Antibacterial Activity of the Alkaloid-Enriched Extract from Prosopis juliflora Pods and Its Influence on in Vitro Ruminal Digestion
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Procedures
3.2. Preparation of the Dragendorff’s Reagent
3.3. Plant Material
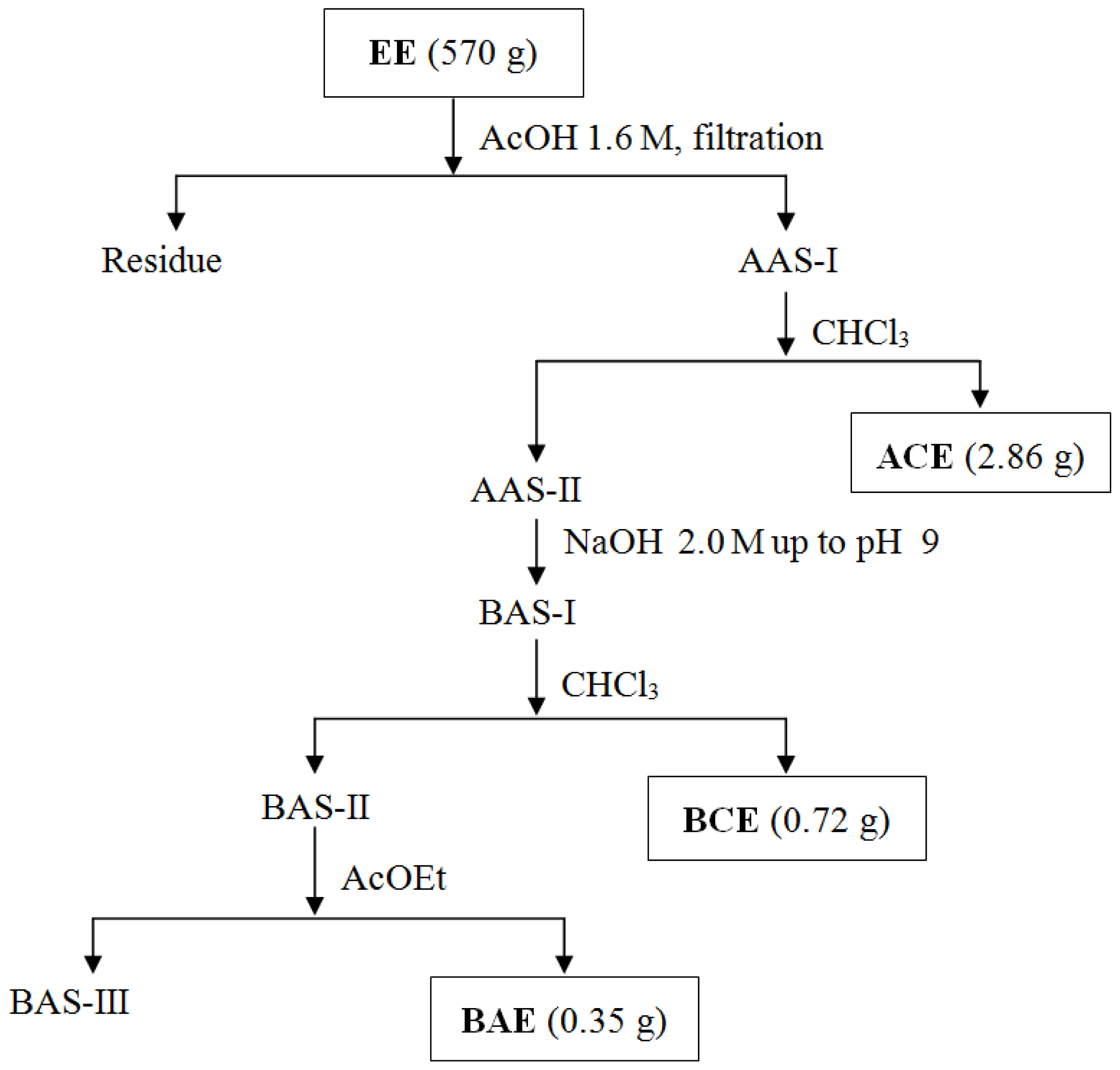
3.4. Obtaining Extracts
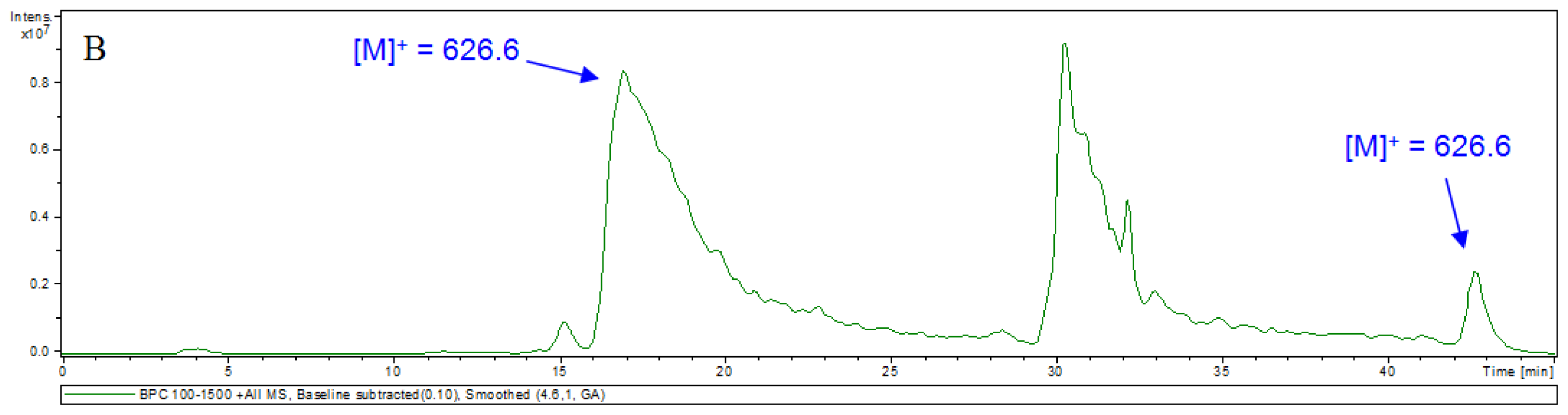
3.5. HPLC-MS Analyses
3.6. In Vitro Antimicrobial Assay
3.7. Evaluation of Effects on Digestion Parameters by in Vitro Gas Production
3.8. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-08496-s001.pdfAcknowledgments
Abbreviations
| AAS | acid aqueous solution |
| ACE | acid chloroformic extract |
| ADDM | apparent degradability of DM |
| BAE | basic ethyl acetate extract |
| BAS | basic aqueous solution |
| BCE | basic chloroformic extract |
| DM | dry matter |
| DMMP | dry microbial mass production |
| EE | ethanolic extract |
| HRESIMS | high resolution electron spray ionization mass spectrometry |
| HPLC-MS | high-performance liquid chromatography mass spectrometer |
| 1H NMR | proton nuclear magnetic resonance |
| MHz | megahertz |
| MIC | minimum inhibitory concentration |
| MMP | microbial mass production |
| RUSITEC | rumen simulation technique |
| SAEG | system of statistical and genetic analysis |
| TDDM | true degradability of DM |
| TLC | thin layer chromatography |
| TMS | tetramethylsilane |
| VFA | volatile fat acids |
References
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci 1995, 73, 2483–2492. [Google Scholar]
- Eliseev, A.V.; Mokhov, I.I.; Arzhanov, M.M.; Demchenko, P.F.; Denisov, S.N. Interaction of the methane cycle and processes in wetland ecosystems in a climate model of intermediate complexity. Izv. Atmos. Ocean. Phys 2008, 44, 139–152. [Google Scholar]
- Beauchemin, K.A.; Mcginn, S.M.; Martinez, T.F.; Mcallister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci 2007, 85, 1990–1996. [Google Scholar]
- Lassey, K.R.; Ulyatt, M.J.; Martin, R.J.; Walker, C.F.; Shelton, I.D. Methane emissions measured directly from grazing livestock in New Zealand. Atmos. Environ 1997, 31, 2905–2914. [Google Scholar]
- Richardson, L.F.; Raun, A.P.; Potter, E.L.; Cooley, C.O.; Rathmacher, R.P. Effect of monensin on rumen fermentation in vitro and in vivo. J. Anim. Sci 1976, 43, 657–664. [Google Scholar]
- Manero, A.; Vilanova, X.; Cerda-Cuellar, M.; Blanch, A.R. Vancomycin- and erythromycin-resistant enterococci in a pig farm and its environment. Environ. Microbiol 2006, 8, 667–674. [Google Scholar]
