Abstract
The TiO2-montmorillonite (TiO2-MMT) complex was prepared by blending TiO2 sol and MMT with certain ratio, and its properties as an enzyme immobilization support were investigated. The pristine MMT and TiO2-MMT calcined at 800 °C (TiO2-MMT800) were used for comparison to better understand the immobilization mechanism. The structures of the pristine MMT, TiO2-MMT, and TiO2-MMT800 were examined by HR-TEM, XRD and BET. SEM was employed to study different morphologies before and after laccase immobilization. Activity and kinetic parameters of the immobilized laccase were also determined. It was found that the TiO2 nanoparticles were successfully introduced into the MMT layer structure, and this intercalation enlarged the “d value” of two adjacent MMT layers and increased the surface area, while the calcination process led to a complete collapse of the MMT layers. SEM results showed that the clays were well coated with adsorbed enzymes. The study of laccase activity revealed that the optimum pH and temperature were pH = 3 and 60 °C, respectively. In addition, the storage stability for the immobilized laccase was satisfactory. The kinetic properties indicated that laccase immobilized on TiO2-MMT complexes had a good affinity to the substrate. It has been proved that TiO2-MMT complex is a good candidate for enzyme immobilization.
1. Introduction
Laccase (E.C.1.10.3.2) is a multicopper oxidase that catalyzes oxidation of various aromatic substrates with concomitant reduction of oxygen to water []. The low substrate specificity exhibited by laccase and its ability to oxidize many pollutants make it an ideal candidate for use in bioremediation [], oxygen cathode in biofuel cells [], biosensors [], biobleaching, etc. However, some drawbacks have limited the use of free enzymes, such as instability, unrecoverable, and no direct electron transfer between free enzyme and bare electrodes. The immobilization of enzymes to some functional supports can not only increase the operational stability and durability, but also can endow it with some new properties, which may have synergy effects on pollutants treatment. Therefore, many efforts have been devoted to enzyme immobilization. A variety of supports with different functionality, morphology, and physical properties have been reported for the immobilization of enzymes such as polymer nanofibers [,], hydrogels [], carbon nanotubes [] and nanoparticles [,]. To evaluate the suitability of a material for enzyme immobilization the following factors are suggested to be important considerations: surface area to volume ratio, enzyme loading, flow rate, mass transfer, ease of separation, as well as reactor design []. In addition, selecting a reasonable support which may significantly improve the stability of immobilized enzyme should take the following factors into consideration: prevention of intermolecular interactions, enzyme structural rigidification, prevention of enzyme subunits dissociation, generation of artificial environments around the enzyme, enzyme reactivation, modulation of enzyme selectivity or specificity and reduction of inhibition problems [].
Montmorillonite (MMT), consisting of a lamellar stack of crystalline, 1 nm thick aluminosilicate sheets, is one of the most commonly used enzyme supports. The properties of MMT can be tailored to meet different needs by simple methods such as acid activation, ion exchange, pillaring and intercalation with organic compounds []. Moreover, it has been realized that, by taking advantage of the high aspect ratio surface area of each layer within a multilayered structure, MMT may serve as a benign host to provide spacious sites for protein accommodation []. In recent years, MMT has been widely studied as a benign enzyme support. A. Naidja et al. [] chose MMT coated with varying levels of hydroxyaluminum as the support, and investigated the effects of coating on adsorption, immobilization and activity of tyrosinase. Sanjay Gopinath et al. [] studied the immobilization of three different kinds of enzymes including α-amylase, glucoamylase and invertase onto acid activated montmorillonite via two independent techniques—adsorption and grafting using glutaraldehyde as spacer, and proposed that the enzymes were situated at the periphery of the clay mineral particles whereas the side chains of different amino acid residues penetrated between the layers.
TiO2 has been widely used for the decomposition of a great variety of organic pollutants because of its particular advantages, such as high chemical stability, strong oxidizing power, low cost, nontoxicity, and so on. Especially in recent years, coupled photochemical and biochemical treatment has been highly focused on and proven to be more efficient for degradation of biorecalcitrant pollutants [–]. The objective of this study is to evaluate the immobilization ability of TiO2-MMT complexes by physical adsorption, which has been considered as a simple process since no toxic solvents are used []. To fully understand the effects of TiO2-MMT complexes on enzyme activities, we choose TiO2-MMT complexes calcined at 800 °C (identified as TiO2-MMT800) as well as pristine MMT for comparison. Due to the fact that the space between two adjacent layers will be broken by high temperature, the effect of interlayer could be ignored for TiO2-MMT800. The effects of pH, temperature and aging on the immobilized laccase were discussed. This kind of material could have great potential in pollution treatment.
2. Results and Discussion
2.1. The Structures of MMT and TiO2-MMT Complexes
The TEM images reveal the internal structures of the pristine and the modified MMT nanohybrids as presented in Figure 1. The layered crystallites of MMT aggregate in large sized particles are shown in Figure 1a. As can be seen in Figs. 1b and 1c, there are numerous well-distributed small particles within the MMT layers, indicating well intercalation of TiO2 particles into the interlayers of MMT. It also needs to be noted that the small particles that existed in TiO2-MMT (Figure 1c) are not as clear as those in and the presence of a wide range of hydrolyzed species of Ti, such as monomeric TiO2+/Ti(OH)22+ and polymeric species, in the TiO2 sol solution []. Figure 1d shows the EDX spectra of TiO2-MMT, indicating that the complex mainly consisted of Si, Al, Ti, Mg, Ca, Na, and further confirmed the presence of TiO2. Moreover, the inset image in Figure 1c clearly shows the lattice fringe of the TiO2 nanoparticles as well as the ordered MMT layers. In addition, the size of the TiO2 particles is less than 5 nm, because the dispersed layer silicates of MMT in this system behave as barriers to prevent TiO2 from agglomeration [].
