Synthesis of 1-isopropyl-3-acyl-5-methyl-benzimidazolone Derivatives and Their Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
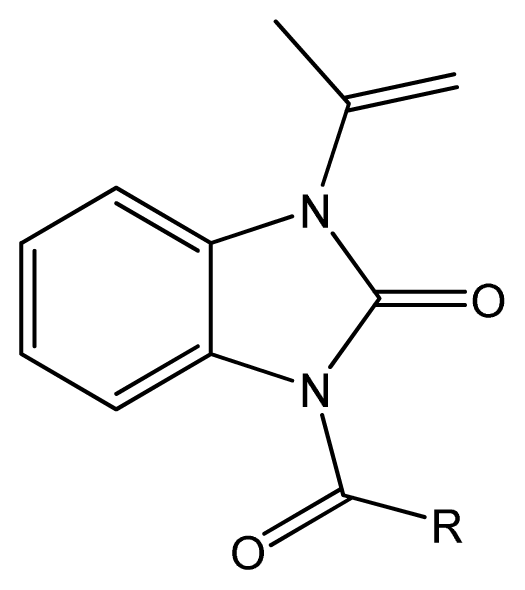
2.1. Chemistry
2.2. Antimicrobial Activities of 1-isopropyl-3-acyl-5-methyl-benzimidazolones
Antibacterial activity
Antifungal activity
3. Experimental Section
3.1. General Experimental Procedures
3.2. Synthetic Procedures
3.2.1. Synthesis of N-isopropyl-4-methyl-2-nitroaniline (2)
3.2.2. Synthesis of N1-isopropyl-4-methylbenzene-1,2-diamine (3)
3.2.3. Synthesis of 1-isopropyl-5-methyl-1H-benzo[d]imidazol-2(3H)-one (4)
3.2.4. Synthesis of 3-acyl-1-isopropyl-5-methyl-benzimidazolones (5-01~5-30)
3.3. Paper Disc-Diffusion Method
3.4. Minimum Inhibitory Concentrations (MICs)
3.5. Spore Germination Assay
4. Conclusions
Acknowledgments
References
- Seki, M.; Mori, K. Synthesis of (-)-cytoxazone, a novel cytokine modulator isolated from Streptomycessp. Eur. J. Org. Chem 1999, 2965–2967. [Google Scholar]
- Liu, W. G.; Lau, F.; Liu, K.; Wood, H. B.; Zhou, G. C.; Chen, Y. L.; Li, Y.; Akiyama, T. E.; Castriota, G.; Einstein, M.; et al. Benzimidazolones: A new class of selective peroxisome proliferator-activated receptor γ (PPARγ) modulators. J. Med. Chem 2011, 54, 8541–8554. [Google Scholar]
- Palin, R.; Clark, J. K.; Evans, L.; Houghton, A. K.; Jones, P. S.; Prosser, A.; Wishart, G.; Yoshiizumi, K. Structure-activity relationships and CoMFA of N-3 substituted phenoxypropyl piperidine benzimidazol-2-one analogues as NOP receptor agonists with analgesic properties. Bioorg. Med. Chem 2008, 16, 2829–2851. [Google Scholar]
- Palin, R.; Bom, A.; Clark, J. K.; Evans, L.; Feilden, H.; Houghton, A. K.; Jones, P. S.; Montgomery, B.; Weston, M. A.; Wishart, G. Synthesis and evaluation of N-3 substituted phenoxypropyl piperidine benzimidazol-2-one analogues as NOP receptor agonists with analgesic and sedative properties. Bioorg. Med. Chem 2007, 15, 1828–1847. [Google Scholar]
- Risi, C. D.; Pollini, G. P.; Trapella, C.; Peretto, I.; Ronzoni, S.; Giardina, G.A.M. A new synthetic approach to 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydrobenzimidazol- 2-one(J-113397), the first non-peptide ORL-1 receptor antagonist. Bioorg. Med. Chem 2001, 9, 1871–1877. [Google Scholar]
- Leibrock, J.; Prucher, H.; Rautenberg, W. EMD 95885, a new eliprodil analogue with higher affinity for the N-methyl-D-aspartate (NMDA) receptor. Pharmazie 1997, 52, 479–480. [Google Scholar]
- Ceccarelli, S. M.; Jaeschke, G.; Buettelmann, B.; Huwyler, J.; Kolczewski, S.; Peters, J.; Prinssen, E.; Porter, R.; Spooren, W.; Vieir, E. Rational design, synthesis and structure-activity relationship of benzoxazolones: new potent mglu5 receptor antagonists based on the fenobam structure. Bioorg. Med. Chem. Lett 2007, 17, 1302–1306. [Google Scholar]