- Yang, S.; Carlson, K. Routine monitoring of antibiotics in water and wastewater with a radioimmunoassay technique. Water Res 2004, 38, 3155–3166. [Google Scholar]
- Jouany, J.P.; Morgavi, D.P. Use of “natural” products as alternatives to antibiotic feed additives in ruminant production. Animal 2007, 1, 1443–1466. [Google Scholar]
- Pen, B.; Sar, C.; Mwenya, B.; Takahashi, J. Effects of Quillaja saponaria extract alone or in combination with Yucca schidigera extract on ruminal fermentation and methanogenesis in vitro. Anim. Sci. J 2008, 79, 193–199. [Google Scholar]
- Gibbons, S. Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem. Rev 2005, 4, 63–78. [Google Scholar]
- Felker, P. Use of tree legumes in semiarid regions. Econ. Bot 1981, 35, 174–186. [Google Scholar]
- Tapia, A.; Feresin, G.E.; Bustos, D.; Astudillo, L.; Theoduloz, C.; Schmeda-Hirschmann, G. Biologically active alkaloids and a free radical scavenger from Prosopis species. J. Ethnopharm 2000, 71, 241–246. [Google Scholar]
- Ortega-Nieblas, M.; Vázquez-Moreno, L.; Robles-Burgueño, M.R. Protein quality and antinutritional factors of wild legume seeds from the Sonoran desert. J. Agric. Food Chem 1996, 44, 3130–3132. [Google Scholar]
- Svensson, B.; Fukuda, K.; Nielsen, P.K.; Bonsager, B.C. Proteinaceous α-amylase inhibitors. Biochim. Biophys. Acta 2004, 1696, 145–156. [Google Scholar]
- Tabosa, I.M.; Quintans-Júnior, L.J.; Pamplona, F.V.; Almeida, R.N.; Cunha, E.V.L.; Silva, M.S.; Souza, J.C.A.; Barbosa-Filho, J.M. Isolamento biomonitorado de alcalóides tóxicos de Prosopis juliflora(algaroba). Rev. Bras. Farmacogn. 2000, 9–10, 11–22. [Google Scholar]
- Hughes, J.B.; Silva, V.D.A.; Silva, A.R.; Souza, C.S.; Silva, A.M.M.; Velozo, E.S.; Batatinha, M.J.M.; Costa, M.F.D.; Tardy, M.; El-Bachá, R.S.; et al. Cytotoxicity effect of alkaloidal extract from Prosopis juliflora Sw.D.C. (Algaroba) pods on glial cells. Braz. J. Vet. Res. Anim. Sci 2006, 43, 50–58. [Google Scholar]
- Cook, R.W.; Scott, C.B.; Hartmann, F.S. Short-term mesquite pod consumption by goats does not induce toxicity. Rangel. Ecol. Manag 2008, 61, 566–570. [Google Scholar]
- Sharma, R.C.; Zaman, A.; Kidwai, A.R. Chemical examination of Daphne paryracea Wall. Indian J. Chem 1964, 2, 83. [Google Scholar]
- Bhardwaj, D.K.; Bisht, M.S.; Jain, R.K.; Sharma, G.C. Prosogerin-D, a new flavone from Prosopis spicigera seeds. Phytochemistry 1980, 19, 1269–1270. [Google Scholar]
- Shukla, R.V.N.; Misra, K. Two flavonoid glycosides from the bark of Prosopis juliflora. Phytochemistry 1981, 20, 339–340. [Google Scholar]