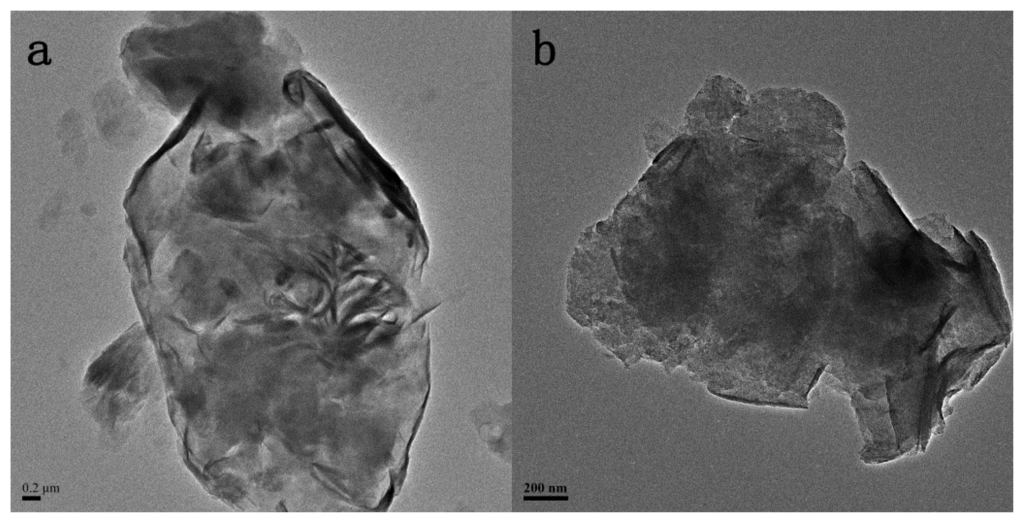
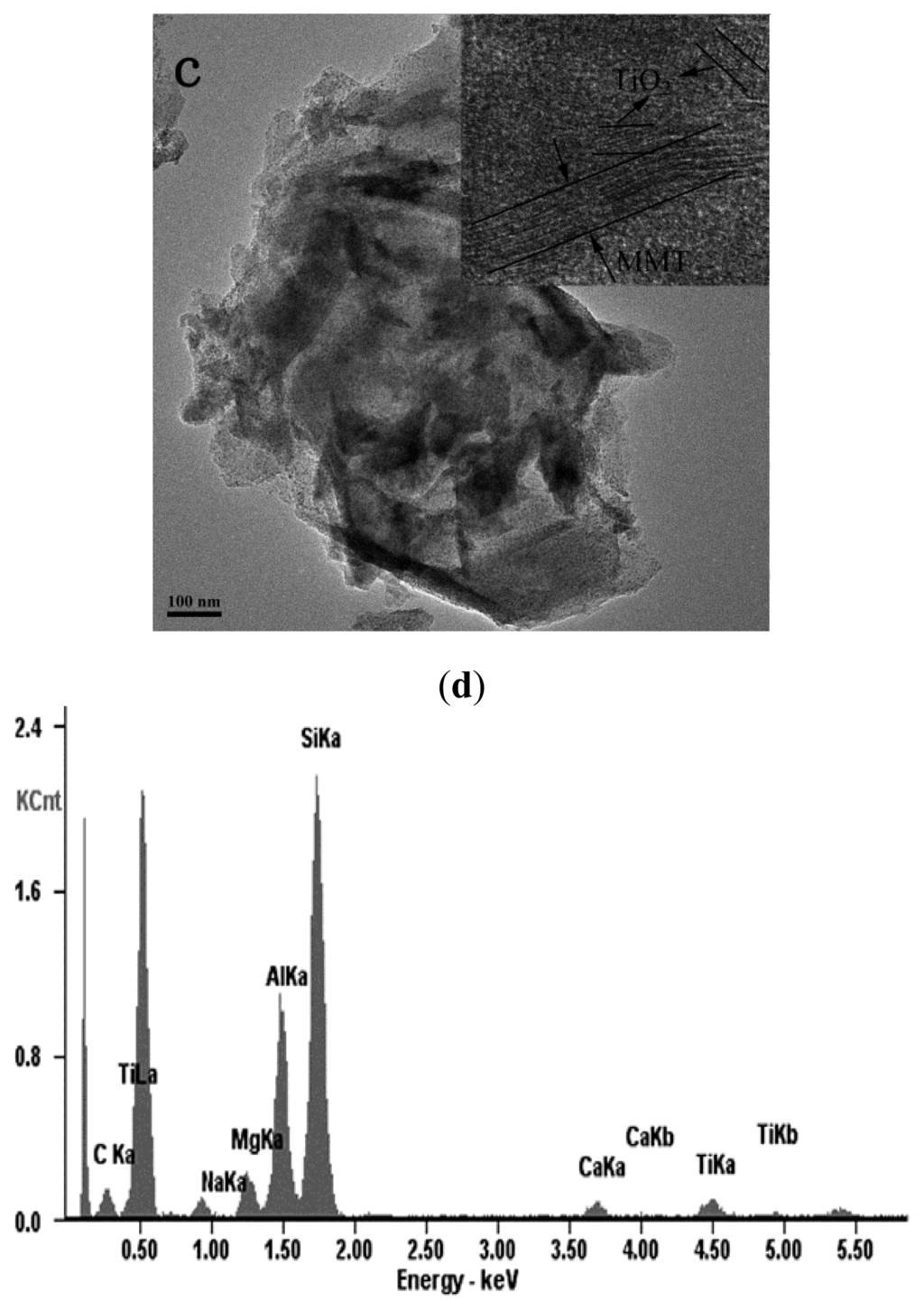
Figure 1.
Transmission electron microscope (TEM) images of (a) pristine Montmorillonite (MMT); (b) TiO2-MMT; (c) TiO2-MMT800 and HR-TEM of TiO2-MMT800 (inset); (d) EDX of (c).
