Bioactive Molecules in Soil Ecosystems: Masters of the Underground
Abstract
:1. Introduction
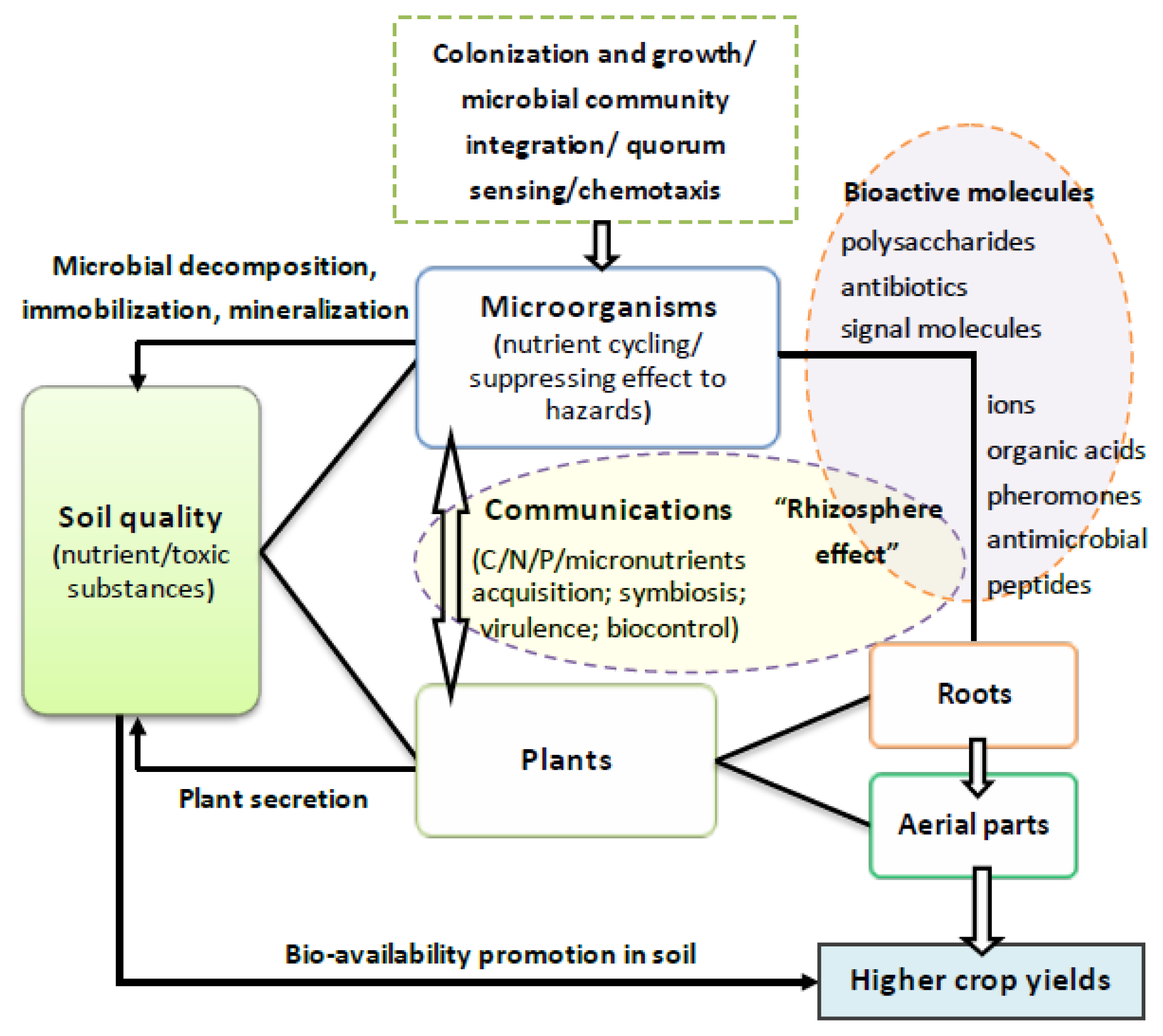
2. Essential and Regulatory Roles of “Bio-Signals”
2.1. Trophic Interactions and C/N/P
2.1.1. Nitrogen Fixation
2.1.2. Phosphate Uptake & Carbon Availability
2.2. Survival Capacity-Virulence/Defenses
2.2.1. Virulence Factors
2.2.2. Biocontrol
2.3. QS—A Messenger in Rhizosphere
2.4. Other Features of Roots Exudates
3. Conclusions and Perspectives
Acknowledgments
Conflict of Interest
References
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol 2006, 57, 233–266. [Google Scholar]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Becard, G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 2007, 12, 224–230. [Google Scholar]
- Gewin, V. An underground revolution. Nature 2010, 466, 552–553. [Google Scholar]
- Feeney, D.S.; Crawford, J.W.; Daniell, T.; Hallett, P.D.; Nunan, N.; Ritz, K.; Rivers, M.; Young, I.M. Three-dimensional microorganization of the soil-root-microbe system. Microb. Ecol 2006, 52, 151–158. [Google Scholar]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol 2009, 68, 1–13. [Google Scholar]
- Marschner, P.; Timonen, S. Interactions between plant species and mycorrhizal colonization on the bacterial community composition in the rhizosphere. Appl. Soil Ecol 2005, 28, 23–36. [Google Scholar]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L. Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A review. Biol. Fertility Soils 2012, 48, 123–149. [Google Scholar]
- Decho, A.W.; Norman, R.S.; Visscher, P.T. Quorum sensing in natural environments: Emerging views from microbial mats. Trends Microbiol 2010, 18, 73–80. [Google Scholar]
- McLean, R.J.C.; Barnes, M.B.; Windham, M.K.; Merchant, M.; Forstner, M.R.J.; Fuqua, C. Cell-cell influences on bacterial community development in aquatic biofilms. Appl. Environ. Microbiol 2005, 71, 8987–8990. [Google Scholar]
- Schaefer, A.L.; Greenberg, E.; Oliver, C.M.; Oda, Y.; Huang, J.J.; Bittan-Banin, G.; Peres, C.M.; Schmidt, S.; Juhaszova, K.; Sufrin, J.R. A new class of homoserine lactone quorum-sensing signals. Nature 2008, 454, 595–599. [Google Scholar]
- Diggle, S.P.; Matthijs, S.; Wright, V.J.; Fletcher, M.P.; Chhabra, S.R.; Lamont, I.L.; Kong, X.; Hider, R.C.; Cornelis, P.; Cámara, M. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol 2007, 14, 87–96. [Google Scholar]
- Flavier, A.B.; Clough, S.J.; Schell, M.A.; Denny, T.P. Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol. Microbiol 1997, 26, 251–259. [Google Scholar]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell. Dev. Biol 2005, 21, 319–346. [Google Scholar]
- Suntharalingam, P.; Cvitkovitch, D.G. Quorum sensing in streptococcal biofilm formation. Trends Microbiol 2005, 13, 3–6. [Google Scholar]
- Gonzalez, J.E.; Marketon, M.M. Quorum sensing in nitrogen-fixing rhizobia. Microbiol. Mol. Biol. Rev 2003, 67, 574–592. [Google Scholar]
- Zhu, J.; Miller, M.B.; Vance, R.E.; Dziejman, M.; Bassler, B.L.; Mekalanos, J.J. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2002, 99, 3129–3134. [Google Scholar]
- Smith, R.S.; Iglewski, B.H. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol 2003, 6, 56–60. [Google Scholar]
- Daniels, R.; Vanderleyden, J.; Michiels, J. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev 2004, 28, 261–289. [Google Scholar]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar]
- Rumbaugh, K.P. Convergence of hormones and autoinducers at the host/pathogen interface. Anal. Bioanal. Chem 2007, 387, 425–435. [Google Scholar]
- Carlsen, S.C.K.; Understrup, A.; Fomsgaard, I.S.; Mortensen, A.G.; Ravnskov, S. Flavonoids in roots of white clover: Interaction of arbuscular mycorrhizal fungi and a pathogenic fungus. Plant Soil 2008, 302, 33–43. [Google Scholar]
- Glazebrook, J.; Walker, G.C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell 1989, 56, 661–672. [Google Scholar]
- Cheng, H.P.; Walker, G.C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol 1998, 180, 5183–5191. [Google Scholar]
- Benson, D.R.; Silvester, W. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev 1993, 57, 293–319. [Google Scholar]
- Downie, J.A.; Walker, S.A. Plant responses to nodulation factors. Curr. Opin. Plant Biol 1999, 2, 483–489. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol 2008, 6, 763–775. [Google Scholar]
