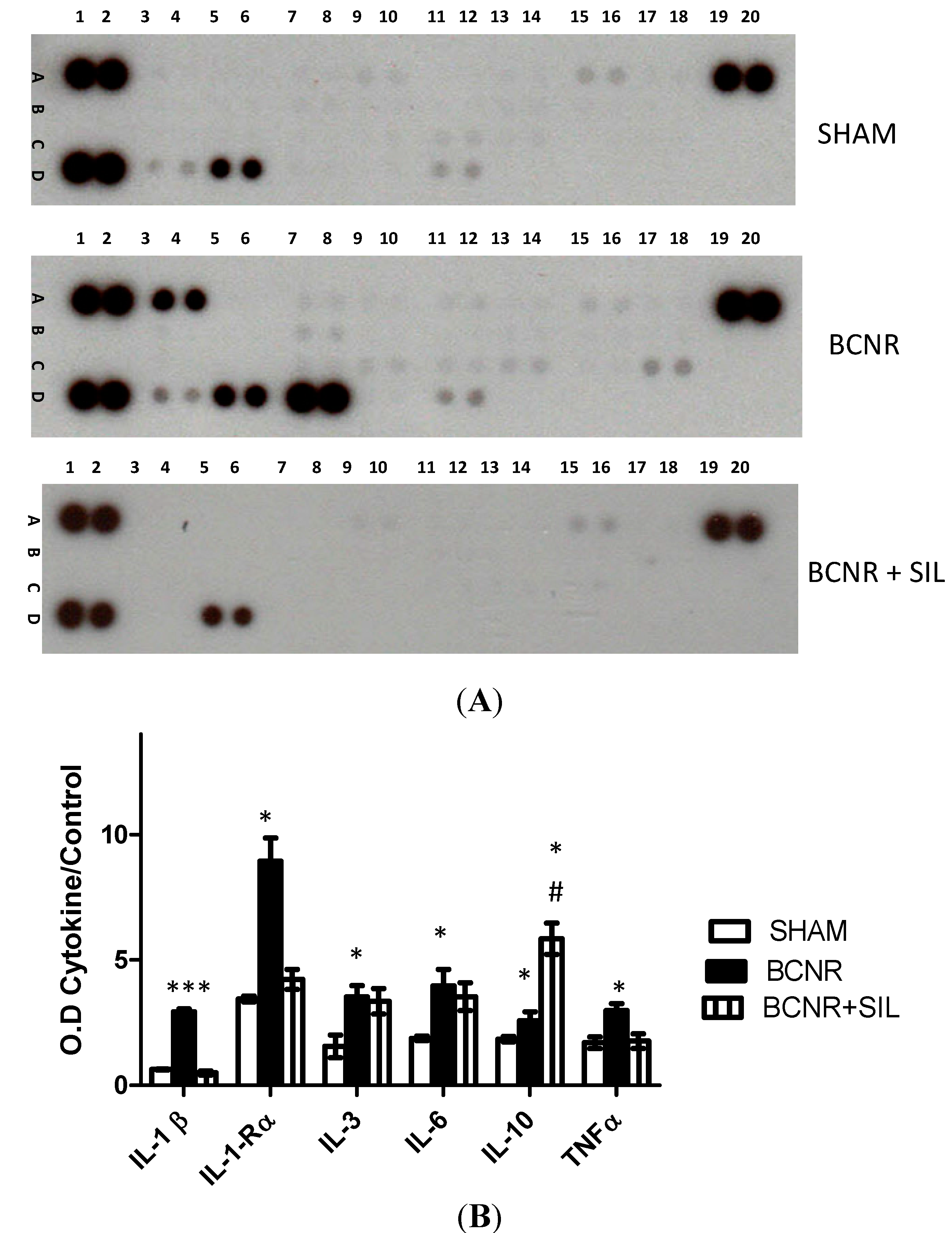


2.2. Changes in Oxidative Stress Markers after BCNR and Sildenafil (SIL) Treatment
| Symbol | Description | Sham vs. BCNR | BCNR vs. BCNR + SIL |
|---|---|---|---|
| Aass | Aminoadipate-semialdehyde synthase | −1.39 | −3.19 |
| Fos | FBJ osteosarcoma oncogene | 2.22 | −1.08 |
| Gpx1 | Glutathione peroxidase 1 | 1.89 | −1.83 |
| Gpx7 | Glutathione peroxidase 7 | 2.08 | −1.71 |
| Mpo | Myeloperoxidase | 1.16 | −4.21 |
| Ncf2 | NADPH oxidase activator 2 | 2.22 | −1.08 |
| Nos2 | Nitric oxide synthase 2, inducible | 2.28 | −1.96 |
| Nos3 | Nitric oxide synthase 3, endothelial cell | 1.25 | −1.05 |
| Noxa1 | NADPH oxidase activator 1 | 3.70 | −4.62 |
| p22-phox | Cytochrome b-245, alpha polypeptide | 3.45 | −2.26 |
| Prdx6 | Peroxiredoxin 6 | 1.54 | −1.92 |
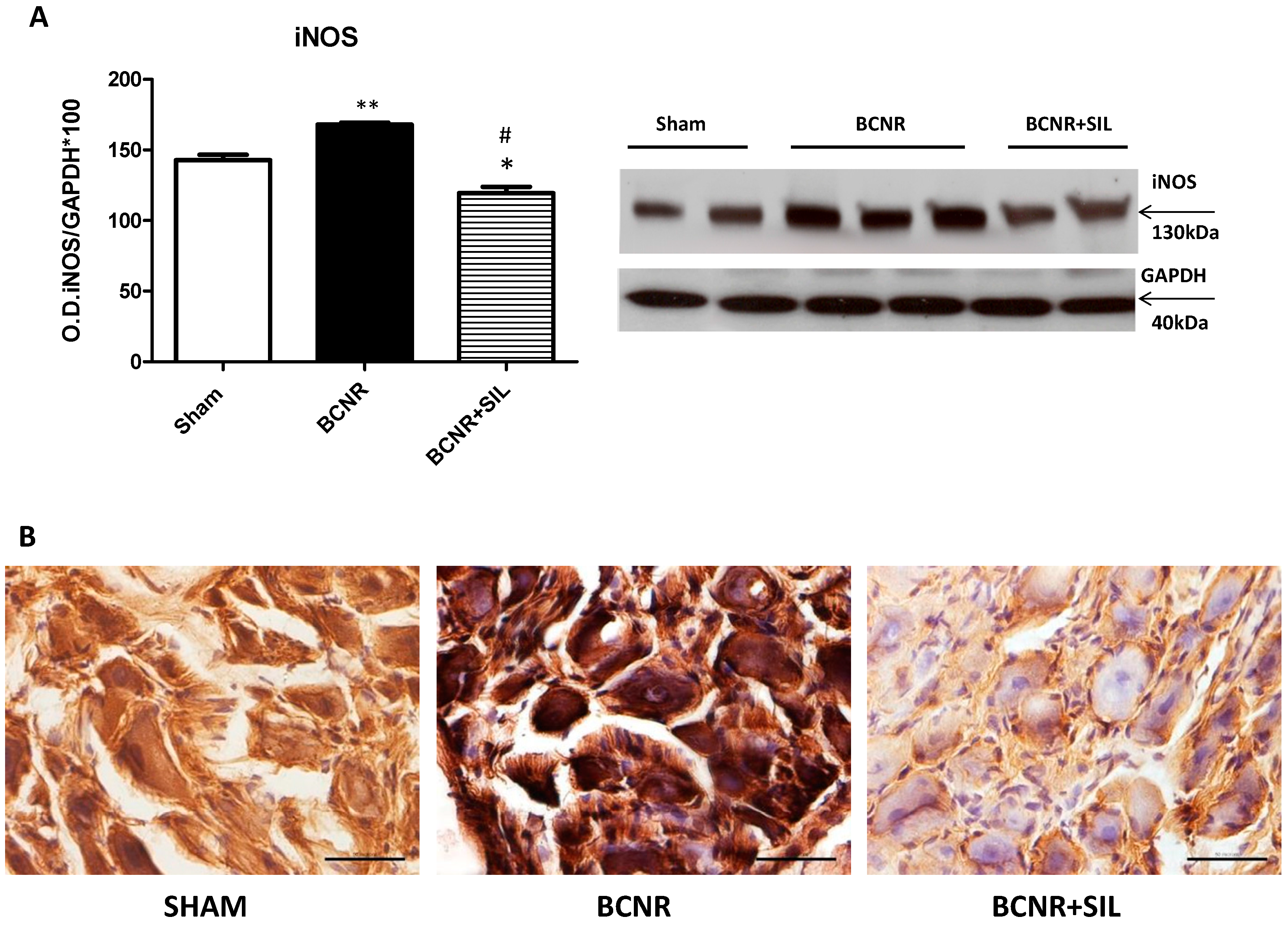
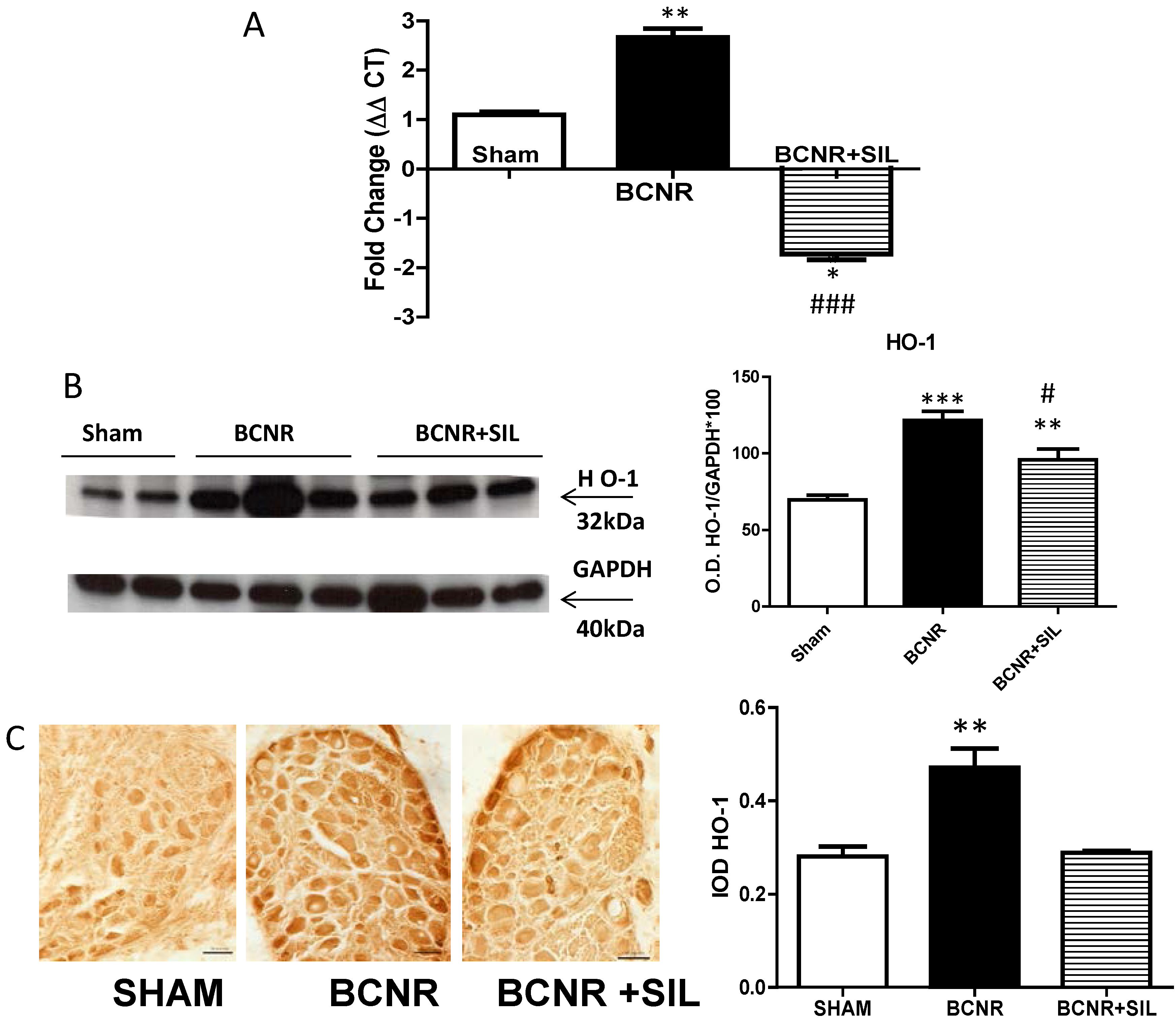
2.3. Expression of Heme Oxygenase 1 (HO-1) Parallels Inducible Nitric Oxide Synthase (iNOS) after BCNR and SIL Treatment
3. Discussion
4. Experimental Section
4.1. Animals Treatment
4.2. RT2 Profiler™ PCR Array Analysis of Cytokines and Nitric Oxide Related Target Genes
4.3. Heme Oxygenase Determination by TaqMan® Real Time PCR
4.4. Proteome Array for Determination of Cytokines and Chemokines
4.5. Western Blot Analysis
4.6. Retrograde Labeling of Pelvic Ganglion Cells
4.7. Immunohistochemistry and Immunofluorescence
4.8. Co-Localization of Fluorogold Positive Neurons with IL-1β and IL-6 by Immunofluorescence
4.9. Quantitative Image Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Authors Contributions
Abbreviations
| SIL | Sildenafil |
| BCNR | bilateral cavernosal nerve resection |
| iNOS | inducible nitric oxide synthase |
| HO-1 | Heme oxygenase 1 |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| RPlP1,3 | Ribosomal protein, large 1/3 |
