Fluvastatin Influences Hair Color in C57Bl/6 Mice
Abstract
:1. Introduction
2. Results and Discussion
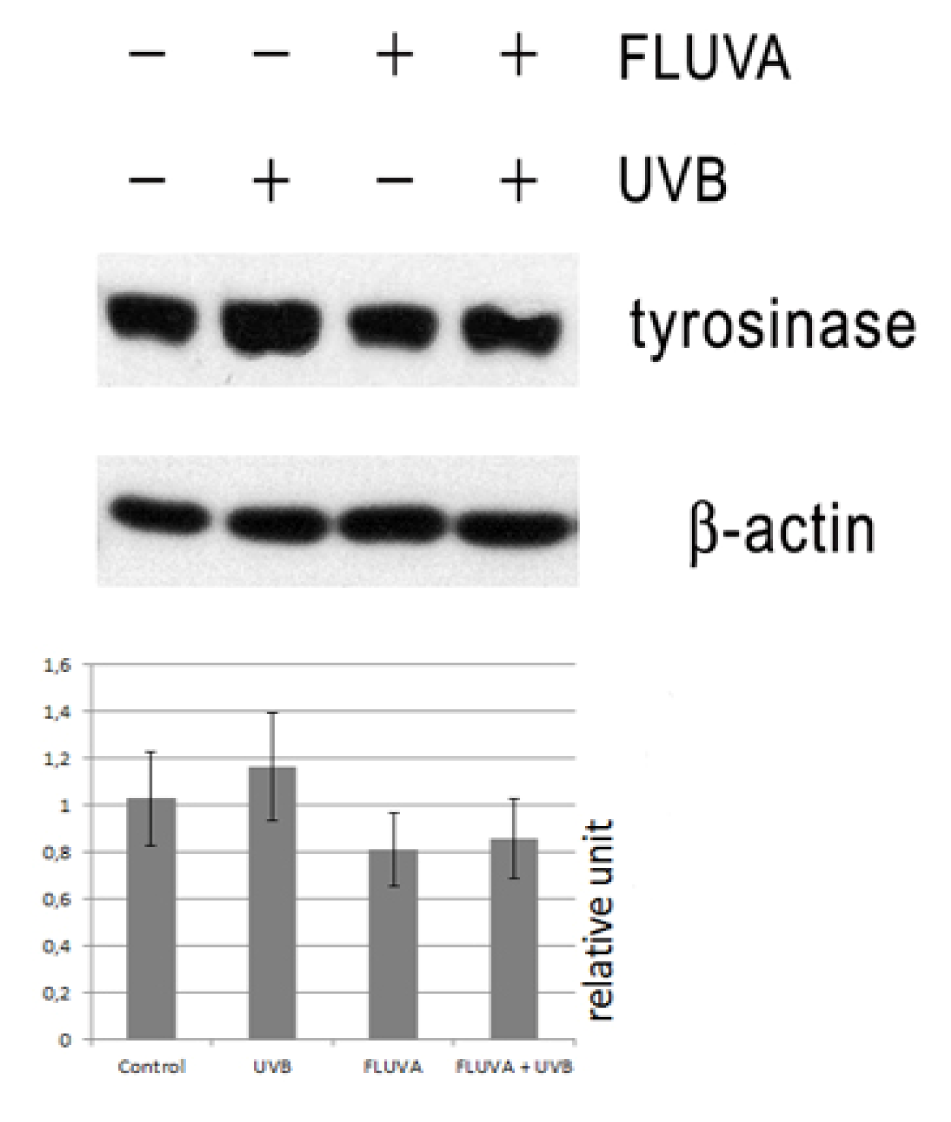
2.1. Western Blot
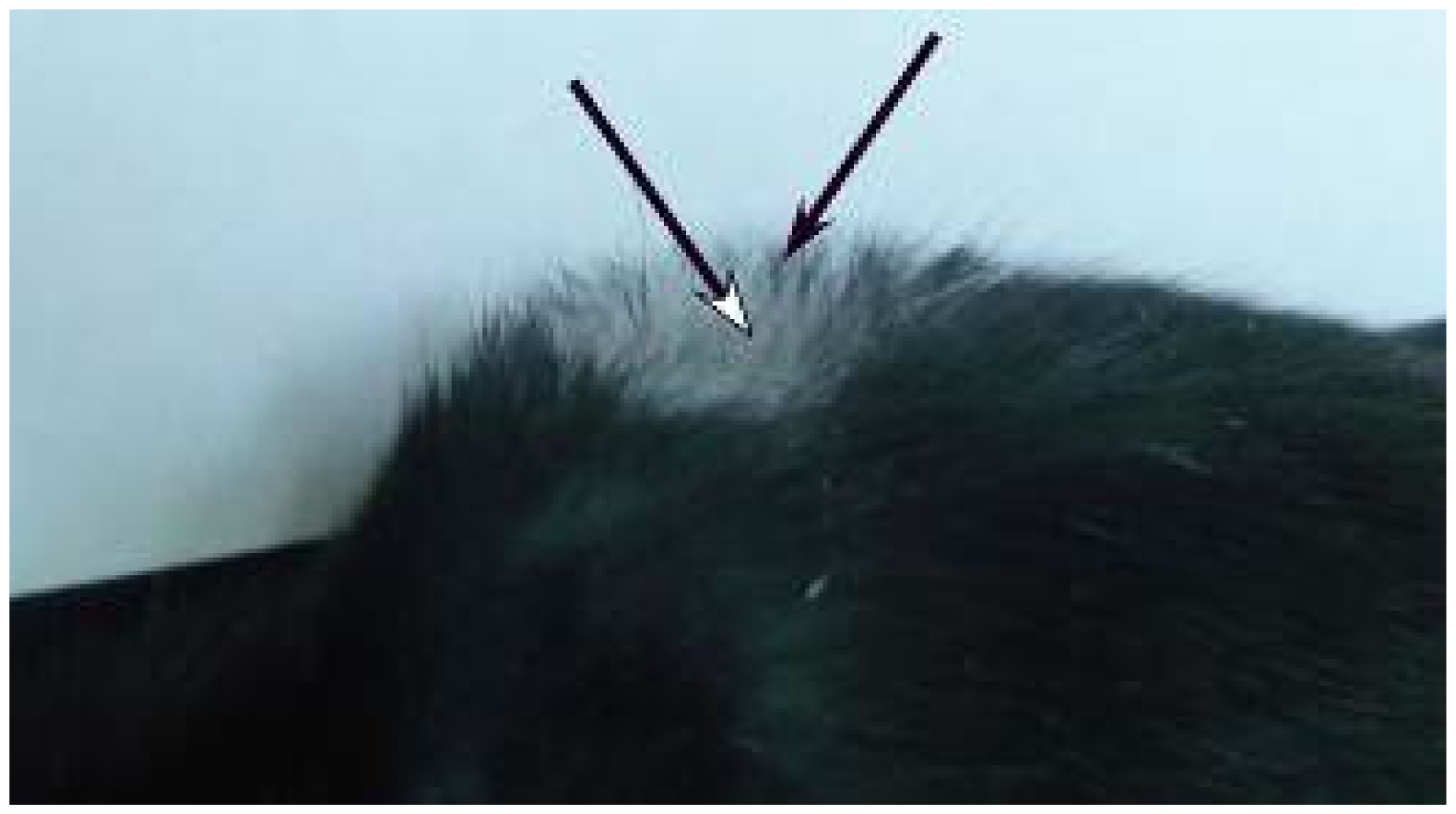
2.2. Macroscopic Examination of Skin and Hair Color
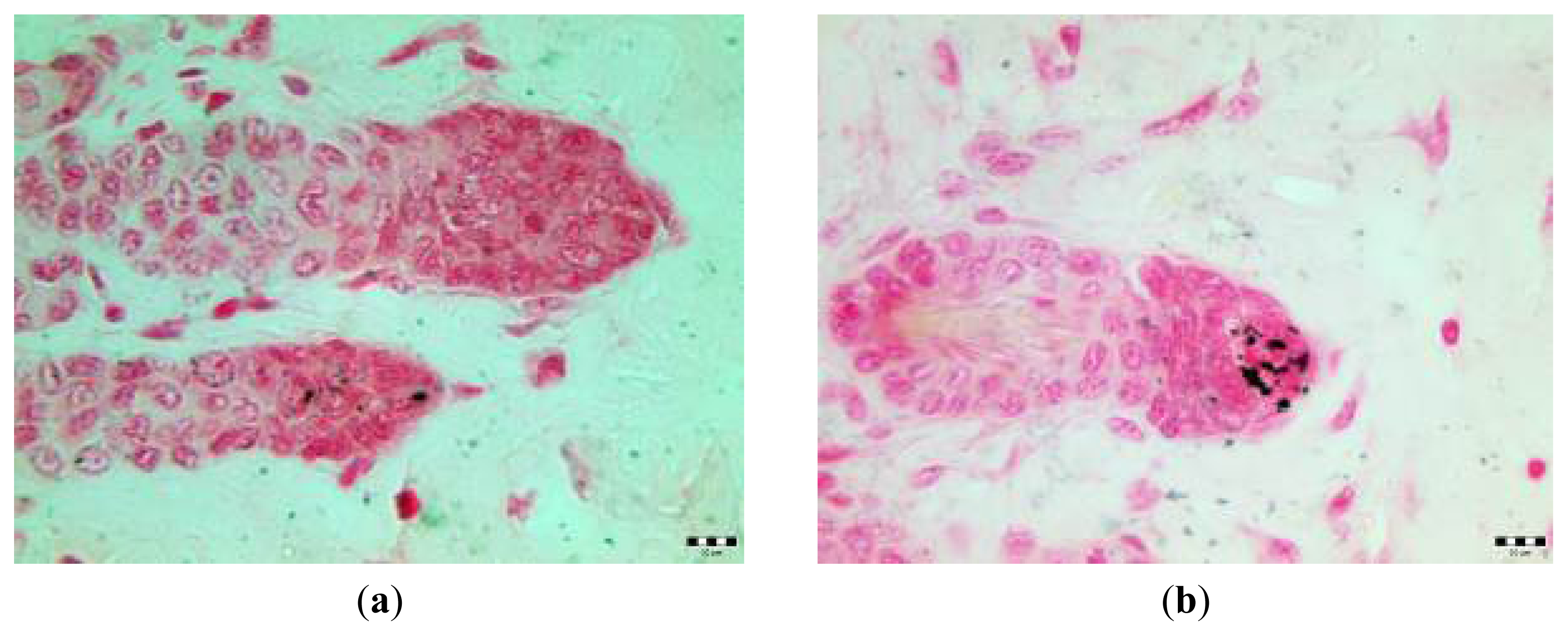
2.3. Microscopic Examination
3. Experimental Section
3.1. Experiments in Vitro
3.1.1. Cell Culture, Irradiation Procedure
3.1.2. Western Blot Analysis
3.2. Experiments in Vivo
3.2.1. Animals, Anagen Induction (AI), Fluvastatin Gel Injections, and Tissue Collection
3.2.2. Histology
3.2.3. Statistical Analysis
4. Conclusions
Conflict of Interest
Abbreviations
| AI | anagen induction |
| HF | hair follicle |
| UVB | ultraviolet B |
References
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Wang, G.J.; Chung, K.C.; Shen, W. Lipid clearing agents in steroid-induced osteoporosis. J. Formos. Med. Assoc 1995, 94, 589–592. [Google Scholar]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar]
- Galus, R.; Wlodarski, P.K.; Wlodarski, K.H. Fluvastatin increases heterotopically induced ossicles in mice. Clin. Exp. Pharmacol. Physiol 2006, 33, 388–390. [Google Scholar]
- Adánez, G.; Castells, M.T.; García Pérez, B.; Sánchez-Polo, M.T.; Martín Castillo, A.; Montes, A.; Ayala, I. Effects of atorvastatin on progression-regression of renal injury in hyperlipidemic chickens. Histol. Histopathol 2008, 23, 1131–1142. [Google Scholar]
- Lin, S.J.; Hsieh, F.Y.; Chen, Y.H.; Lin, C.C.; Kuan, I.I.; Wang, S.H.; Wu, C.C.; Chien, H.F.; Lin, F.Y.; Chen, Y.L. Atorvastatin induces thrombomodulin expression in the aorta of cholesterol-fed rabbits and in TNFalpha-treated human aortic endothelial cells. Histol. Histopathol 2009, 24, 1147–1159. [Google Scholar]