- Kosuta, S.; Chabaud, M.; Lougnon, G.; Gough, C.; Dénarié, J.; Barker, D.G.; Bécard, G. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol 2003, 131, 952–962. [Google Scholar]
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett 2006, 254, 173–181. [Google Scholar]
- Denny, T.P.; Baek, S.R. Genetic evidence that extracellular polysaccharide is a virulence factor of Pseudomonas solanacearum. Mol. Plant Microbe Interact 1991, 4, 198–206. [Google Scholar]
- Liu, H.; Zhang, S.; Schell, M.A.; Denny, T.P. Pyramiding unmarked deletions in Ralstonia solanacearum shows that secreted proteins in addition to plant cell-wall-degrading enzymes contribute to virulence. Mol. Plant Microbe Interact 2005, 18, 1296–1305. [Google Scholar]
- Grant, S.R.; Fisher, E.J.; Chang, J.H.; Mole, B.M.; Dangl, J.L. Subterfuge and manipulation: Type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol 2006, 60, 425–449. [Google Scholar]
- Notz, R.; Maurhofer, M.; Dubach, H.; Haas, D.; Defago, G. Fusaric acid-producing strains of Fusarium oxysporum alter 2,4-diacetylphloroglucinol biosynthetic gene expression in Pseudomonas fluorescens CHA0 in vitro and in the rhizosphere of wheat. Appl. Environ. Microbiol 2002, 68, 2229–2235. [Google Scholar]
- Mandava, N.B.; Orellana, R.G.; Warthen, J.D., Jr; Worley, J.F.; Dutky, S.R.; Finegold, H.; Weathington, B.C. Phytotoxins in Rhizoctonia solani: Isolation and biological activity of m-hydroxy-and m-methoxyphenylacetic acids. J. Agric. Food Chem 1980, 28, 71–75. [Google Scholar]
- Ligon, J.M.; Hill, D.S.; Hammer, P.E.; Torkewitz, N.R.; Hofmann, D.; Kempf, H.J.; Pée, K.H. Natural products with antifungal activity from Pseudomonas biocontrol bacteria. Pest Manag. Sci 2000, 56, 688–695. [Google Scholar]
- Asaka, O.; Shoda, M. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl. Environ. Microbiol 1996, 62, 4081–4085. [Google Scholar]
- Papavizas, G.C. Trichoderma and Gliocladium: Biology, ecology, and potential for biocontrol. Annu. Rev. Phytopathol 1985, 23, 23–54. [Google Scholar]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 2009, 57, 171–183. [Google Scholar]
- Morandi, D.; Bailey, J.; Gianinazzi-Pearson, V. Isoflavonoid accumulation in soybean roots infected with vesicular-arbuscular mycorrhizal fungi. Physiol. Plant Pathol 1984, 24, 357–364. [Google Scholar]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar]
- Hause, B.; Maier, W.; Miersch, O.; Kramell, R.; Strack, D. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol 2002, 130, 1213–1220. [Google Scholar]
- Fitze, D.; Wiepning, A.; Kaldorf, M.; Ludwig-Müller, J. Auxins in the development of an arbuscular mycorrhizal symbiosis in maize. J. Plant Physiol 2005, 162, 1210–1219. [Google Scholar]
- Shaul-Keinan, O.; Gadkar, V.; Ginzberg, I.; Grünzweig, J.M.; Chet, I.; Elad, Y.; Wininger, S.; Belausov, E.; Eshed, Y.; Atzmon, N. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol 2002, 154, 501–507. [Google Scholar]
- Herrera-Medina, M.J.; Steinkellner, S.; Vierheilig, H.; Ocampo Bote, J.A.; García Garrido, J.M. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol 2007, 175, 554–564. [Google Scholar]
- Schaarschmidt, S.; González, M.C.; Roitsch, T.; Strack, D.; Sonnewald, U.; Hause, B. Regulation of arbuscular mycorrhization by carbon. The symbiotic interaction cannot be improved by increased carbon availability accomplished by root-specifically enhanced invertase activity. Plant Physiol 2007, 143, 1827–1840. [Google Scholar]
- Douds, D.D.; Pfeffer, P.E.; Shachar-Hill, Y. Application of in vitro methods to study carbon uptake and transport by AM fungi. Plant Soil 2000, 226, 255–261. [Google Scholar]
- Pasold, S.; Siegel, I.; Seidel, C.; Ludwig-Muller, J. Flavonoid accumulation in Arabidopsis thaliana root galls caused by the obligate biotrophic pathogen Plasmodiophora brassicae. Mol. Plant Pathol 2010, 11, 545–562. [Google Scholar]
- Bressan, M.; Roncato, M.A.; Bellvert, F.; Comte, G.; el Zahar Haichar, F.; Achouak, W.; Berge, O. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. ISME J 2009, 3, 1243–1257. [Google Scholar]
- Glenn, S.A.; Gurich, N.; Feeney, M.A.; Gonzalez, J.E. The ExpR/Sin quorum-sensing system controls succinoglycan production in Sinorhizobium meliloti. J. Bacteriol 2007, 189, 7077–7088. [Google Scholar]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar]