Introducing of TiO2 nanoparticles not only has effects on the surface morphology of MMT, but also changes its internal structure. As presented in Table 1, the thickness of adjacent MMT layer sheet is increased after interaction with TiO2 sol, and greatly decreased after the calcination process. Moreover, the BET data reveals the similar results as the d value derived from XRD, more specifically, the surface area are increased after exchange of CTAB with TiO2 sol, and decreased considerably after calcination. As is known, the thickness of two adjacent MMT layers without gallery heights is about 0.96 nm [], indicating that the layer structure of TiO2-MMT800 has totally collapsed and no gallery heights existed between the layers.

Table 1.
Typical characteristics of three different supports, and adsorption capacity, activity as well as kinetic parameters for free and immobilized laccase.
2.2. Surface Morphology of the Nanohybrids before and after Enzyme Immobilization
The morphology of the clays before and after enzyme immobilization was investigated by SEM, as shown in Figure 2. The pristine MMT (Figure 2a) clusters together with irregular shape, and the modified MMT before (Figure 2c) and after (Figure 2e) calcinations exhibits similar morphologies except that the diameters of the particles are decreased, which can be attributed to the dispersion effect during the preparation process of TiO2-MMT complexes. In general, the morphology of modified MMT is little affected by hydrolysis or intercalating process.
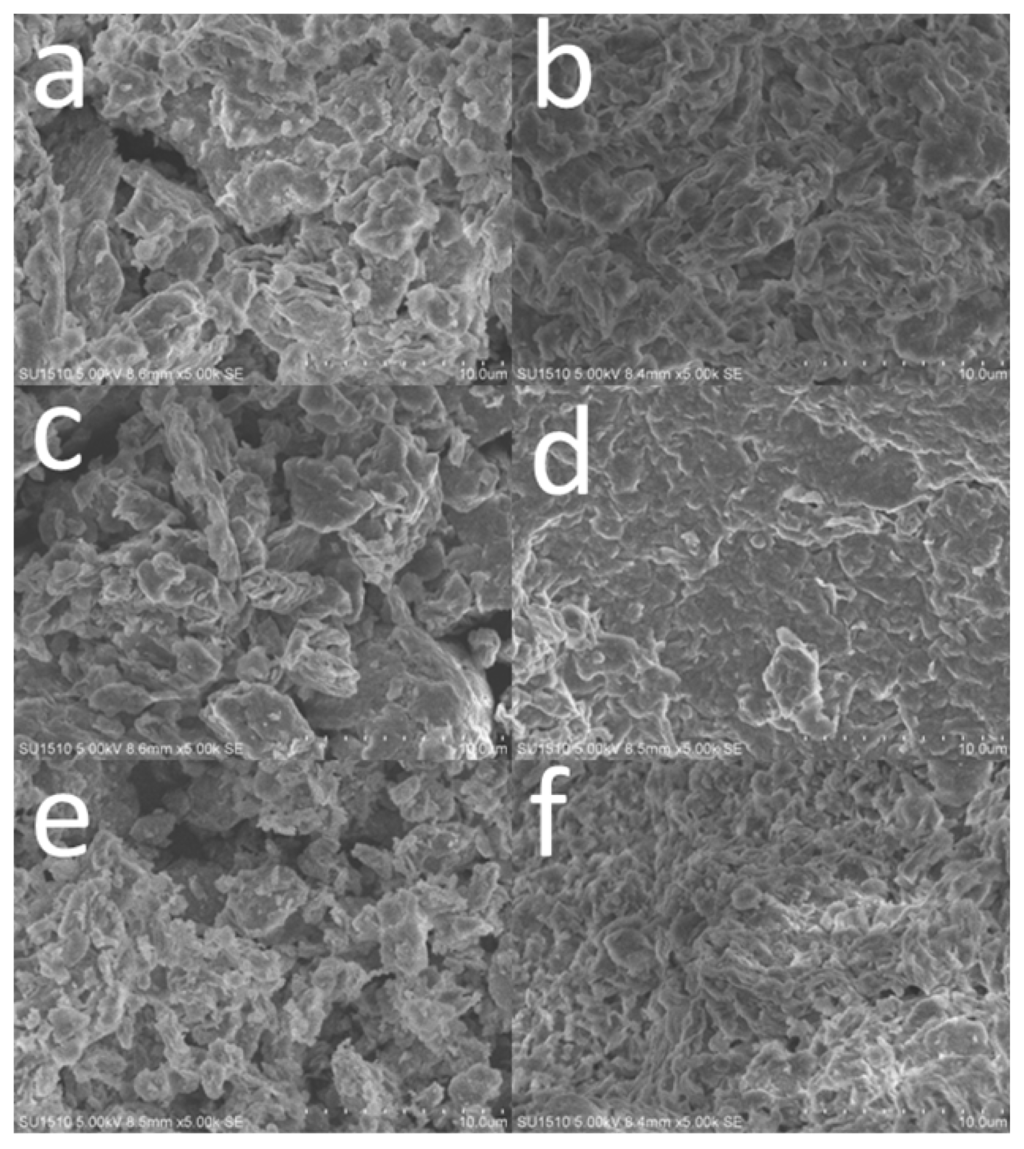
Figure 2.
Scanning electron microscope (SEM) images of (a,b) pristine MMT, (c,d) TiO2-MMT, (e,f) TiO2-MMT800 before and after enzyme immobilization.
However, after enzyme immobilization, the surface morphologies show a significant change. The clays coated with adsorbed enzymes adhere to each other, as illustrated in Figure 2b,d,f. For pristine MMT (Figure 2b), the original structure can still be identified, while the other two samples (Figure 2d,e) show a compact and continuous structure after the enzyme immobilization, especially for TiO2-MMT complexes (Figure 2d).
So, it can be inferred that the existence of TiO2 enhanced the enzyme immobilization. In addition, the immobilized enzymes are mainly adsorbed on the surface of clays rather than intercalated in-between the clays, because TiO2-MMT800 also shows good immobilization capability though its layered structure is completely broken during the process of calcination under high temperature.