- Malhotra, S.; Misra, K. 3,3′-di-O-methylellagic acid 4-O-rhamnoside from the roots of Prosopis juliflora. Phytochemistry 1981, 20, 2043–2044. [Google Scholar]
- Malhotra, S.; Misra, K. New flavanones from Prosopis juliflora roots. Planta Medica 1983, 47, 46–48. [Google Scholar]
- Ahmad, V.U.; Sultana, A. A terpenoid diketone from the leaves of Prosopis juliflora. Phytochemistry 1989, 28, 278–279. [Google Scholar]
- Ahmad, V.U.; Sultana, A.; Qazi, S. Alkaloids from the leaves of Prosopis juliflora. J. Nat. Prod 1989, 52, 497–501. [Google Scholar]
- Ahmad, A.; Khursheed, A.K.; Sabiha, Q.; Viqaruddin, A. Antifungial activity of some hydrosoluble Prosopis juliflora alkaloids. Fitoterapia 1989, 60, 86–89. [Google Scholar]
- Zia, N.B. Investigation of the chemical constituents of Prosopis juliflora; Circular dichroismic studies of cholestanoquinoxalines. Ph.D Thesis, University of Karachi, Karachi, Pakistan, 1992. [Google Scholar]
- Nakano, H.; Nakajima, E.; Hiradate, S.; Fujii, Y.; Yamada, K.; Shigemori, H.; Hasegawa, K. Growth inhibitory alkaloids from mesquite (Prosopis juliflora (Sw.) DC.) leaves. Phytochemistry 2003, 65, 587–591. [Google Scholar]
- Aqeel, A.; Khursheed, A.K.; Viqaruddin, A.; Sabiha, Q. Antimicrobial activity of julifloricine isolated from Prosopis juliflora. Drug Res 1989, 39, 652–655. [Google Scholar]
- Satish, S.; Raveesha, K.A.; Janardhana, G.R. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Lett. Appl. Microbiol 1999, 28, 145–147. [Google Scholar]
- Kanthasamy, A.; Subramanian, S.; Govindasamy, S. Bactericidal and fungicidal effects of Prosopis juliflora alkaloidal fraction. Indian Drugs 1988, 26, 390–394. [Google Scholar]
- Cáceres, A.; Menéndez, H.; Méndez, E.; Cohobón, E.; Samayoa, B.E.; Jauregui, E. Antigonorrhoeal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. J. Ethnopharm 1995, 48, 85–88. [Google Scholar]
- Ahmad, A. Study of antimicrobial activity of the alkaloids isolated from Prosopis juliflora. Ph.D. Thesis, University of Karachi, Karachi, Pakistan, 1991. [Google Scholar]
- Batatinha, M.J.M. Investigations about toxic influences of Prosopis juliflora D.C: (Algarobeira) on cell cultures as well as on the fermentation in the rumen of cattle (in vitro). Ph.D. Thesis, University of Veterinary Medicine, Foundation Hannover, Hannover, Germany, 1997. [Google Scholar]
- Singh, S.; Swapnil, S.K.V. Antibacterial properties of alkaloid rich fractions obtained from various parts of Prosopis juliflora. Int. J. Pharma. Sci. Res 2011, 2, 114–120. [Google Scholar]