| GAPDH | glyceraldehyde-3-phosphate-dehydrogenase |
| IMM | immunohistochemistry |
Conflicts of Interest
References
- Tal, R.; Alphs, H.H.; Krebs, P.; Nelson, C.J.; Mulhall, J.P. Erectile function recovery rate after radica lprostatectomy: A meta-analysis. J. Sex. Med. 2009, 6, 2538–2546. [Google Scholar]
- Walsh, P.C.; Donker, P.J. Impotence following radical prostatectomy: Insight into etiology and prevention. J. Urol. 1982, 128, 492–497. [Google Scholar]
- Ozkara, H.; Alan, C.; Atukeren, P.; Uyaner, I.; Demirci, C.; Gümüştaş, M.K.; Alici, B. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manipulation in rat penis. Chin. J. Physiol. 2006, 49, 160–166. [Google Scholar]
- Ferrini, M.G.; Kovanecz, I.; Sanchez, S.; Umeh, C.; Rajfer, J.; Gonzalez-Cadavid, N.F. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J. Sex. Med. 2009, 6, 415–428. [Google Scholar]
- Mulhall, J.P. Penile rehabilitation following radical prostatectomy. Curr. Opin. Urol. 2008, 18, 613–620. [Google Scholar]
- Jung, G.W.; Kwak, J.Y.; Yoon, S.; Yoon, J.H.; Lue, T.F. IGF-I and TGF-β2 have a key role on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in rats. Int. J. Impot. Res. 1999, 11, 247–259. [Google Scholar]
- Lagoda, G.; Jin, L.; Lehrfeld, T.J.; Liu, T.; Burnett, A.L. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J. Sex. Med. 2007, 4, 908–916. [Google Scholar]
- Lagoda, G.; Xie, Y.; Sezen, S.F.; Hurt, K.J.; Liu, L.; Musicki, B.; Burnett, A.L. FK506 neuroprotection after cavernous nerve injury is mediated by thioredoxin and glutathione redox systems. J. Sex. Med. 2011, 8, 3325–3334. [Google Scholar]
- Navarro, X.; Vivó, M.; Valero-Cabré, A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007, 82, 163–201. [Google Scholar]