2.3. Kinetic Properties of Immobilized Enzyme
As for the enzyme immobilization capability of the three supports, the TiO2-MMT adsorbs more enzymes than that of the other two and the enzyme activity retention is also very good, as indicated in Table 1, which could be ascribed to the biofriendly microenvironment for the immobilized laccase created by the TiO2 sol on the upper surface or the interlayer in-between the clays.
The kinetic parameters of Km and Vmax are also presented in Table 1. Km value is most useful in probing the ability of an enzyme to bind its substrate. An increase in Km value for immobilized laccase is observed with ABTS as substrate. The result is in agreement with other investigators [], indicating a lower affinity for the substrate caused by diffusional limitations and decreased protein flexibility after immobilization []. The Km value for the immobilized enzymes on TiO2-MMT800 is the lowest among the three different supports, which signifies that the enzymes immobilized on TiO2-MMT800 is less distorted than the other two due to a lower interaction with the support and also a lower diffusion limitation. It is proposed by other reporters [] that the enzymes are situated at the periphery of the clay mineral particles whereas the side chains of different amino acid residues penetrate between the layers. “d value” calculated from XRD results represents the interlayer distance of MMT. It can be inferred that for TiO2-MMT800, the space between two adjacent layers is completely destroyed by high temperature during calcination, as proved by the “d value” presented in Table 1. So, the enzymes immobilized on TiO2-MMT800 are mainly situated at the fringe of the MMT surface and no penetrating of side chains in-between the layers, which is considered to be the main reason of the lowest Km value and the highest Vmax value among the three different kinds of supports.
Therefore, TiO2-MMT complexes can be considered as a relatively better support for laccase immobilization.
2.4. The Distribution of Immobilized Laccase on MMT and TiO2-MMT Complexes
A schematic diagram is proposed, as presented in Figure 3, showing the immobilization of laccase on the three different kinds of supports. For pristine MMT, the existence of CTAB enlarges the d-spacing, so the side chains of laccase will penetrate in-between the adjacent layer, leading to increased diffusion limitation during catalytic process. The intercalation of TiO2 sol further increases the d-spacing of MMT, and so the amount of enzymes with side chains penetrated into the MMT interlayer is increased. Though enzymes immobilized in this way will lose activity to some extent, the support can bind more enzymes in a larger quantity. In addition, the TiO2 nanoparticles situated on the upper layer of MMT can be ideal sites for the enzyme to locate; the same conclusion has been drawn in the literature []. So, TiO2-MMT adsorbs more enzymes than that of the other two supports, though the specific activity is not the highest. As for TiO2-MMT800, the MMT layered structure collapses during the calcination process, so an enzyme immobilized on TiO2-MMT800 is mainly due to the effect of surface entrapment. An enzyme immobilized in this way will maintain its initial activity to a great extent, while the drawback is that this kind of combination between enzyme and support is not very strong.
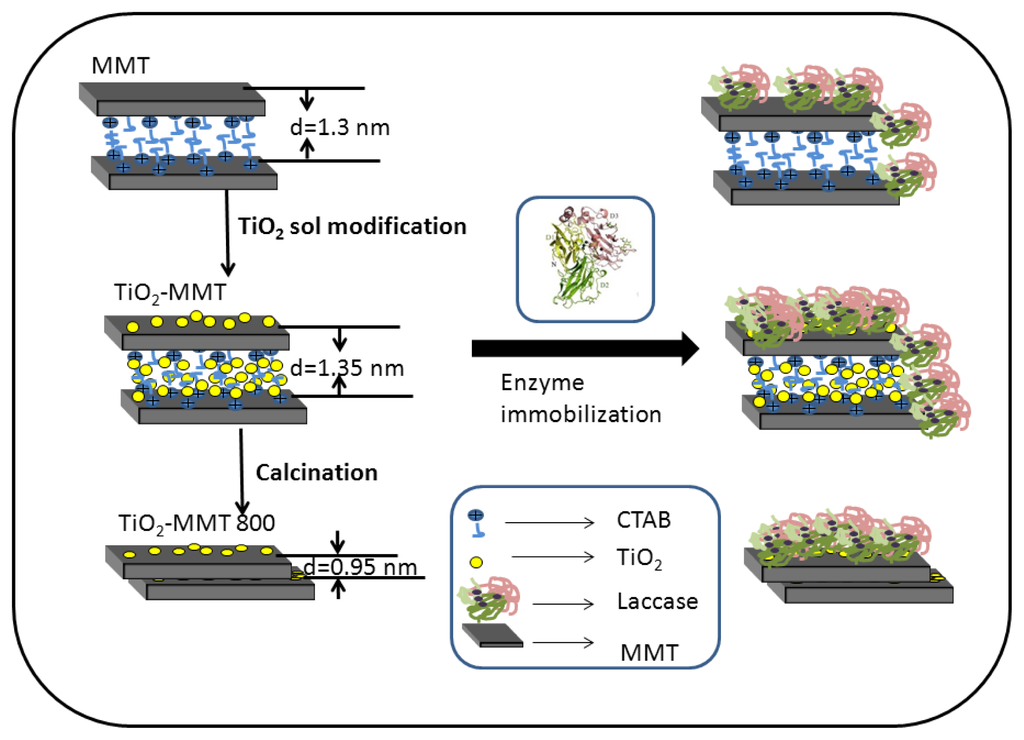
Figure 3.
Schematic diagram of the enzyme immobilization mechanism.
2.5. Effect of pH on Free and Immobilized Laccase
Changes in pH value have effects on the enzyme conformation and the dissociation degree of substrate and coenzyme, and thus affect the binding and catalysis between the enzyme molecules and substrate. Only at the specific pH value could the enzyme and substrate be most appropriately combined, and the optimum catalysis reaction occur.