- Sivendran, S.; Jones, V.; Sun, D. Q.; Wang, Y.; Grzegorzewicz, A. E.; Scherman, M. S.; Napper, A. D.; McCammon, J. A.; Lee, R. E.; Diamond, S. L.; McNeil, M. Identification of triazinoindol-benzimidazolones as nanomolar inhibitors of the Mycobacterium tuberculosis enzyme TDP-6-deoxy-D- xylo-4-hexopyranosid-4-ulose 3,5-epimerase (RmlC). Bioorg. Med. Chem. 2010, 18, 896–908. [Google Scholar]
- Monforte, A.; Rao, A.; Logoteta, P.; Ferro, S.; Luca, L. D.; Barreca, M. L.; Iraci, N.; Maga, G.; Clercq, E. D.; Pannecouque, C.; Chimirri, A. Novel N1- substituted 1,3-dihydro-2H-benzimidazol-2-ones as potent non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem 2008, 16, 7429–7435. [Google Scholar]
- Berry, J. F.; Ferraris, D. V.; Duvall, B.; Hin, N.; Rais, R.; Alt, J.; Thomas, A G.; Rojas, C.; Hashimoto, K.; Slusher, B.S.; et al. Synthesis and SAR of 1-Hydroxy-1H-benzo[d]imidazol-2(3H)- ones as inhibitors of D-amino acid oxidase. ACS Med. Chem. Lett. 2012, 3, 839–843. [Google Scholar]
- Bruncko, M.; Tahir, S. K.; Song, X. H.; Chen, J; Ding, H.; Huth, J. R.; Jin, S.; Judge, R. A.; Madar, D. J.; Park, C.H.; et al. N-Aryl-benzimidazolones as novel small molecule HSP90 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 7503–7506. [Google Scholar]
- Messaoudi, S.; Sancelme, M.; Polard-Housset, V.; Aboab, B.; Moreau, P.; Prudhomme, M. Synthesis and biological evaluation of oxindoles and benzimidazolinones derivatives. Eur. J. Med. Chem 2004, 39, 453–458. [Google Scholar] [Green Version]
- Vira, J. J.; Patel, D. R.; Bhimani, N. V.; Ajudia, P.V. Synthesis and biological evaluation of potent benzimidazolone derivatives. Der Pharma Chemica 2010, 2, 178–183. [Google Scholar]
- Li, S. K.; Ji, Z. Q.; Zhang, J. W.; Guo, Z. Y.; Wu, W.J. Synthesis of 1-acyl-3- isopropenyl benzimidazolone derivatives and their activity against Botrytis cinerea. J. Agric. Food Chem 2010, 58, 2668–2672. [Google Scholar]
- Wei, S. P.; Wu, W. J.; Ji, Z.Q. Synthesis and antibacterial activities of 1-alkyl-3-methacryloyl (acryloyl) of benzimidazolone (thione) derivatives. Int. J. Mol. Sci 2012, 13, 4819–4830. [Google Scholar]
- Li, F. F.; Wei, S. P.; Zong, Z. F.; Ji, Z.Q. Synthesis and antimicrobial activity of 1-acyl benzimidazolone amide derivatives. Chin. J. Pest. Sci 2012, 14, 597–601. [Google Scholar]
- Yang, D. L.; Kefi, S.; Audinot, V.; Millan, M. J.; Langlois, M. Benzamides derived from 1,2-diaminocyclopropane as novel ligands for human D2 and D3 dopamine receptors. Bioorg. Med. Chem 2000, 8, 321–327. [Google Scholar]