- Gonzalez-Perez, F.; Udina, E.; Navarro, X. Extracellular matrix components in peripheral nerve regeneration. Int. Rev. Neurobiol. 2013, 108, 257–275. [Google Scholar]
- Bitterman, H.; Kinarty, A.; Lazarovich, H.; Lahat, N. Acute release of cytokines is proportional to tissue injury induced by surgical trauma and shock in rats. J. Clin. Immunol. 1991, 11, 184–192. [Google Scholar]
- Fracalanza, S.; Ficarra, V.; Cavalleri, S.; Galfano, A.; Novara, G.; Mangano, A.; Plebani, M.; Artibani, W. Is robotically assisted laparoscopic radical prostatectomy less invasive than retropubic radical prostatectomy? Results from a prospective, unrandomized, comparative study. BJU Int. 2008, 101, 1145–1149. [Google Scholar]
- Narita, S.; Tsuchiya, N.; Kumazawa, T.; Maita, S.; Numakura, K.; Obara, T.; Tsuruta, H.; Saito, M.; Inoue, T.; Horikawa, Y.; et al. Comparison of surgical stress in patients undergoing open versus laparoscopic radical prostatectomy by measuring perioperative serum cytokine levels. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 33–37. [Google Scholar]
- Gadient, R.A.; Otten, U.H. Interleukin-6 (IL-6)—A molecule with both beneficial and destructive potentials. Prog. Neurobiol. 1997, 52, 379–390. [Google Scholar]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar]
- Chéret, C.; Gervais, A.; Lelli, A.; Colin, C.; Amar, L.; Ravassard, P.; Mallet, J.; Cumano, A.; Krause, K.H.; Mallat, M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J. Neurosci. 2008, 28, 12039–12051. [Google Scholar]
- Iadecola, C.; Zhang, F.; Casey, R.; Nagayama, M.; Ross, M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997, 17, 9157–9164. [Google Scholar]
- Rubbo, H.; Raddi, R. Protein and lipid nitration: Role in redox signaling and injury. Biochim. Biophys. Acta 2008, 80, 1318–1324. [Google Scholar]