The free and immobilized laccases were incubated in buffers at 4 °C for 12 h with pH values ranging from 1 to 7. The immobilized laccase demonstrates much higher pH stability than free enzymes, especially in the pH range of 3–7, as indicated in Figure 4. This phenomenon can partially be attributed to the acid centers in MMT clay, that is, Lewis and Bronsted acid sites. Lewis acid is originated from the pillars structure of the pillared MMT, while Bronsted acid comes from the layered structure of pillared clay and the H+ released during the process of calcination. So, MMT can provide laccase with a relatively stable acid environment and improve its pH stability. In the range of pH 1–5, the pillared MMT shows better stability in the relatively alkali buffer (compared with optimum pH) and comparatively lower stability in the relatively acid buffer than the pristine MMT, which phenomenon can further confirm the effect of the Lewis and Bronsted acid sites in MMT clay.
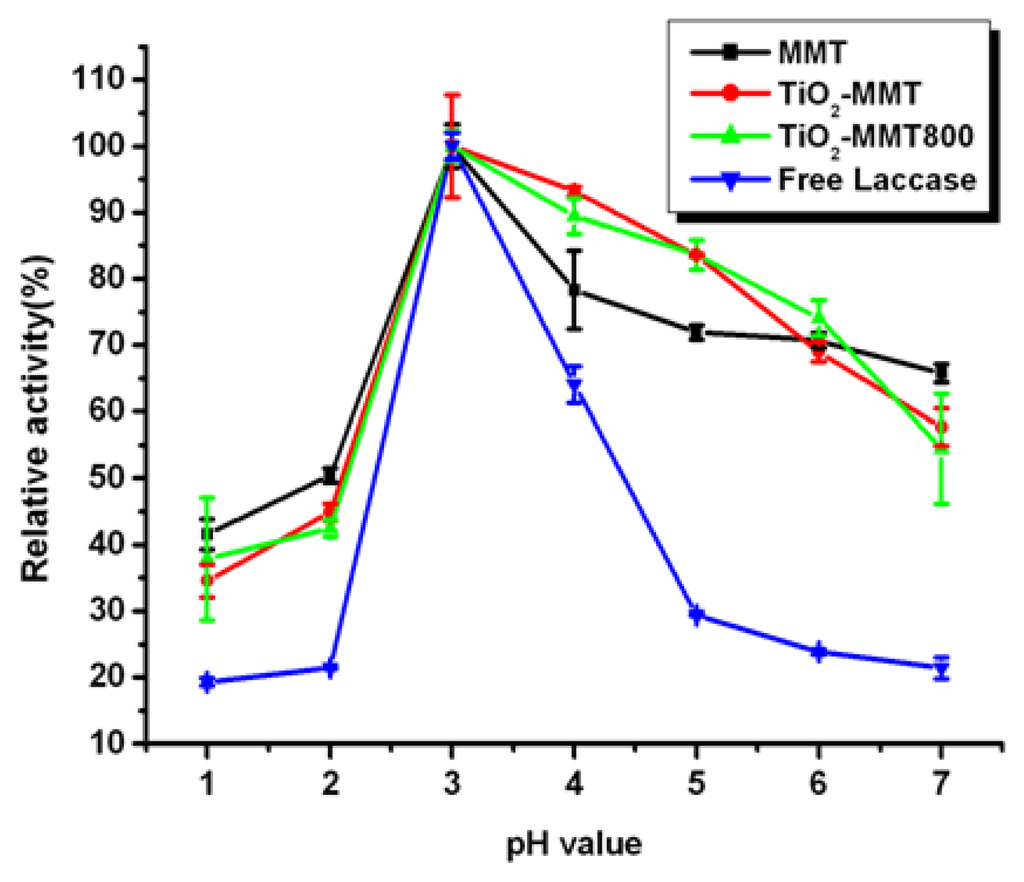
Figure 4.
Effect of pH on the activity of free and immobilized laccase.
However, in the pH range from 6 to 7, the results do not coincide with our previously suggested inference. So, there might be other factors which contribute to the pH stability as well. The enzyme immobilized by clay can be divided into two types, one is to be adsorbed on the surface of clay and the other is intercalated in-between its interlayers. According to the data presented in Table 1, pillared MMT shows better immobilization ability, from which it can be inferred that immobilized laccase mainly exist in the form of surface adsorption rather than intercalation. In addition, TiO2 has a good affinity for enzymes []. This means that the pH stability is also influenced by the existence state of TiO2, the structure of MMT as well as the way laccase bonded with the clay. The experimental results reveal that the immobilized laccase has higher activity stability across a broader pH range than the free laccase.
2.6. Effect of Temperature on the Activity of Free and Immobilized Laccase
The effect of temperature on relative activity of free and immobilized laccase is shown in Figure 5. As is known, MMT has been widely used as flame retardant material for its insulation property and favorable heat resistance property, which might lead to the displacement of the temperature-activity profile. The immobilized enzymes show a similar trend of temperature stability in the temperature range studied, and they keep high activity in the temperature range of 50 to 65 °C. However, at the lower temperature range (30–50 °C) and the slightly higher temperatures (65–80 °C), the relative activity loss of immobilized laccase is higher than the free one.