- Hogan, D.A.; Vik, Å.; Kolter, R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol 2004, 54, 1212–1223. [Google Scholar]
- Drissner, D.; Kunze, G.; Callewaert, N.; Gehrig, P.; Tamasloukht, M.B.; Boller, T.; Felix, G.; Amrhein, N.; Bucher, M. Lyso-phosphatidylcholine is a signal in the arbuscular mycorrhizal symbiosis. Science 2007, 318, 265–268. [Google Scholar]
- Pietro, A.D.; Madrid, M.P.; Caracuel, Z.; Delgado-Jarana, J.; Roncero, M.I.G. Fusarium oxysporum: Exploring the molecular arsenal of a vascular wilt fungus. Mol. Plant Pathol 2003, 4, 315–325. [Google Scholar]
- Somssich, I.E.; Hahlbrock, K. Pathogen defence in plants-a paradigm of biological complexity. Trends Plant Sci 1998, 3, 86–90. [Google Scholar]
- Anderson, J.P.; Lichtenzveig, J.; Gleason, C.; Oliver, R.P.; Singh, K.B. The B-3 ethylene response factor MtERF1-1 mediates resistance to a subset of root pathogens in Medicago truncatula without adversely affecting symbiosis with rhizobia. Plant Physiol 2010, 154, 861–873. [Google Scholar]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot 2012, 63, 3429–3444. [Google Scholar]
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci 2004, 9, 26–32. [Google Scholar]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol 2010, 72, 313–327. [Google Scholar]
- Davies, J.; Ryan, K.S. Introducing the parvome: Bioactive compounds in the microbial world. ACS Chem. Biol 2011, 7, 252–259. [Google Scholar]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Plant Biol 2008, 59, 341–363. [Google Scholar]
- Oldroyd, G.E.D.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol 2008, 59, 519–546. [Google Scholar]
- Rome, S.; Fernandez, M.P.; Brunel, B.; Normand, P.; Cleyet-Marel, J.C. Sinorhizobium medicae sp. nov., isolated from annual Medicago spp. Int. J. Syst. Bacteriol 1996, 46, 972–980. [Google Scholar]
- Yan, A.M.; Wang, E.T.; Kan, F.L.; Tan, Z.Y.; Sui, X.H.; Reinhold-Hurek, B.; Chen, W.X. Sinorhizobium meliloti associated with Medicago sativa and Melilotus spp. in arid saline soils in Xinjiang, China. Int. J. Syst. Evol. Microbiol 2000, 50, 1887–1891. [Google Scholar]
- Álvarez-Martínez, E.R.; Valverde, Á.; Ramírez-Bahena, M.H.; García-Fraile, P.; Tejedor, C.; Mateos, P.F.; Santillana, N.; Zúñiga, D.; Peix, A.; Velázquez, E. The analysis of core and symbiotic genes of rhizobia nodulating Vicia from different continents reveals their common phylogenetic origin and suggests the distribution of Rhizobium leguminosarum strains together with Vicia seeds. Arch. Microbiol 2009, 191, 659–668. [Google Scholar]
- Segovia, L.; Young, J.P.W.; Martínez-Romero, E. Reclassification of American Rhizobium leguminosarum biovar phaseoli type I strains as Rhizobium etli sp. nov. Int. J. Syst. Bacteriol 1993, 43, 374–377. [Google Scholar]
- Jones, K.M.; Sharopova, N.; Lohar, D.P.; Zhang, J.Q.; VandenBosch, K.A.; Walker, G.C. Differential response of the plant Medicago truncatula to its symbiont Sinorhizobium meliloti or an exopolysaccharide-deficient mutant. Proc. Natl. Acad. Sci. USA 2008, 105, 704–709. [Google Scholar]
- Battisti, L.; Lara, J.C.; Leigh, J.A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc. Natl. Acad. Sci. USA 1992, 89, 5625–5629. [Google Scholar]
- Gonzalez, J.E.; York, G.M.; Walker, G.C. Rhizobium meliloti exopolysaccharides: Synthesis and symbiotic function. Gene 1996, 179, 141–146. [Google Scholar]
- Pellock, B.J.; Cheng, H.P.; Walker, G.C. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol 2000, 182, 4310–4318. [Google Scholar]
- Díaz, C.L.; Melchers, L.S.; Hooykaas, P.J.J.; Lugtenberg, B.J.J.; Kijne, J.W. Root lectin as a determinant of host-plant specificity in the Rhizobium-legume symbiosis. 1989, 338, 579–581. [Google Scholar]
- Brewin, N.J.; Kardailsky, I.V. Legume lectins and nodulation by Rhizobium. Trends Plant Sci 1997, 2, 92–98. [Google Scholar]
- Mathesius, U.; Watt, M. Rhizosphere Signals for Plant-Microbe Interactions: Implications for Field-Grown Plants. In Progress in Botany; Lüttge, U.E., Beyschlag, W., Eds.; Springer: Berlin, Germany, 2011; Volume 72, pp. 125–161. [Google Scholar]
- Mitra, R.M.; Shaw, S.L.; Long, S.R. Six nonnodulating plant mutants defective for Nod factor-induced transcriptional changes associated with the legume-rhizobia symbiosis. Proc. Natl. Acad. Sci. USA 2004, 101, 10217–10222. [Google Scholar]