- Ferrini, M.; Wang, C.; Swerdloff, R.S.; Sinha-Hikim, A.P.; Rajfer, J.; Gonzalez-Cadavid, N.F. Aging-related increased expression of inducible nitric oxide synthase and cytotoxicity markers in rat hypothalamic regions associated with male reproductive function. Neuroendocrinology 2001, 74, 1–11. [Google Scholar]
- Iadecola, C.; Xu, X.; Zhang, F.; El-Fakahany, E.E.; Ross, M.E. Marked induction of calcium-independent nitric oxide synthase activity after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1995, 15, 52–59. [Google Scholar]
- Iadecola, C.; Zhang, F.; Xu, X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am. J. Physiol. 1995, 268, R286–R292. [Google Scholar]
- Xu, J.; Kim, G.M.; Chen, S.; Yan, P.; Ahmed, S.H.; Ku, G.; Beckman, J.S.; Xu, X.M.; Hsu, C.Y. iNOS nitrotyrosine expression after spinal cord injury. J. Neurotrauma 2001, 18, 523–532. [Google Scholar]
- Gonzalez-Cadavid, N.F.; Rajfer, J. The pleiotropic effects of inducible nitric oxide synthase (iNOS) on the physiology and pathology of penile erection. Curr. Pharm. Des. 2005, 11, 4041–4046. [Google Scholar]
- Sirad, F.; Hlaing, S.; Kovanecz, I.; Artaza, J.N.; Garcia, L.A.; Rajfer, J.; Ferrini, M.G. Sildenafil promotes smooth muscle preservation and ameliorates fibrosis through modulation of extracellular matrix and tissue growth factor gene expression after bilateral cavernosal nerve resection in the rat. J. Sex. Med. 2011, 8, 1048–1060. [Google Scholar]
- Hlaing, S.M.; Garcia, L.A.; Kovanecz, I.; Martinez, R.A.; Shah, S.; Artaza, J.N.; Ferrini, M.G. Sildenafil promotes neuroprotection of the pelvic ganglia neurons after bilateral cavernosal nerve resection in the rat. BJU Int. 2013, 111, 159–170. [Google Scholar]