- Russo, E.; Donato di Paola, E.; Gareri, P.; Siniscalchi, A.; Labate, A.; Gallelli, L.; Citraro, R.; de Sarro, G. Pharmacodynamic potentiation of antiepileptic drugs’ effects by some HMG-CoA reductase inhibitors against audiogenic seizures in DBA/2 mice. Pharmacol. Res 2013, 70, 1–12. [Google Scholar]
- Pelaia, G.; Gallelli, L.; Renda, T.; Fratto, D.; Falcone, D.; Caraglia, M.; Busceti, M.T.; Terracciano, R.; Vatrella, A.; Maselli, R.; et al. Effects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non-small lung cancer cells. Cell Prolif 2012, 45, 557–565. [Google Scholar]
- Falcone, D.; Gallelli, L.; Di Virgilio, A.; Tucci, L.; Scaramuzzino, M.; Terracciano, R.; Pelaia, G.; Savino, R. Effects of simvastatin and rosuvastatin on RAS protein, matrix metalloproteinases and NF-κB in lung cancer and in normal pulmonary tissues. Cell Prolif 2013, 46, 172–182. [Google Scholar]
- Namazi, M.R. Statins: Novel additions to the dermatologic arsenal? Exp. Dermatol 2004, 13, 337–339. [Google Scholar]
- Galus, R.; Sajjad, E.; Niderla, J.; Borowska, K.; Włodarski, K.; Włodarski, P.; JóŸwiak, J. Fluvastatin increases tyrosinase synthesis induced by UVB irradiation of B16F10 melanoma cells. Folia Histochem. Cytobiol 2009, 47, 363–365. [Google Scholar]
- Galus, R.; Niderla, J.; Sladowski, D.; Sajjad, E.; Włodarski, K.; JóŸwiak, J. Fluvastatin increases tyrosinase synthesis induced by alpha-melanocyte-stimulating hormone in B16F10 melanoma cells. Pharmacol. Rep 2010, 62, 164–169. [Google Scholar]
- Suzuki, S.; Tajima, T.; Sassa, S.; Kudo, H.; Okayasu, I.; Sakamoto, S. Preventive effect of fluvastatin on ulcerative colitis-associated carcinogenesis in mice. Anticancer Res 2006, 26, 4223–4228. [Google Scholar]
- Konturek, P.C.; Burnat, G.; Hahn, E.G. Inhibition of Barret’s adenocarcinoma cell growth by simvastatin: involvement of COX-2 and apoptosis-related proteins. J. Physiol. Pharmacol 2007, 58, 141–148. [Google Scholar]