- Smit, P.; Raedts, J.; Portyanko, V.; Debellé, F.; Gough, C.; Bisseling, T.; Geurts, R. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 2005, 308, 1789–1791. [Google Scholar]
- Madsen, E.B.; Madsen, L.H.; Radutoiu, S.; Olbryt, M.; Rakwalska, M.; Szczyglowski, K.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N. A receptor kinase gene of the LysM type is involved in legumeperception of rhizobial signals. Nature 2003, 425, 637–640. [Google Scholar]
- Gherbi, H.; Markmann, K.; Svistoonoff, S.; Estevan, J.; Autran, D.; Giczey, G.; Auguy, F.; Péret, B.; Laplaze, L.; Franche, C. SymRK defines a common genetic basis for plant root endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 4928–4932. [Google Scholar]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Jurkiewicz, A.; Fukai, E.; Quistgaard, E.M.H.; Albrektsen, A.S.; James, E.K.; Thirup, S.; Stougaard, J. LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J 2007, 26, 3923–3935. [Google Scholar]
- Arrighi, J.F.; Barre, A.; Ben Amor, B.; Bersoult, A.; Soriano, L.C.; Mirabella, R.; de Carvalho-Niebel, F.; Journet, E.P.; Ghérardi, M.; Huguet, T. The Medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol 2006, 142, 265–279. [Google Scholar]
- Phillips, D.A.; Tsai, S.M. Flavonoids as plant signals to rhizosphere microbes. Mycorrhiza 1992, 1, 55–58. [Google Scholar]
- Hoang, H.H.; Gurich, N.; González, J.E. Regulation of motility by the ExpR/Sin quorum-sensing system in Sinorhizobium meliloti. J. Bacteriol 2008, 190, 861–871. [Google Scholar]
- Lithgow, J.K.; Wilkinson, A.; Hardman, A.; Rodelas, B.; Wisniewski-Dyé, F.; Williams, P.; Downie, J.A. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol 2000, 37, 81–97. [Google Scholar]
- Torrey, J.G. Nitrogen fixation by actinomycete-nodulated angiosperms. Bioscience 1978, 28, 586–592. [Google Scholar]
- Normand, P.; Lapierre, P.; Tisa, L.S.; Gogarten, J.P.; Alloisio, N.; Bagnarol, E.; Bassi, C.A.; Berry, A.M.; Bickhart, D.M.; Choisne, N. Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res 2007, 17, 7–15. [Google Scholar]
- Popovici, J.; Comte, G.; Bagnarol, É.; Alloisio, N.; Fournier, P.; Bellvert, F.; Bertrand, C.; Fernandez, M.P. Differential effects of rare specific flavonoids on compatible and incompatible strains in the Myrica gale-Frankia actinorhizal symbiosis. Appl. Environ. Microbiol 2010, 76, 2451–2460. [Google Scholar]
- Harrison, M.J.; Dixon, R.A. Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular-arbuscular mycorrhizal associations in roots of Medicago truncatula. Mol. Plant Microbe Interact 1993, 6, 643–654. [Google Scholar]
- Buee, M.; Rossignol, M.; Jauneau, A.; Ranjeva, R.; Bécard, G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant Microbe Interact 2000, 13, 693–698. [Google Scholar]
- Graham, J.H. Effect of citrus root exudates on germination of chlamydospores of the vesicular-arbuscular mycorrhizal fungus, Glomus epigaeum. Mycologia 1982, 74, 831–835. [Google Scholar]
- Bécard, G.; Piché, Y. Fungal growth stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl. Environ. Microbiol 1989, 55, 2320–2325. [Google Scholar]
- St-Arnaud, M.; Hamel, C.; Vimard, B.; Caron, M.; Fortin, J.A. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol. Res 1996, 100, 328–332. [Google Scholar]
- Kosuta, S.; Hazledine, S.; Sun, J.; Miwa, H.; Morris, R.J.; Downie, J.A.; Oldroyd, G.E.D. Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proc. Natl. Acad. Sci. USA 2008, 105, 9823–9828. [Google Scholar]
- Op den Camp, R.; Streng, A.; de Mita, S.; Cao, Q.; Polone, E.; Liu, W.; Ammiraju, J.S.S.; Kudrna, D.; Wing, R.; Untergasser, A. LysM-type mycorrhizal receptor recruited for Rhizobium symbiosis in nonlegume Parasponia. Science 2011, 331, 909–912. [Google Scholar]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar]
- Bago, B.; Zipfel, W.; Williams, R.M.; Jun, J.; Arreola, R.; Lammers, P.J.; Pfeffer, P.E.; Shachar-Hill, Y. Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol 2002, 128, 108–124. [Google Scholar]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar]
- Chen, Z.; Agnew, J.L.; Cohen, J.D.; He, P.; Shan, L.; Sheen, J.; Kunkel, B.N. Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proc. Natl. Acad. Sci. USA 2007, 104, 20131–20136. [Google Scholar]