- Callaway, T.R.; Adams, K.A.; Russell, J.B. The ability of “low g + c gram-positive” ruminal bacteria to resist monensin and counteract potassium depletion. Curr. Microbiol 1999, 39, 226–230. [Google Scholar]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol 1999, 79, 321–330. [Google Scholar]
- Nelson, D.A. TLC detection of phthalate esters with anisaldehyde and Dragendorff reagents. Anal. Biochem 1969, 29, 171–173. [Google Scholar]
- Wyrwas, B.; Szymanski, A.; Lukaszewski, Z. Tensammetric studies of the separation of surfactants. Part 1. Investigation of sources of error in precipitation of non-ionic surfactants with modified Dragendorff reagent. Anal. Chim. Acta 1993, 278, 197–203. [Google Scholar]
- Simonovska, B.; Vovk, I. High-performance thin-layer chromatographic determination of potato glycoalkaloids. J. Chromatogr. A 2000, 903, 219–225. [Google Scholar]
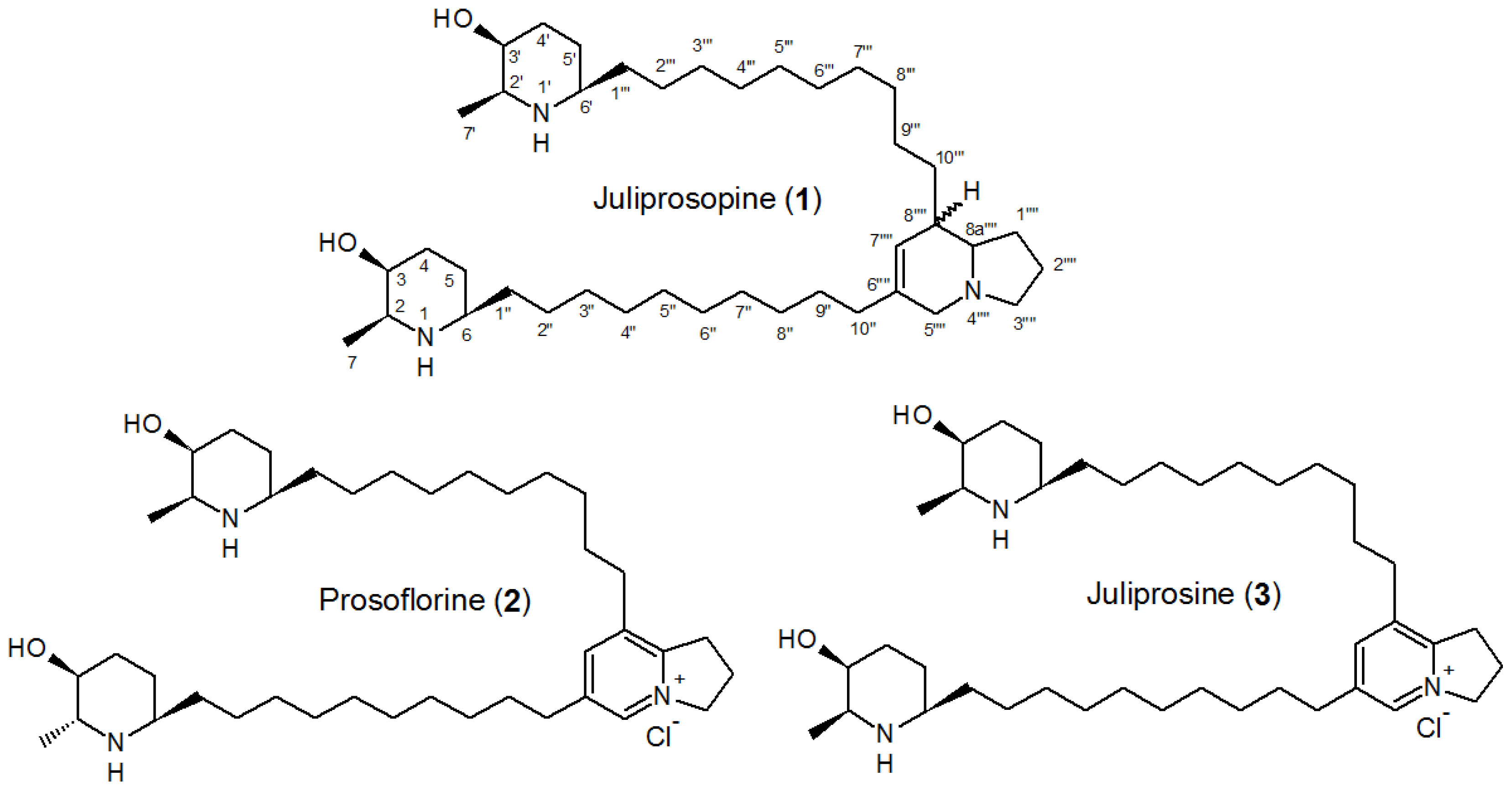
- Ott-Longoni, R.; Viswanathan, N.; Hesse, M. The structure of the alkaloid juliprosopin from Prosopis juliflora A. DC. Helv. Chim. Acta 1980, 63, 2119–2129. [Google Scholar]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci 1994, 72, 2980–2991. [Google Scholar]
- Chen, M.; Wolin, M.J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl. Environ. Microbiol 1979, 38, 72–77. [Google Scholar]
- Júnior, D.S.; Queiroz, A.C.; Lana, R.P.; Pacheco, C.G.; Camardelli, M.M.L.; Detmann, E.; Eifert, E.C.; Nunes, P.M.M.; Oliveira, M.V.M. Ação do extrato de própolis sobre a fermentação in vitro de deferentes alimentos pela técnica de produção de gases. Rev. Bras. Zootec 2004, 33, 1093–1099. [Google Scholar]
- Gupta, R.S. Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes. Microbiol. Mol. Biol. Rev 1998, 62, 1435–1491. [Google Scholar]
- Choudhary, M.I.; Nawaz, S.A.; Zaheer-ul-Haq; Azim, M.K.; Ghayur, M.N.; Lodhy, M.A.; Jalil, S.; Khalid, A.; Ahmed, A.; Rode, B.M.; et al. Juliflorine: A potent natural peripheral anionic-site-binding inhibitor of acetylcholinesterase with calcium-channel blocking potential, a leading candidate for Alzheimer’s disease therapy. Biochem. Biophys. Res. Commun. 2005, 332, 1171–1179. [Google Scholar]
- Moss, R.A. Methane: Global Warming and Production by Animals; Chalcombe Publications: Canterbury, UK, 1993. [Google Scholar]
- Reilly, P.E.B. Rumen biochemistry; The University of Queensland: Brisbane, 1998. Available online: http://www.vet.ed.ac.uk/clive/cal/RUMENCAL/vetcal.html (accessed on 30 May 2007).