- Jeong, K.H.; Lee, T.W.; Ihm, C.G.; Lee, S.H.; Moon, J.Y.; Lim, S.J. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am. J. Nephrol. 2009, 29, 274–282. [Google Scholar]
- Küçük, A.; Yucel, M.; Erkasap, N.; Tosun, M.; Koken, T.; Ozkurt, M.; Erkasap, S. The effects of PDE5 inhibitory drugs on renal ischemia/reperfusion injury in rats. Mol. Biol. Rep. 2012, 39, 9775–9782. [Google Scholar]
- Dubový, P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann. Anat. 2011, 193, 267–275. [Google Scholar]
- Chertoff, M.; di Paolo, N.; Schoeneberg, A.; Depino, A.; Ferrari, C.; Wurst, W.; Pfizenmaier, K.; Eisel, U.; Pitossi, F. Neuroprotective and neurodegenerative effects of the chronic expression of tumor necrosis factor α in the nigrostriatal dopaminergic circuit of adult mice. Exp. Neurol. 2011, 227, 237–251. [Google Scholar]
- Cámara-Lemarroy, C.R.; Guzmán-de la Garza, F.J.; Fernández-Garza, N.E. Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation 2010, 17, 314–324. [Google Scholar]
- Dubový, P.; Jančálek, R.; Kubek, T. Role of inflammation and cytokines in peripheral nerve regeneration. Int. Rev. Neurobiol. 2013, 108, 173–206. [Google Scholar]
- Reichert, F.; Levitzky, R.; Rotshenker, S. Interleukin 6 in intact and injured mouse peripheral nerves. Eur. J. Neurosci. 1996, 8, 530–535. [Google Scholar]
- Santos, C.L.; Souza, M.H.L.P.; Gomes, A.S.; Lemos, H.P.; Santos, A.A.; Cunha, F.Q.; Wallace, J.L. Sildenafil prevents indomethacin-induced gastropathy in rats: Role of leukocyte adherence and gastric blood flow. Br. J. Pharmacol. 2005, 146, 481–486. [Google Scholar]
- Yildirim, A.; Ersoy, Y.; Ercan, F.; Atukeren, P.; Gumustas, K.; Uslu, U.; Alican, I. Phosphodiesterase-5 inhibition by sildenafil citrate in a rat model of bleomycin-induced lung fibrosis. Pulm. Pharmacol. Ther. 2010, 23, 215–221. [Google Scholar]