- Alabouvette, C.; Olivain, C.; Migheli, Q.; Steinberg, C. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol 2009, 184, 529–544. [Google Scholar]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 2004, 134, 307–319. [Google Scholar]
- Vargas Gil, S.; Pastor, S.; March, G.J. Quantitative isolation of biocontrol agents Trichoderma spp., Gliocladium spp. and actinomycetes from soil with culture media. Microbiol. Res 2009, 164, 196–205. [Google Scholar]
- Igarashi, Y.; Ogawa, M.; Sato, Y.; Saito, N.; Yoshida, R.; Kunoh, H.; Onaka, H.; Furumai, T. Fistupyrone, a novel inhibitor of the infection of Chinese cabbage by Alternaria brassicicola, from Streptomyces sp. TP-A0569. J. Antibiot 2000, 53, 1117–1122. [Google Scholar]
- Ziedan, E.-S.H.; Farrag, E.S.; El-Mohamedy, R.S.; Abd Alla, M.A. Streptomyces alni as a biocontrol agent to root-rot of grapevine and increasing their efficiency by biofertilisers inocula. Arch. Phytopathol. Plant Protect 2010, 43, 634–646. [Google Scholar]
- Zeng, R.S.; Mallik, A.U. Selected ectomycorrhizal fungi of black spruce (Picea mariana) can detoxify phenolic compounds of Kalmia angustifolia. J. Chem. Ecol 2006, 32, 1473–1489. [Google Scholar]
- Azcón-Aguilar, C.; Barea, J. Arbuscular mycorrhizas and biological control of soil-borne plant pathogens—An overview of the mechanisms involved. Mycorrhiza 1997, 6, 457–464. [Google Scholar]
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu. Rev. Plant Physiol. Plant Mol. Biol 1988, 39, 221–244. [Google Scholar]
- Thomas, L.; Mallesha, B.C.; Bagyaraj, D.J. Biological control of damping-off of Cardamom by the VA mycorrhizal fungus, Glomus fasciculatum. Microbiol. Res 1994, 149, 413–417. [Google Scholar]
- Volpin, H.; Elkind, Y.; Okon, Y.; Kapulnik, Y. A vesicular arbuscular mycorrhizal fungus (Glomus intraradix) induces a defense response in alfalfa roots. Plant Physiol 1994, 104, 683–689. [Google Scholar]
- Kim, K.; Yim, W.; Trivedi, P.; Madhaiyan, M.; Deka Boruah, H.P.; Islam, M.R.; Lee, G.; Sa, T. Synergistic effects of inoculating arbuscular mycorrhizal fungi and Methylobacterium oryzae strains on growth and nutrient uptake of red pepper (Capsicum annuum L.). Plant Soil 2010, 327, 429–440. [Google Scholar]
- Medina, A.; Probanza, A.; Gutierrez Maņero, F.; Azcón, R. Interactions of arbuscular-mycorrhizal fungi and Bacillus strains and their effects on plant growth, microbial rhizosphere activity (thymidine and leucine incorporation) and fungal biomass (ergosterol and chitin). Appl. Soil Ecol 2003, 22, 15–28. [Google Scholar]
- Marschner, P.; Crowley, D.E. Physiological activity of a bioluminescent Pseudomonas fluorescens (strain 2–79) in the rhizosphere of mycorrhizal and non-mycorrhizal pepper (Capsicum annuum L.). Soil Biol. Biochem 1996, 28, 869–876. [Google Scholar]
- Raupach, G.S.; Kloepper, J.W. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 1998, 88, 1158–1164. [Google Scholar]
- Dunne, C.; Moenne-Loccoz, Y.; McCarthy, J.; Higgins, P.; Powell, J.; Dowling, D.N.; O’Gara, F. Combining proteolytic and phloroglucinol-producing bacteria for improved biocontrol of Pythium-mediated damping-off of sugar beet. Plant Pathol 1998, 47, 299–307. [Google Scholar]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot 2001, 52, 487–511. [Google Scholar]
- Gilardi, G.; Manker, D.; Garibaldi, A.; Gullino, M. Efficacy of the biocontrol agents Bacillus subtilis and Ampelomyces quisqualis applied in combination with fungicides against powdery mildew of zucchini. J. Plant Dis. Prot 2011, 5, 208–213. [Google Scholar]
- Roberts, D.P.; Lohrke, S.M.; Meyer, S.L.F.; Buyer, J.S.; Bowers, J.H.; Jacyn Baker, C.; Li, W.; de Souza, J.T.; Lewis, J.A.; Chung, S. Biocontrol agents applied individually and in combination for suppression of soilborne diseases of cucumber. Crop Protect 2005, 24, 141–155. [Google Scholar]
- Snyder, B.A.; Nicholson, R.L. Synthesis of phytoalexins in sorghum as a site-specific response to fungal ingress. Science 1990, 248, 1637–1639. [Google Scholar]
- Lanoue, A.; Burlat, V.; Henkes, G.J.; Koch, I.; Schurr, U.; Röse, U.S.R. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol 2010, 185, 577–588. [Google Scholar]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol 2006, 57, 303–333. [Google Scholar]