- Faria, B.N.; Reis, R.B.; Maurício, R.M.; Lana, A.M.Q.; Leite, L.A.; Coelho, S.G.; Saturnino, H.M. Efeitos da adição de monensina ou propilenoglicol à polpa cítrica sobre a cinética de degradação dos carboidratos totais e da produção cumulativa de gases in vitro. Arq. Bras. Med. Vet. Zootec 2008, 60, 691–697. [Google Scholar]
- Nagaraja, T.G.; Newbold, C.J.; Van Nevel, C.J.; Demeyer, D.I. Manipulation of Ruminal Fermentation. In The Rumen Microbial Ecosystem, 2nd ed.; Hobson, P.N., Stewart, C.S., Eds.; Chapmam and Hall: London, UK, 1997; pp. 523–632. [Google Scholar]
- Hook, S.E.; Northwood, K.S.; Wright, D.G.; McBride, B.W. Long-term monensin supplementation does not significantly affect the quantity or diversity of methanogens in the rumen of the lactating dairy cow. Appl. Env. Microbiol 2009, 75, 374–380. [Google Scholar]
- Asanuma, N.; Iwamoto, M.; Hino, T. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci 1999, 82, 780–787. [Google Scholar]
- Li, S.-H.; Zhang, H.-J.; Qiu, S.-X.; Niu, X.-M.; Santarsiero, B.D.; Mesecar, A.D.; Fong, H.H.S.; Farnsworth, N.R.; Sun, H.-D. Vitexlactam A, a novel labdane diterpene lactam from the fruits of Vitex agnus -castus. Tetrahedron Lett 2002, 43, 5131–5134. [Google Scholar]
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 6th Ed ed; NCCLS document M7-A6; Volume 23, National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003.
- Bicalho, B.; Gonçalves, R.A.C.; Zibordi, A.P.M.; Manfiio, G.P.; Marsaioli, A.J. Antimicrobial compounds of fungi vectored by Clusia ssp. (Clusiaceae) pollinating bees. Z. Naturforsch 2003, 58c, 746–751. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; Mcallan, A.B. A simple gas production method using a pressure transducer to determine fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol 1994, 48, 185–197. [Google Scholar]
- Mass, R.A.; Lardy, G.P.; Grant, R.J.; Klopfenstein, T.J. In situ neutral detergent insoluble nitrogen as a method for measuring forage protein degradability. J. Anim. Sci 1999, 77, 1565–1571. [Google Scholar]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS® System for Mixed Models, 2nd Ed ed; SAS Institute, Inc: Cary, NC, USA; p. 2006.

| Part used | Extract (Method) | Susceptible Microorganisms | Concentration (MIC) | Reference |
|---|---|---|---|---|
| Leaves | Aqueous (maceration) | Xanthomonas campestris | 50 g of leaves in 100 mL H2O | [29] |
| Leaves | Aqueous * | Fungi: | [25] | |
| Allescheria boydii | 10 g/mL | |||
| Aspergillus niger | 7.5 g/mL | |||
| Aspergillus fumigatus | 10 g/mL | |||
| Aspergillus flavus | 15 g/mL | |||
| Candida albicans | 0.5 g/disc | |||
| Candida tropicalis | 0.5 g/disc | |||