- Kim, D.; You, B.; Jo, E.K.; Han, S.K.; Simon, M.I.; Lee, S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14851–14856. [Google Scholar]
- Babalola, O.; Mamalis, A.; Lev-Tov, H.; Jagdeo, J. NADPH oxidase enzymes in skin fibrosis: Molecular targets and therapeutic agents. Arch. Dermatol. Res. 2014, 306, 313–330. [Google Scholar]
- Luo, L.; Dai, D.Z.; Cheng, Y.S.; Zhang, Q.; Yuan, W.J.; Dai, Y. Sildenafil improves diabetic vascular activity through suppressing endothelin receptor A, iNOS and NADPH oxidase which is comparable with the endothelin receptor antagonist CPU0213 in STZ-injected rats. J. Pharm. Pharmacol. 2011, 63, 943–951. [Google Scholar]
- Arruda, M.A.; Barcellos-de-Souza, P.; Sampaio, A.L.F.; Rossi, A.G.; Graça-Souza, A.V.; Barja-Fidalgo, C. NADPH oxidase-derived ROS: Key modulators of heme-induced mitochondrial stability in human neutrophils. Exp. Cell Res. 2006, 312, 3939–3948. [Google Scholar]
- Wu, C.C.; Wu, Y.N.; Ho, H.O.; Chen, K.C.; Sheu, M.T.; Chiang, H.S. The neuroprotective effect of platelet-rich plasma on erectile function in bilateral cavernous nerve injury rat model. J. Sex. Med. 2012, 9, 2838–2848. [Google Scholar]
- Wu, Y.N.; Wu, C.C.; Sheu, M.T.; Chen, K.C.; Ho, H.O.; Chiang, H.S. Optimization of platelet-rich plasma and its effects on the recovery of erectile function after bilateral cavernous nerve injury in a rat model. J. Tissue Eng. Regen. Med. 2013. [Google Scholar] [CrossRef]
- Ferrini, M.G.; Davila, H.H.; Kovanecz, I.; Sanchez, S.P.; Gonzalez-Cadavid, N.F.; Rajfer, J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology 2006, 68, 429–435. [Google Scholar]
- Kovanecz, I.; Rambhatla, A.; Ferrini, M.G.; Vernet, D.; Sanchez, S.; Rajfer, J.; Gonzalez-Cadavid, N. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008, 101, 203–210. [Google Scholar]