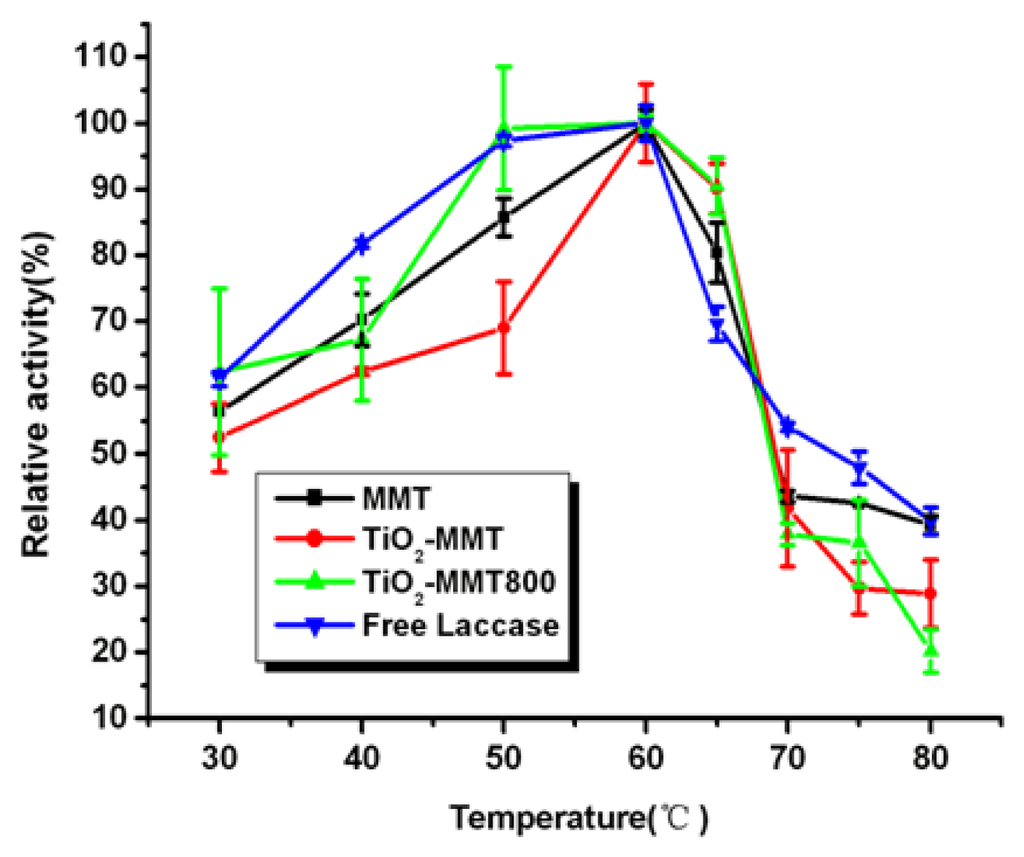
Figure 5.
Effect of temperature on the activity of free and immobilized laccase.
2.7. Storage Stability
For enzyme immobilization, storage stability is one of the most important parameters to be considered, because it can greatly affect the activity retention during the storage. The stabilities of the immobilized laccase were compared under the predetermined conditions in sodium acetate buffer (100 mM, pH 4.5) at room temperature. As is shown by Figure 6a, the activity retention of the enzyme immobilized on the pristine MMT, TiO2-MMT and TiO2-MMT800 after 20 days is 45%, 21% and 27%, respectively, indicating that the enzyme immobilized on pristine MMT shows the best storage stability under the same storage conditions. It can also be observed that during the first 10 days, the activity loss of enzyme immobilized on TiO2-MMT800 is more than those of the other two, because the enzyme is just adsorbed on the surface of the TiO2-MMT800 and not as firmly bonded as those immobilized on MMT and TiO2-MMT supports, which have certain interlayer space for side chains of enzymes to penetrate.
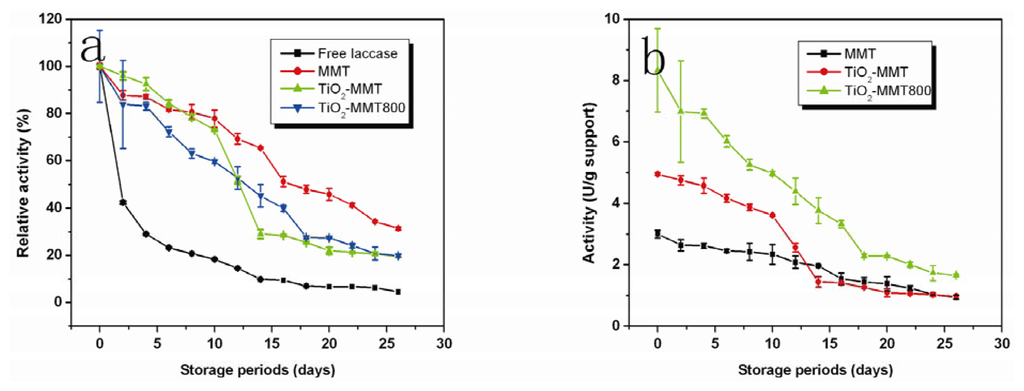
Figure 6.
Effect of aging on (a) relative activity and (b) specific activity.
As seen in Figure 6b, the initial activities of the laccase on pristine MMT, TiO2-MMT and TiO2-MMT800 are 3.004, 4.947 and 8.329 U, respectively. The activity of the immobilized laccase on TiO2-MMT800 is about 2–3 times higher than those of immobilized laccase on MMT and TiO2-MMT, which is also in agreement with the results shown in Table 1. The high initial activity of laccase immobilized on TiO2-MMT800 can be well explained by the supports’ high enzyme immobilization ability and enzyme activity retention, as indicated in Table 1. Therefore, though the immobilized laccase on the pristine MMT shows better storage stability, the enzyme activity of immobilized laccase on TiO2-MMT complexes is higher.
3. Experimental Section
3.1. Materials
Laccase (EC 1.10.3.2) was provided by Novozymes (Guangzhou, China). 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was obtained from Shanghai Richu Biosciences Co., Ltd. (Shanghai, China). The organically modified montmorillonite (O-MMT) by hexadecyl trimethyl ammonium bromide (cation exchange capacity, CEC, 97 mequiv./100 g of clay) was purchased from Zhejiang Fenghong Clay Chemicals Co., Ltd. (Huzhou, China). The polyacrylonitrile (PAN, Mw = 79,100) powder was obtained from Aldrich (Shanghai, China). The 99.5% N,N-dimethyl formamide (DMF), tetrabutyl titanate [Ti(OC4H9)4, CP], ethanol (EtOH, AR) and hydrochloric acid (HCl, AR) were all used as received.