| Leaves | Methanolic | Gram-positive Bacteria: | [30] | |
| Staphylococcus aureus | 1 g/disc | |||
| Bacillus subtilis | ||||
| Sarcina lutea | ||||
| Streptococcus pyogenes | ||||
| Streptococcus faecalis | 10 g/disc | |||
| Gram-negative Bacteria: | ||||
| Escherichia coli | 1 g/disc | |||
| Klebsiela pneumonia | ||||
| Samonella typhi | ||||
| Proteus ulgaris | ||||
| Fungi: | ||||
| Candida albicans | 30 g/disc | |||
| Rhizopus nigricans | 30 g/disc | |||
| Aspergillus flavus | 40 g/disc | |||
| Aspergillus nidulans | 20 g/disc | |||
| Leaves | Hydroalcoholic (maceration) | Neisseria gonorrhoeae | 50 mg dried leaves/disc | [31] |
| Pods | Alkaloid Rich Fraction * | Gram-negative Bacteria: | [34] | |
| Escherichia coli | 75 g/mL | |||
| Klebsiella pneumonia | 75 g/mL | |||
| Pseudomonas putida | 50 g/mL | |||
| Pods | Basic Chloroformic * | Gram-positive Bacteria: | Present study | |
| Micrococcus luteus | 25 g/mL | |||
| Staphylococcus aureus | 50 g/mL | |||
| Streptococcus mutans | 50 g/mL | |||
| Alkaloid | Part | Susceptible Microorganisms | Concentration | Reference |
|---|---|---|---|---|
| Julifloricine | Leaves | Gram-positive Bacteria: | [28] | |
| Staphylococcus aureus | 1 g/mL | |||
| Staphylococcus citrus | ||||
| Staphylococcus epidermidis | ||||
| Staphylococcus pyogenes | ||||
| Sarcita lutea | ||||
| Staphylococcus faecalis | 5 g/mL | |||
| Staphylococcus pneumoniae | ||||
| Staphylococcus lactis | ||||
| Corynebacterium diphtheriae | ||||
| Corynebacterium hofmanii | ||||
| Bacillus subtilis | ||||
| Fungi: | ||||
| Candida albicans | 2.5 g/mL | |||
| Candida tropicalis | 1.0 g/mL | |||
| Juliprosinene | Leaves | Bacteria: | [24] | |
| Escherichia coli | ||||
| Klebsiella pneumoniae | ||||
| Pseudomonas aeruginosa | N.D. * | |||
| Staphylococcus aureus | ||||
| Shigella sonnei | ||||
| Juliflorine (Juliprosopine) | Leaves | Gram-positive Bacteria: | [32] | |
| Streptococcus pyogenes | 1 to 30 g/mL | |||
| Staphylococcus aureus | ||||
| Corynebacterium diphtheriae | ||||
| Corynebacterium hofmanni | ||||
| Bacillus subtilis | ||||
| Streptococcus faecalis | ||||
| Fungus: Candida sp. | 0.5 to 5 g/mL | |||
| Dermatophyte Fungi | 2.5 g/mL | |||
| Protozoa: | ||||
| Entamoeba histolytica | 10 g/mL | |||
| Microorganisms | BCE | BAE | Chloramphenicol a | Loprox b |
|---|---|---|---|---|
| M. luteus | 25 | >100 | 0.8 | - |
| S. aureus | 50 | >100 | 6.3 | - |
| S. mutans | 50 | >100 | 6.3 | - |
| B. subtilis | >100 | >100 | 6.3 | - |
| E. coli | >100 | >100 | 3.1 | - |
| S. choleaesuis | >100 | >100 | 6.3 | - |
| P. aeruginosa | >100 | >100 | 100 | - |
| A. Niger | >100 | >100 | - | 12.5 |
| C. cladosporioides | >100 | >100 | - | 6.3 |