- Garwood, E.R.; Kumar, A.S.; Baehner, F.L.; Moore, D.H.; Au, A.; Hylton, N.; Flowers, C.I.; Garber, J.; Lesnikoski, B.A.; Hwang, E.S.; et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res. Treat 2010, 119, 137–144. [Google Scholar]
- Rodríguez-Vita, J.; Sánchez-Galán, E.; Santamaría, B.; Sánchez-López, E.; Rodrigues-Díez, R.; Blanco-Colio, L.M.; Egido, J.; Ortiz, A.; Ruiz-Ortega, M. Essential role of TGF-beta/Smad pathway on statin dependent vascular smooth muscle cell regulation. PLoS One 2008, 3, e3959. [Google Scholar]
- Rodrigues Díez, R.; Rodrigues-Díez, R.; Lavoz, C.; Rayego-Mateos, S.; Civantos, E.; Rodríguez-Vita, J.; Mezzano, S.; Ortiz, A.; Egido, J.; Ruiz-Ortega, M. Statins inhibit angiotensin II/Smad pathway and related vascular fibrosis, by a TGF-β-independent process. PLoS One 2010, 5, e14145. [Google Scholar]
- Burke, J.P.; Watson, R.W.; Murphy, M.; Docherty, N.G.; Coffey, J.C.; O’Connell, P.R. Simvastatin impairs smad-3 phosphorylation and modulates transforming growth factor beta1-mediated activation of intestinal fibroblasts. Br. J. Surg 2009, 96, 541–551. [Google Scholar]
- Martínez-Esparza, M.; Jiménez-Cervantes, C.; Beermann, F.; Aparicio, P.; Lozano, J.A.; García-Borrón, J.C. Transforming growth factor-beta1 inhibits basal melanogenesis in B16/F10 mouse melanoma cells by increasing the rate of degradation of tyrosinase and tyrosinase-related protein-1. J. Biol. Chem 1997, 272, 3967–3972. [Google Scholar]
- Dalla, Y.; Singh, N.; Jaggi, A.S.; Singh, D. Memory restorative role of statins in experimental dementia: an evidence of their cholesterol dependent and independent actions. Pharmacol. Rep 2010, 62, 784–796. [Google Scholar]
- Jin, K.M.; Kim, Y.H. Injectable, thermo-reversible and complex coacervate combination gels for protein drug delivery. J. Control. Release 2008, 127, 249–256. [Google Scholar]