- Nicol, R.W.; Yousef, L.; Traquair, J.A.; Bernards, M.A. Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochemistry 2003, 64, 257–264. [Google Scholar]
- Wood, D.W.; Gong, F.; Daykin, M.M.; Williams, P.; Pierson, L., 3rd. N-acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30–84 in the wheat rhizosphere. J. Bacteriol 1997, 179, 7663–7670. [Google Scholar]
- Wu, X.; Duan, H.; Tian, T.; Yao, N.; Zhou, H.; Zhang, L. Effect of the hfq gene on 2,4-diacetylphloroglucinol production and the PcoI/PcoR quorum-sensing system in Pseudomonas fluorescens 2P24. FEMS Microbiol. Lett 2010, 309, 16–24. [Google Scholar]
- Wei, H.L.; Zhang, L.Q. Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 2006, 89, 267–280. [Google Scholar]
- Walker, T.S.; Bais, H.P.; Déziel, E.; Schweizer, H.P.; Rahme, L.G.; Fall, R.; Vivanco, J.M. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol 2004, 134, 320–331. [Google Scholar]
- Steindler, L.; Bertani, I.; de Sordi, L.; Schwager, S.; Eberl, L.; Venturi, V. LasI/R and RhlI/R quorum sensing in a strain of Pseudomonas aeruginosa beneficial to plants. Appl. Environ. Microbiol 2009, 75, 5131–5140. [Google Scholar]
- Müller, H.; Westendorf, C.; Leitner, E.; Chernin, L.; Riedel, K.; Schmidt, S.; Eberl, L.; Berg, G. Quorum-sensing effects in the antagonistic rhizosphere bacterium Serratia plymuthica HRO-C48. FEMS Microbiol. Ecol 2009, 67, 468–478. [Google Scholar]
- Mäe, A.; Montesano, M.; Koiv, V.; Palva, E.T. Transgenic plants producing the bacterial pheromone N-acyl-homoserine lactone exhibit enhanced resistance to the bacterial phytopathogen Erwinia carotovora. Mol. Plant Microbe Interact 2001, 14, 1035–1042. [Google Scholar]
- Toth, I.; Newton, J.; Hyman, L.; Lees, A.; Daykin, M.; Ortori, C.; Williams, P.; Fray, R. Potato plants genetically modified to produce N-acylhomoserine lactones increase susceptibility to soft rot erwiniae. Mol. Plant Microbe Interact 2004, 17, 880–887. [Google Scholar]
- Hogan, D.A. Talking to themselves: Autoregulation and quorum sensing in fungi. Eukaryot. Cell 2006, 5, 613–619. [Google Scholar]
- Sanchez-Contreras, M.; Bauer, W.D.; Gao, M.; Robinson, J.B.; Downie, J.A. Quorum-sensing regulation in rhizobia and its role in symbiotic interactions with legumes. Philos. Trans. R. Soc. Lond. B 2007, 362, 1149–1163. [Google Scholar]
- Uroz, S.; Heinonsalo, J. Degradation of N-acyl homoserine lactone quorum sensing signal molecules by forest root-associated fungi. FEMS Microbiol. Ecol 2008, 65, 271–278. [Google Scholar]
- Mathesius, U.; Mulders, S.; Gao, M.; Teplitski, M.; Caetano-Anollés, G.; Rolfe, B.G.; Bauer, W.D. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA 2003, 100, 1444–1449. [Google Scholar]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.N.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; van Breusegem, F.; Eberl, L. Induction of systemic resistance in tomato by N-acyl-l-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 2006, 29, 909–918. [Google Scholar]
- Von Rad, U.; Klein, I.; Dobrev, P.I.; Kottova, J.; Zazimalova, E.; Fekete, A.; Hartmann, A.; Schmitt-Kopplin, P.; Durner, J. Response of Arabidopsis thaliana to N-hexanoyl-dl-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 2008, 229, 73–85. [Google Scholar]
- Teplitski, M.; Robinson, J.B.; Bauer, W.D. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant Microbe Interact 2000, 13, 637–648. [Google Scholar]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol 1996, 178, 6618–6622. [Google Scholar]
- Keshavan, N.D.; Chowdhary, P.K.; Haines, D.C.; González, J.E. l-Canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J. Bacteriol 2005, 187, 8427–8436. [Google Scholar]
- Mark, G.L.; Dow, J.M.; Kiely, P.D.; Higgins, H.; Haynes, J.; Baysse, C.; Abbas, A.; Foley, T.; Franks, A.; Morrissey, J. Transcriptome profiling of bacterial responses to root exudates identifies genes involved in microbe-plant interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 17454–17459. [Google Scholar]
- Yao, J.; Allen, C. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J. Bacteriol 2006, 188, 3697–3708. [Google Scholar]
- Estabrook, E.M.; Yoder, J.I. Plant-plant communications: Rhizosphere signaling between parasitic angiosperms and their hosts. Plant Physiol 1998, 116, 1–7. [Google Scholar]