| C. albicans | >100 | >100 | - | 6.3 |
| Carbon | Literature [24,26,40] | BCE | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 1″″ | 33.2 | 32.4 | 32.4 | 33.2 | 32.3 | 32.3 |
| 2″″ | 21.5 | 21.3 | 21.4 | 21.4 | 21.3 | 21.4 |
| 3″″ | 54.5 | 59.8 | 59.8 | 54.5 | 60.5 | 59.8 |
| 5″″ | 55.3 | 138.9 | 139.0 | 55.2 | 138.7 | 138.9 |
| 6″″ | 136.3 | 139.1 | 139.0 | 136.0 | 138.9 | 138.9 |
| 7″″ | 123.8 | 144.0 | 144.0 | 123.9 | 143.9 | 143.9 |
| 8″″ | 42.6 | 141.9 | 141.8 | 42.5 | 141.7 | 141.7 |
| 8a″″ | 65.5 | 154.2 | 154.0 | 65.5 | 154.1 | 154.1 |
| 2,2′ | 57.2 | 57.3 | 57.2 | 57.1 | 57.1 | 57.1 |
| 3,3′ | 67.8 | 67.6, 77.3 | 67.2 | 67.7 | 67.7, 77.4 | 67.3 |
| 4,4′ | 32.2 | 32.0, 31.7 | 31.8 | 32.3 | 31.9, 31.7 | 31.9 |
| 5,5′ | 26.2 | 25.7 | 25.6 | 26.6 | 25.6 | 25.5 |
| 6,6′ | 55.7 | 55.9 | 55.8 | 55.8 | 55.8 | 55.8 |
| 1″,1‴ | 37.1 | 36.2 | 36.1, 36.0 | 36.6 | 36.1 | 36.1, 35.9 |
| 2″,2‴ | 25.8 | 25.6 | 25.1, 25.0 | 25.8 | 25.6 | 25.1, 24.9 |
| 3″,8″ | 30.0–29.4 | 31.9–28.9 | 32.0, 30.8 | 30.0–28.8 | 30.0–28.8 | 31.9, 30.7 |
| 3‴–8‴ | 30.0–29.4 | 31.9–28.9 | 30.5–29.0 | 30.0–28.8 | 30.0–28.8 | 30.0–28.8 |
| 9″,9‴ | 26.6 | N.A.* | N.A.* | 26.6 | N.A.* | N.A.* |
| 10″ | 35.1 | N.A.* | N.A.* | 35.2 | N.A.* | N.A.* |
| 10‴ | 28.0 | N.A.* | N.A.* | 27.9 | N.A.* | N.A.* |
| 7,7′ | 18.7 | 18.1, 17.9 | 18.1, 18.0 | 18.4 | 18.0, 17.9 | 18.0, 17.9 |
| Treatment | Kinetic Parameters of Gas Production * | ||||
|---|---|---|---|---|---|
| VFF 1 | KdFF 2 | L 3 | VSF 4 | KdSF 5 | |
| BCE 0 | 43.31 ± 0.69 a | 0.17 ± 0.01 a | 6.55 ± 0.22 c | 96.73 ± 3.46 a | 0.0434 ± 0.0005 c |
| BCE 25 | 38.53 ± 2.58 a | 0.19 ± 0.01 a | 6.93 ± 0.27 b,c | 81.67 ± 13.58 a | 0.0453 ± 0.0005 b,c |
| BCE 50 | 33.75 ± 1.44 a | 0.20 ± 0.01 a | 6.99 ± 0.25 b,c | 90.72 ± 2.38 a | 0.0497 ± 0.0005 b |
| BCE 100 | 34.76 ± 1.21 a | 0.19 ± 0.01 a | 7.16 ± 0.10 b,c | 89.56 ± 2.99 a | 0.04933 ± 0.00009 b |
| BCE 200 | 36.92 ± 3.81 a | 0.16 ± 0.01 a | 8.15 ± 0.54 b | 86.28 ± 7.64 a | 0.0445 ± 0.0019 c |
| Mon | 20.12 ± 0.62 b | 0.26 ± 0.07 a | 10.90 ± 0.32 a | 106.53 ± 1.30 a | 0.056 ± 0.001 a |
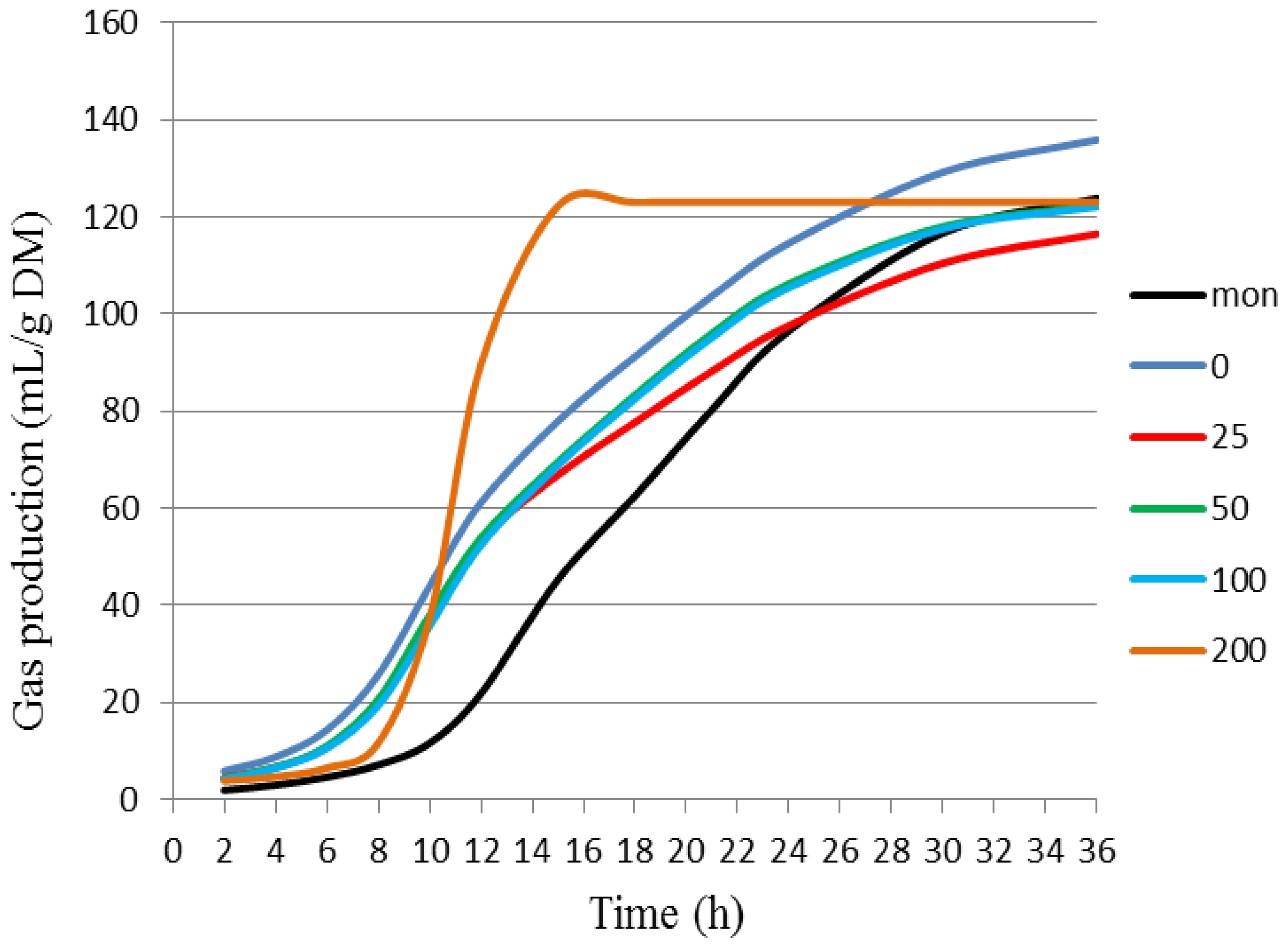
| Treatment | Cumulative Gas Production (mL/g of DM) * | |||||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 18 h | 24 h | 36 h | ||
| BCE | 0 | 15.31 ± 1.26 a | 60.17 ± 1.52 a | 91.76 ± 1.75 a | 114.22 ± 2.63 a | 137.79 ± 2.80 a |
| 25 | 11.00 ± 2.08 b | 53.23 ± 2.06 b | 81.21 ± 3.61 b | 100.44 ± 5.80 b,c | 120.89 ± 7.70 b | |
| 50 | 11.52 ± 0.67 b | 52.92 ± 1.07 b | 83.39 ± 0.67 b | 106.15 ± 1.07 b | 124.28 ± 1.31 b | |
| 100 | 10.81 ± 0.55 b | 51.66 ± 1.62 c | 82.89 ± 2.21 b | 105.64 ± 2.61 b | 123.48 ± 2.96 b | |