- Paus, R.; Müller-Röver, S.; van Der Veen, C.; Maurer, M.; Eichmüller, S.; Ling, G.; Hofmann, U.; Foitzik, K.; Mecklenburg, L.; Handjiski, B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol 1999, 113, 523–532. [Google Scholar]
- Slominski, A.; Paus, R.; Costantino, R. Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J. Invest. Dermatol 1991, 96, 172–179. [Google Scholar]
- Paus, R.; van der Veen, C.; Eichmüller, S.; Kopp, T.; Hagen, E.; Müller-Röver, S.; Hofmann, U. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Invest. Dermatol 1998, 111, 7–18. [Google Scholar]
- Toker, S.; Gulcan, E.; Cayc, M.K.; Olgun, E.G.; Erbilen, E.; Ozay, Y. Topical atorvastatin in the treatment of diabetic wounds. Am. J. Med. Sci 2009, 338, 201–204. [Google Scholar]
- Roméro, C.; Aberdam, E.; Larnier, C.; Ortonne, J.P. Retinoic acid as modulator of UVB-induced melanocyte differentiation. Involvement of the melanogenic enzymes expression. J. Cell Sci 1994, 107, 1095–1103. [Google Scholar]
- Paus, R.; Stenn, K.S.; Link, R.E. Telogen skin contains an inhibitor of hair growth. Br. J. Dermatol 1990, 122, 777–784. [Google Scholar]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol 2001, 117, 3–15. [Google Scholar]
- Thylin, M.R.; McConnell, J.C.; Schmid, M.J.; Reckling, R.R.; Ojha, J.; Bhattacharyya, I.; Marx, D.B.; Reinhardt, R.A. Effects of simvastatin gels on murine calvarial bone. J. Periodontol 2002, 73, 1141–1148. [Google Scholar]



| Gender | Frequency of depigmentation % (number of mice with depigmentation after injection of fluva-gel/total number of mice after injection of respective fluva-gel) | Frequency of depigmentation % (number of mice with depigmentation after injection of placebo-gel/total number of mice after injection of placebo-gel) |
|---|---|---|
| Anagen induction in seven-week old mice—group 1 | ||
| fluva-gel 1 | placebo-gel (control 1) | |
| F | 25 (1/4) | 0 (0/3) |