- Hua, C.; Wang, Y.; Zheng, X.; Dou, D.; Zhang, Z.; Govers, F. A Phytophthora sojae G-protein α subunit is involved in chemotaxis to soybean isoflavones. Eukaryot. Cell 2008, 7, 2133–2140. [Google Scholar]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, L.; Pinton, R.; Varanini, Z.; Nannipieri, P.; Torrent, J.; Martinoia, E.; Cesco, S. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem 2008, 40, 1971–1974. [Google Scholar]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol 2013, 39, 283–297. [Google Scholar]
- Weston, L.A.; Ryan, P.R.; Watt, M. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J. Exp. Bot 2012, 63, 3445–3454. [Google Scholar]
- Tang, C.S.; Young, C.C. Collection and identification of allelopathic compounds from the undisturbed root system of bigalta limpograss (Hemarthria altissima). Plant Physiol 1982, 69, 155–160. [Google Scholar]
- Yu, J.Q.; Matsui, Y. Effects of root exudates of cucumber (Cucumis sativus) and allelochemicals on ion uptake by cucumber seedlings. J. Chem. Ecol 1997, 23, 817–827. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar]
- Mader, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar]
- Watt, M.; Kirkegaard, J.; Passioura, J. Rhizosphere biology and crop productivity—A review. Soil Res 2006, 44, 299–317. [Google Scholar]
- Shen, J.; Li, C.; Mi, G.; Li, L.; Yuan, L.; Jiang, R.; Zhang, F. Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. J. Exp. Bot 2013, 64, 1181–1192. [Google Scholar]
- Ryan, P.R.; Dessaux, Y.; Thomashow, L.S.; Weller, D.M. Rhizosphere engineering and management for sustainable agriculture. Plant Soil 2009, 321, 363–383. [Google Scholar]
- Zhuang, X.; Chen, J.; Shim, H.; Bai, Z. New advances in plant growth-promoting rhizobacteria for bioremediation. Environ. Int 2007, 33, 406–413. [Google Scholar]
- Hense, B.A.; Kuttler, C.; Müller, J.; Rothballer, M.; Hartmann, A.; Kreft, J.U. Does efficiency sensing unify diffusion and quorum sensing? Nat. Rev. Microbiol 2007, 5, 230–239. [Google Scholar]
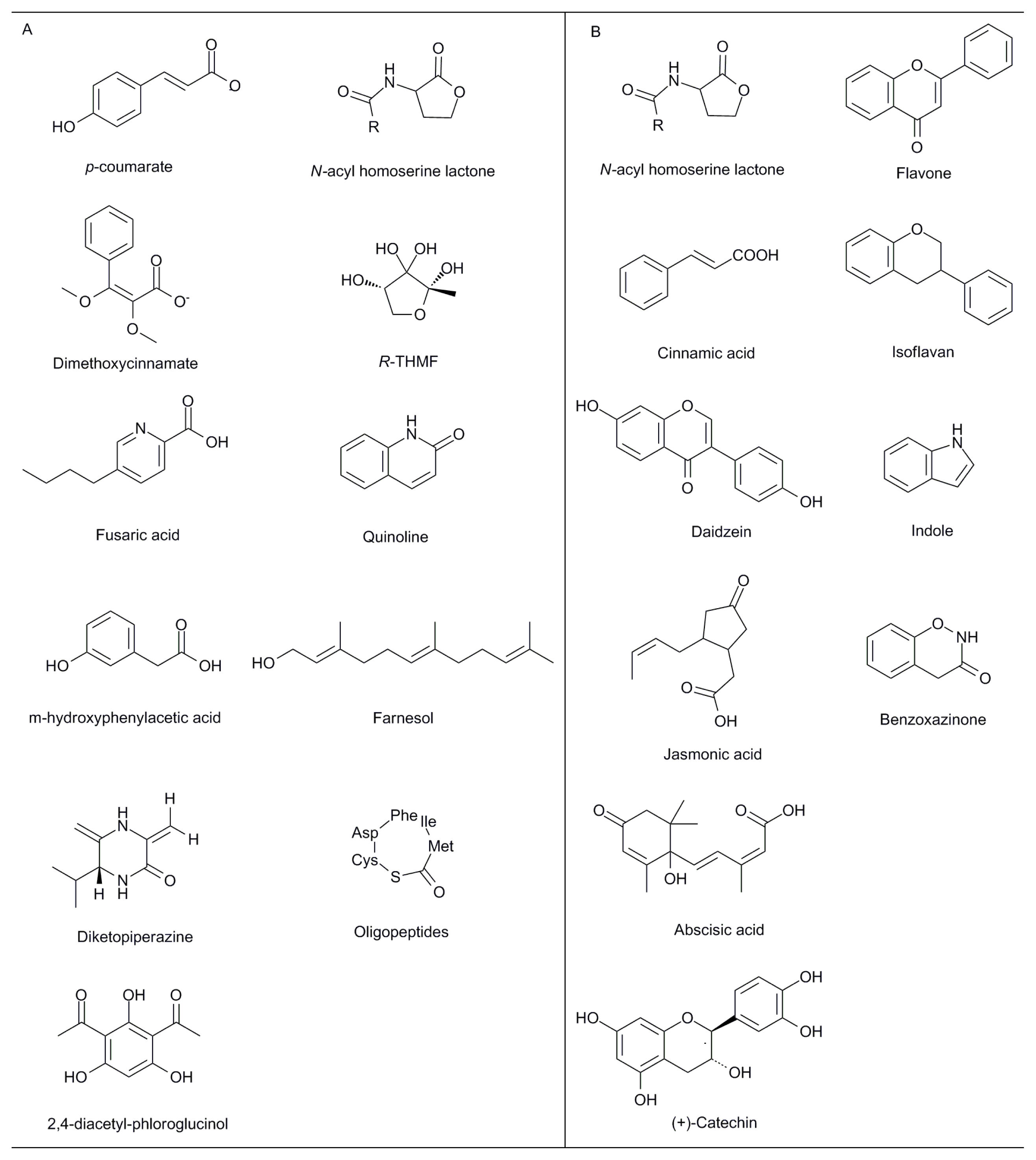
| Primary rhizosphere effects | Bio-molecules | Agents involved | Functional description/Recipients | References |
|---|---|---|---|---|
| Microorganisms driving | ||||
| Nitrogen fixation | Exopolysaccharides: EPS II; succinoglycan | Sinorhizobium meliloti; Rhizobium sp. | Nodulation with a majority of leguminous plants (Medicago and Melilotus spp.; Vicia; Pisum; Parasponia) and other plants | [23–25] |
| Nodulation factors: lipochitooligosaccharide | Rhizobium meliloti | Inducing a variety of effects including deformation of root hairs, division of root cortical cells, and nodule morphogenesis | [26] | |
| Symbionts (with Arbuscular mycorrhizal fungi) | “Myc factor” (soluble signaling molecules) | Arbuscular mycorrhizal fungi | Fungal signaling factor that triggers gene activation in the root required for mycorrhization | [27,28] |
| Metal uptake | Glutathione; metallothioneins | Ectomycorrhizal fungi | Influence on metallic element bioavailability in soil | [29] |
| Virulence factors | Extracellular polysaccharide | Pseudomonas solanacearum | Responsible for the wilt symptoms | [30] |
| Extracellular plant cell wall-degrading enzymes | Ralstonia solanacearum | [31] | ||
| Effector proteins | Pseudomonas syringae; Xanthomonas spp.; Ralstonia solanacearum; Erwinia species | Essential for the virulence and suppression of host defense responses | [32] | |
| Phytotoxin (fusaric acid) | Fusarium oxysporum | Inhibiting the growth of rice seedlings and repressing antimicrobial activity of the biocontrol strain Pseudomonas fluorescens CHA0 | [33] | |
| m-hydroxyphenylacetic acid; m-methoxyphenylacetic acid | Rhizoctonia solani | Infection of soybeans and decreasing of nodule formation | [34] | |
| Biological control activities | Antibiotics: phenazine; pyoluteorin; 2,4-diacetyl-phloroglucinol; pyrrolnitrin; 2,3-de-epoxy-2,3-didehydro-rhizoxin; hydrogen cyanide | Pseudomonas spp. | Interfering growth of various pathogens and contributing to disease suppression | [35] |
| Lipopeptides: surfactin; iturin A | Bacillus subtilis | Antibacterial and antifungal agents | [36] | |
| Antibiotics: gliovirin; gliotoxin | Trichoderma spp.; Gliocladium spp. | Protection of plants against pathogens | [37] | |
| Roots driving | ||||