3.2. Preparation of TiO2–MMT Complexes
The TiO2 sol was prepared by the hydrolysis of Ti(OC4H9)4 in the blended solution of deionized water and HCl. The detailed process was described in our previous research [].
One gram MMT powders were added to the TiO2 sol with the volume of 50 mL and vigorously stirred for 3 h at room temperature and aged for 24 h. Subsequently, the MMT suspensions were centrifuged and filtered. The obtained wet cakes were dried under vacuum at 80 °C and then calcined at 800 °C in air for 2 h, identified as TiO2-MMT800, while the uncalcined as TiO2-MMT.
3.3. Laccase Immobilization by Physical Adsorption
Pristine MMT and TiO2-MMT complexes were used as the supports. For a typical immobilization process, 100 mg supports were mixed with equal volumes (80 mL) of enzyme solution (3 g/L) dissolved in 100 mM sodium acetate (pH = 4.5) buffer and magnetically stirred for 5 h in an ice bath. Then the supports were filtered and washed several times with buffer until no laccase activity was detected in the washing buffer.
3.4. Structural Characterization
The Brunauer-Emmett-Teller (BET) specific surface areas of the pristine MMT and modified MMT were obtained using a ASAP 2020 instrument (Micromeritics, Norcross, GA, USA). The samples were degassed at 115 °C for 8 h before the measurement.
The X-ray diffraction (XRD) experiments were carried out in a Philips MPD-18801 diffractometer (BRUKER AXS GMBH, Beijing, China) using Cu Kα (λ = 0.15406 nm) radiation and a fixed power source (40 kV, 40 mA).
The structures of the composite nanoparticles was evaluated from TEM micrographs obtained by a transmission electron microscope (TEM) (JEOL2100, Tokyo, Japan) operating at 80 kV and characterized by a point-to-point resolution.
Scanning electron microscope (SEM, Quanta 200, Holland FEI Company, Beijing, China) was used to investigate the surface morphology of composite nanoparticles before and after enzyme immobilization.
3.5. Protein Concentration and Laccase Activity
3.5.1. Protein Concentration
The protein content in the immobilized supports was determined by subtracting the protein of the supernatant after immobilization from the initial enzyme solution, using Bradford’s method. To be more specific, the protein concentration was measured by the absorbance of the Coomassie brilliant blue (CBB) at 595 nm, with bovine serum albumin as the protein standard.
3.5.2. Activity Assay of Free and Immobilized Laccase
The activity of free and immobilized laccase was determined according to the method reported by Lu et al. [] with some modifications. The reaction mixture was composed of 0.5 mM ABTS, 100 mM sodium acetate buffer (pH = 4.5) and a suitable amount of free and immobilized laccase. The reaction was started by adding 0.1 mL 15 mM ABTS into 2.9 mL sodium acetate buffer containing certain amount of free or immobilized laccase. For immobilized laccase, the supernatant used for activity assay was obtained by centrifugation at 12,000 rpm for about 40 s, and the incubation time and the centrifugation time were controlled to be altogether 3 min.
3.6. Kinetic Parameters
Kinetic tests were carried out at 25 °C in 100 mM sodium acetate (pH = 3.0) buffer using ABTS as the substrate, with the substrate concentration varied from 0.1 to 1 mM.
The kinetic parameters of Km and Vmax were calculated according to the Lineweaver-Burk double reciprocal models as follows:
where v is the initial catalytic rate and S is the substrate concentration.
3.7. Effect of Temperature and pH on the Activity of Immobilized and Free Enzyme
To determine the resistance to pH changes, activities of the free and immobilized enzymes were determined by measuring the activities after they were put in different buffer solutions (pH 1–7) for 12 h, while the effect of temperature on the activity of free and immobilized laccase was determined by measuring the enzyme activity over the temperature range of 30–80 °C at pH = 4.5.
3.8. Storage Stability
The stability of immobilized enzyme was determined by calculating their activity retention ratio during storage at 4 °C in 100 mM sodium acetate buffer solution (pH 4.5), at regular intervals up to 26 days.
4. Conclusions
TiO2-MMT complexes were successfully fabricated and proved to be better supports than the pristine MMT for laccase immoblization. The amount of enzyme adsorbed by TiO2-MMT was higher than those of the other two supports, while the enzyme immobilized on TiO2-MMT800 showed better kinetic properties and the activity retention was as high as 88%. After immobilization, the pH stability was greatly improved, while no significant improvement could be observed for temperature stability. Although the storage stability of laccase immobilized on TiO2-MMT complexes was not as good as the pristine MMT, the activity displayed by TiO2-MMT complexes was higher than that of pristine MMT. Laccase immobilized on TiO2-MMT support combined the advantages of biocatalytic and photocatalytic properties as well as high adsorbability, so this kind of material is believed to be ideal candidate for waste treatment.
Acknowledgments
This work was financially supported by the National High-tech R&D Program of China (No. 2012AA030313), Changjiang Scholars and Innovative Research Team in University (No. IRT1135), National Natural Science Foundation of China (No. 51006046 and No. 51163014), and China Scholarship Council for the State Scholarship Fund.
Conflict of Interest
The authors declare no conflict of interest.