- Ferrini, M.G.; Kovanecz, I.; Sanchez, S.; Vernet, D.; Davila, H.H.; Rajfer, J.; Gonzalez-Cadavid, N.F. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol. Reprod. 2007, 76, 915–923. [Google Scholar]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar]
- Garcia, L.A.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C2C12 skeletal muscle cells. J. Steroid. Biochem. Mol. Biol. 2013, 133, 1–11. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, L.A.; Hlaing, S.M.; Gutierrez, R.A.; Sanchez, M.D.; Kovanecz, I.; Artaza, J.N.; Ferrini, M.G. Sildenafil Attenuates Inflammation and Oxidative Stress in Pelvic Ganglia Neurons after Bilateral Cavernosal Nerve Damage. Int. J. Mol. Sci. 2014, 15, 17204-17220. https://doi.org/10.3390/ijms151017204
Garcia LA, Hlaing SM, Gutierrez RA, Sanchez MD, Kovanecz I, Artaza JN, Ferrini MG. Sildenafil Attenuates Inflammation and Oxidative Stress in Pelvic Ganglia Neurons after Bilateral Cavernosal Nerve Damage. International Journal of Molecular Sciences. 2014; 15(10):17204-17220. https://doi.org/10.3390/ijms151017204
Chicago/Turabian StyleGarcia, Leah A., Su M. Hlaing, Richard A. Gutierrez, Maria D. Sanchez, Istvan Kovanecz, Jorge N. Artaza, and Monica G. Ferrini. 2014. "Sildenafil Attenuates Inflammation and Oxidative Stress in Pelvic Ganglia Neurons after Bilateral Cavernosal Nerve Damage" International Journal of Molecular Sciences 15, no. 10: 17204-17220. https://doi.org/10.3390/ijms151017204
APA StyleGarcia, L. A., Hlaing, S. M., Gutierrez, R. A., Sanchez, M. D., Kovanecz, I., Artaza, J. N., & Ferrini, M. G. (2014). Sildenafil Attenuates Inflammation and Oxidative Stress in Pelvic Ganglia Neurons after Bilateral Cavernosal Nerve Damage. International Journal of Molecular Sciences, 15(10), 17204-17220. https://doi.org/10.3390/ijms151017204