| 200 | 8.73 ± 1.39 c | 40.31 ± 3.10 d | 74.79 ± 2.75 b | 97.27 ± 3.75 c | 120.32 ± 4.00 b | |
| Mon | 2.69 ± 0.36 d | 24.78 ± 1.88 e | 61.84 ± 1.98 c | 96.51 ± 1.53 c | 126.08 ± 1.19 b | |
| Treatment | TDDM (g/100 g DM) * | |
|---|---|---|
| 18 h | 36 h | |
| BCE 0 | 69.25 ± 0.81 a | 72.72 ± 0.34 a |
| BCE 25 | 70.61 ± 0.63 a | 71.77 ± 1.34 a |
| BCE 50 | 69.54 ± 0.65 a | 73.07 ± 0.11 a |
| BCE 100 | 69.15 ± 0.64 a | 72.38 ± 0.35 a |
| BCE 200 | 65.73 ± 0.42 b | 71.41 ± 0.04 a |
| Mon | 57.04 ± 0.23 c | 62.21 ± 0.58 b |
| Treatment | DMMP (mg/100 mg Degradable DM) * | |
|---|---|---|
| 18 h | 36 h | |
| BCE 0 | 16.08 ± 0.72 a | 7.69 ± 0.62 a |
| BCE 25 | 18.85 ± 1.38 a | 8.46 ± 0.33 a |
| BCE 50 | 12.50 ± 0.05 b | 8.40 ± 0.24 a |
| BCE 100 | 12.32 ± 0.70 b | 8.71 ± 1.95 a |
| BCE 200 | 11.81 ± 0.10 b | 10.93 ± 2.06 a |
| Mon | 23.90 ± 0.64 a | 18.01 ± 1.64 b |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dos Santos, E.T.; Pereira, M.L.A.; Da Silva, C.F.P.G.; Souza-Neta, L.C.; Geris, R.; Martins, D.; Santana, A.E.G.; Barbosa, L.C.A.; Silva, H.G.O.; Freitas, G.C.; et al. Antibacterial Activity of the Alkaloid-Enriched Extract from Prosopis juliflora Pods and Its Influence on in Vitro Ruminal Digestion. Int. J. Mol. Sci. 2013, 14, 8496-8516. https://doi.org/10.3390/ijms14048496
Dos Santos ET, Pereira MLA, Da Silva CFPG, Souza-Neta LC, Geris R, Martins D, Santana AEG, Barbosa LCA, Silva HGO, Freitas GC, et al. Antibacterial Activity of the Alkaloid-Enriched Extract from Prosopis juliflora Pods and Its Influence on in Vitro Ruminal Digestion. International Journal of Molecular Sciences. 2013; 14(4):8496-8516. https://doi.org/10.3390/ijms14048496
Chicago/Turabian StyleDos Santos, Edilene T., Mara Lúcia A. Pereira, Camilla Flávia P.G. Da Silva, Lourdes C. Souza-Neta, Regina Geris, Dirceu Martins, Antônio Euzébio G. Santana, Luiz Cláudio A. Barbosa, Herymá Giovane O. Silva, Giovana C. Freitas, and et al. 2013. "Antibacterial Activity of the Alkaloid-Enriched Extract from Prosopis juliflora Pods and Its Influence on in Vitro Ruminal Digestion" International Journal of Molecular Sciences 14, no. 4: 8496-8516. https://doi.org/10.3390/ijms14048496
APA StyleDos Santos, E. T., Pereira, M. L. A., Da Silva, C. F. P. G., Souza-Neta, L. C., Geris, R., Martins, D., Santana, A. E. G., Barbosa, L. C. A., Silva, H. G. O., Freitas, G. C., Figueiredo, M. P., De Oliveira, F. F., & Batista, R. (2013). Antibacterial Activity of the Alkaloid-Enriched Extract from Prosopis juliflora Pods and Its Influence on in Vitro Ruminal Digestion. International Journal of Molecular Sciences, 14(4), 8496-8516. https://doi.org/10.3390/ijms14048496