References
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar]
- Jia, J.B.; Zhang, S.P.; Wang, P.; Wang, H.J. Degradation of high concentration 2,4-dichlorophenol by simultaneous photocatalytic-enzymatic process using TiO2/UV and laccase. J. Hazard. Mater 2012, 205, 150–155. [Google Scholar]
- Brunel, L.; Denele, J.; Servat, K.; Kokoh, K.B.; Jolivalt, C.; Innocent, C.; Cretin, M.; Rolland, M.; Tingry, S. Oxygen transport through laccase biocathodes for a membrane-less glucose/O2 biofuel cell. Electrochem. Commun 2007, 9, 331–336. [Google Scholar]
- Wu, X.M.; Hu, Y.J.; Jin, J.; Zhou, N.L.; Wu, P.; Zhang, H.; Cai, C.X. Electrochemical approach for detection of extracellular oxygen released from erythrocytes based on graphene film integrated with laccase and 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid). Anal. Chem 2010, 82, 3588–3596. [Google Scholar]
- Feng, Q.A.; Xia, X.; Wei, A.F.; Wang, X.Q.; Wei, Q.F.; Huo, D.Y.; Wei, A.J. Preparation of Cu(II)-chelated poly(vinyl alcohol) nanofibrous membranes for catalase immobilization. J. Appl. Polym. Sci 2011, 120, 3291–3296. [Google Scholar]
- Dai, Y.R.; Niu, J.F.; Liu, J.; Yin, L.F.; Xu, J.J. In situ encapsulation of laccase in microfibers by emulsion electrospinning: Preparation, characterization, and application. Bioresour. Technol 2010, 101, 8942–8947. [Google Scholar]
- Kanwar, S.S.; Pathak, S.; Verma, H.K.; Kumar, S.; Gupta, R.; Chimni, S.S.; Chauhan, G.S. Characteristics of poly(aac(5)-co-hpma(3)-cl-egdma(15)) hydrogel-immobilized lipase of pseudomonas aeruginosa mtcc-4713. J. Appl. Polym. Sci 2006, 100, 4636–4644. [Google Scholar]
- Kim, B.C.; Zhao, X.Y.; Ahn, H.K.; Kim, J.H.; Lee, H.J.; Kim, K.W.; Nair, S.; Hsiao, E.; Jia, H.F.; Oh, M.K.; et al. Highly stable enzyme precipitate coatings and their electrochemical applications. Biosens. Bioelectron 2011, 26, 1980–1986. [Google Scholar]
- Jia, H.F.; Zhu, G.Y.; Wang, P. Catalytic behaviors of enzymes attached to nanoparticles: The effect of particle mobility. Biotechnol. Bioeng 2003, 84, 406–414. [Google Scholar]
- Wang, F.; Manku, S.; Hall, D.G. Solid phase syntheses of polyamine toxins HO-416b and PHTX-433. Use of an efficient polyamide reduction strategy that facilitates access to branched analogues. Org. Lett 2000, 2, 1581–1583. [Google Scholar]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol 2013, 97, 23–39. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal 2011, 353, 2885–2904. [Google Scholar]
- Gopinath, S.; Sugunan, S. Enzymes immobilized on montmorillonite K 10: Effect of adsorption and grafting on the surface properties and the enzyme activity. Appl. Clay Sci 2007, 35, 67–75. [Google Scholar]
- Chen, G.J.; Yen, M.C.; Wang, J.M.; Lin, J.J.; Chiu, H.C. Layered inorganic/enzyme nanohybrids with selectivity and structural stability upon interacting with biomolecules. Bioconjug. Chem 2008, 19, 138–144. [Google Scholar]
- Naidja, A.; Huang, P.M.; Bollag, J.M. Activity of tyrosinase immobilized on hydroxyaluminum-montmorillonite complexes. J. Mol. Catal 1997, 115, 305–316. [Google Scholar]
- Farre, M.J.; Maldonado, M.I.; Gernjak, W.; Oller, I.; Malato, S.; Domenech, X.; Peral, J. Coupled solar photo-fenton and biological treatment for the degradation of diuron and linuron herbicides at pilot scale. Chemosphere 2008, 72, 622–629. [Google Scholar]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A.; Gernjak, W.; Maldonado, M.L.; Perez-Estrada, L.A.; Pulgarin, C. A combined solar photocatalytic-biological field system for the mineralization of an industrial pollutant at pilot scale. Catal. Today 2007, 122, 150–159. [Google Scholar]
- Guieysse, B.; Viklund, G. Sequential UV-biological degradation of polycyclic aromatic hydrocarbons in two-phases partitioning bioreactors. Chemosphere 2005, 59, 369–376. [Google Scholar]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci 2013, 13, 49–61. [Google Scholar]
- Zhang, G.K.; Ding, X.M.; He, F.S.; Yu, X.Y.; Zhou, J.; Hu, Y.J.; Xie, J.W. Low-temperature synthesis and photocatalytic activity of TiO2 pillared montmorillonite. Langmuir 2008, 24, 1026–1030. [Google Scholar]
- Pluta, M.; Galeski, A.; Alexandre, M.; Paul, M.A.; Dubois, P. Polylactide/montmorillonite nanocomposites and microcomposites prepared by melt blending: Structure and some physical properties. J. Appl. Polym. Sci 2002, 86, 1497–1506. [Google Scholar]
- Makas, Y.G.; Kalkan, N.A.; Aksoy, S.; Altinok, H.; Hasirci, N. Immobilization of laccase in kappa-carrageenan based semi-interpenetrating polymer networks. J. Biotechnol 2010, 148, 216–220. [Google Scholar]
- Lu, L.; Zhao, M.; Wang, Y. Immobilization of laccase by alginate-chitosan microcapsules and its use in dye decolorization. World J. Microbiol. Biotechnol 2007, 23, 159–166. [Google Scholar]
- Lee, H.C.; Lin, J.L.; Chin, Y.L.; Sun, T.P. Comparison of carbon and platinum performance as TiO2 membrane substrate material and applied to glucose biosensor using amperometric readout circuit. Sens. Lett 2008, 6, 1001–1004. [Google Scholar]
- Wang, Q.Q.; Gao, D.W.; Gao, C.T.; Wei, Q.F.; Cai, Y.B.; Xu, J.; Liu, X.Y.; Xu, Y. Removal of a cationic dye by adsorption/photodegradation using electrospun PAN/O-MMT composite nanofibrous membranes coated with TiO2. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).