| M | 50 (2/4) | 0 (0/3) |
| Anagen induction in four-week old mice—group 2 | ||
| fluva-gel 1 | placebo-gel (control 2) | |
| F | 60 (3/5) | 0 (0/3) |
| M | 82 (9/11) | 0 (0/7) |
| Anagen induction in four-week old mice—group 3 | ||
| fluva-gel 2 | ||
| F | 25 (3/12) | |
| M | 20 (2/10) | |
| Anagen induction in four-week old mice—group 4 | ||
| fluva-gel 3 | ||
| F | 0 (0/8) | |
| M | 0 (0/8) | |
| Mice without anagen induction: | ||
| Four-week old mice in first postnatal anagen—group 5 | ||
| fluva-gel 1 | placebo-gel (control 3) | |
| F | 0 (0/2) | 0 (0/1) |
| M | 37,5 (3/8) | 0 (0/2) |
| Six-week old mice in catagen/telogen after first postnatal anagen—group 6 | ||
| fluva-gel 1 (control 4) | placebo-gel (control 5) | |
| M | 0 (0/4) | 0 (0/2) |
| Eight – week old mice in telogen after first postnatal anagen—group 7 | ||
| fluva-gel 1 (control 6) | placebo-gel (control 7) | |
| M | 0 (0/8) | 0 (0/5) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Galus, R.; Włodarski, K.; Malejczyk, J.; Jóźwiak, J. Fluvastatin Influences Hair Color in C57Bl/6 Mice. Int. J. Mol. Sci. 2013, 14, 14333-14345. https://doi.org/10.3390/ijms140714333
Galus R, Włodarski K, Malejczyk J, Jóźwiak J. Fluvastatin Influences Hair Color in C57Bl/6 Mice. International Journal of Molecular Sciences. 2013; 14(7):14333-14345. https://doi.org/10.3390/ijms140714333
Chicago/Turabian StyleGalus, Ryszard, Krzysztof Włodarski, Jacek Malejczyk, and Jarosław Jóźwiak. 2013. "Fluvastatin Influences Hair Color in C57Bl/6 Mice" International Journal of Molecular Sciences 14, no. 7: 14333-14345. https://doi.org/10.3390/ijms140714333
APA StyleGalus, R., Włodarski, K., Malejczyk, J., & Jóźwiak, J. (2013). Fluvastatin Influences Hair Color in C57Bl/6 Mice. International Journal of Molecular Sciences, 14(7), 14333-14345. https://doi.org/10.3390/ijms140714333