| Bacterial symbionts | Flavonoids | Medicago truncatula | Stimulating presymbiotic steps in rhizobia | [38] |
| Fungal symbionts (with Arbuscular mycorrhizal fungi) | Flavonoids: glyceollin; coumestrol; daidzein | Glycine max | Root colonization by mycorrhizal fungi | [39] |
| Strigolactone | Lotus japonicas; | Branching factor (hyphal branching of AMF) that precedes successful root colonization | [40] | |
| Jasmonic acid | Hordeum vulgare cv Salome | Colonization rate and arbuscule formation in mycorrhizal roots | [41] | |
| Auxin and auxin conjugates | Zea mays | Enhanced fungal growth | [42] | |
| Gibberellin | Nicotiana tabacum | Strengthening the carbohydrate sink of the fungi | [43] | |
| Abscisic acid; ethylene | Lycopersicon esculentum | Development of the complete arbuscule and its functionality | [44] | |
| Carbon availability | Hexose | Medicago truncatula; Daucus carota | Carbon uptake and metabolism | [45,46] |
| Pathogenicity factors and defence response | Flavonoids | Arabidopsis | An intense accumulation of flavonoids in Arabidopsis root infected by Plasmodiophora brassicae | [47] |
| Phytoalexins: indole; saponins; terpenoid; benzoxazinone; flavonoid; rosmarinic acid; naphthoquinones, | -- | Defence compounds of the rhizosphere against pathogenic microorganisms | [1] | |
| Glucosinolates and hydrolysis products (isothiocyanates; nitriles; ionic thiocyanates) | Arabidopsis thaliana | Against fungal and bacterial pathogens | [48] | |
| Indirect effects | Bioactive compounds | Agents involved | Functional description/Recipients | References |
|---|---|---|---|---|
| Microorganisms driving | ||||
| Quorum sensing | N-acyl homoserine lactones (AHLs); p-coumarate; quinolone | Gram-negative bacteria | Cell-cell communication between bacteria to regulate symbiotism, virulence, swarming behavior, biofilm formation and antibiotic production | [11,12,14–17,49,50] |
| Oligopeptides | Gram-positive bacteria | |||
| AI-2: furanosyl borate diester | -- | |||
| Fungal QS systems | Farnesol; tyrosol; dimethoxycinnamate; trisporic acid | Candida albicans; Uromyces phaseoli; zygomycetes | Controlling biofilm formation and pathogenesis in fungus | [51] |
| Phosphate acquisition (with Arbuscular mycorrhizal fungi) | Lysophosphatidylcholine | Arbuscular mycorrhizal fungi; Solanum tuberosum L.; Solanum lycopersicum L. * | Induction of plant phosphate transporter gene and mycorrhiza formation | [52] |
| Virulence | Signal transduction cascades: cAMP-PKA and MAPK cascade | Fusarium strains | Sensing environmental cues and respond by appropriate changes in gene expression to establish disease | [53] |
| Roots driving | ||||
| Defence response | NAD(P)H oxidases, phospholipases, phosphatases and protein kinases; linolenic acid; jasmonic acid; methyl jasmonate | -- | The low doses might act as signals for activation of other defence reactions | [54] |
| B-3 ethylene response factors (ERFs) | Medicago | Resistance to Rhizoctonia solani and Phytophthora medicaginis | [55] | |
| Complex effects | Flavonoids | -- | Stimulating or inhibiting rhizobial nod gene expression, causing chemoattraction of rhizobia towards the root, inhibiting root pathogens, stimulating mycorrhizal spore germination and hyphal branching, mediating allelopathic interactions between plants, affecting quorum sensing, and chelating soil nutrients | [56] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhuang, X.; Gao, J.; Ma, A.; Fu, S.; Zhuang, G. Bioactive Molecules in Soil Ecosystems: Masters of the Underground. Int. J. Mol. Sci. 2013, 14, 8841-8868. https://doi.org/10.3390/ijms14058841
Zhuang X, Gao J, Ma A, Fu S, Zhuang G. Bioactive Molecules in Soil Ecosystems: Masters of the Underground. International Journal of Molecular Sciences. 2013; 14(5):8841-8868. https://doi.org/10.3390/ijms14058841
Chicago/Turabian StyleZhuang, Xuliang, Jie Gao, Anzhou Ma, Shenglei Fu, and Guoqiang Zhuang. 2013. "Bioactive Molecules in Soil Ecosystems: Masters of the Underground" International Journal of Molecular Sciences 14, no. 5: 8841-8868. https://doi.org/10.3390/ijms14058841
APA StyleZhuang, X., Gao, J., Ma, A., Fu, S., & Zhuang, G. (2013). Bioactive Molecules in Soil Ecosystems: Masters of the Underground. International Journal of Molecular Sciences, 14(5), 8841-8868. https://doi.org/10.3390/ijms14058841