- Zhu, H.; Huang, W. Z.; Pu, J.Q. Synthesis of benzimidazoles from o-nitroanilines in a one-step reductive cyclization process. J. Shanghai Univ. (Nat. Sci. ) 2007, 13, 77–81. [Google Scholar]
- Buckman, B.; Nicholas, J. B.; Beigelman, L.; Serebryany, V.; Stoycheva, A. D.; Thrailkill, T.; Seiwert, S.D. Cyclic peptide inhibitors of hepatitis C virus replication. PCT Int. Appl WO 2011038293 A1 20110331, 2011. [Google Scholar]
- Ferreira, I. C.; Calhelha, R. C.; Estevinho, L. M.; Queiroz, M.R. Screening of antimicrobial activity of diarylamines in the 2,3,5-trimethylbenzo[b]thiophene series: a structure-activity evaluation study. Bioorg. Med. Chem. Lett 2004, 14, 5831–5833. [Google Scholar]
- Ji, Z. Q.; Wang, M. A.; Zhang, J. W.; Wei, S. P.; Wu, W.J. Two New Members of Streptothricin Class Antibiotics from Streptomyces qinlingensis sp. Nov. J. Antibiot 2007, 60, 739–744. [Google Scholar]
- Lee, S. K.; Sohn, H. B.; Kim, G. G.; Chung, Y.R. Enhancement of biological control of Botrytis cinerea on cucumber by foliar sprays and bed potting mixes of Trichoderma harzianum YC459 and its application on tomato in the greenhouse. Plant Pathol 2006, 22, 283–288. [Google Scholar]

| Compounds | Zone of inhibition (mm)a | Inhibition rate (%)b | ||||
|---|---|---|---|---|---|---|
| B. cereus | B. subtilis | S. aureus | E. coli | P. aeruginosa | B. cinerea | |
| 5-01 | — | — | — | — | — | 40.38 |
| 5-02 | 11 (+++) | 10 (+++) | 10 (+) | 10 (++) | 11 (+) | 71.54 |
| 5-03 | — | — | — | — | — | 37.06 |
| 5-04 | — | — | — | — | — | 51.92 |
| 5-05 | — | — | — | — | — | 43.24 |
| 5-06 | — | — | — | — | — | 47.69 |
| 5-07 | 14 (+++) | 16 (+++) | 12 (+++) | 11 (++) | 10 (++) | 80.58 |
| 5-08 | — | — | — | — | — | 38.46 |
| 5-09 | — | — | — | — | — | 47.12 |
| 5-10 | — | — | — | — | — | 29.71 |
| 5-11 | — | — | — | — | — | 41.28 |
| 5-12 | 10 (++) | 8 (++) | — | — | — | 93.65 |
| 5-13 | — | — | — | — | — | 17.65 |
| 5-14 | 13 (++) | 11 (++) | 8 (++) | — | — | 44.12 |
| 5-15 | 15 (+++) | 15 (+++) | 12 (+++) | 10 (+) | 10 (++) | 73.18 |
| 5-16 | — | — | — | — | — | 17.06 |
| 5-17 | 10 (++) | 12 (++) | 11 (++) | — | — | 47.31 |
| 5-18 | — | — | — | — | — | 53.08 |
| 5-19 | 17 (+++) | 15 (+++) | 10 (+++) | 11 (++) | 11 (++) | 88.46 |
| 5-20 | 9 (++) | 8 (+++) | 8 (++) | 11 (+) | 9 (++) | 75.77 |
| 5-21 | 8 (++) | 9 (++) | 9 (++) | — | — | 44.87 |
| 5-22 | 8 (++) | 10 (++) | 11 (++) | — | — | 48.08 |
| 5-23 | — | — | — | — | — | 45.88 |
| 5-24 | — | — | — | — | — | 43.24 |
| 5-25 | 9 (++) | 9 (++) | 11 (++) | 11 (+) | 11 (+) | 77.95 |
| 5-26 | 11 (++) | 10 (++) | 12 (+) | 11 (+) | 10 (+) | 32.35 |
| 5-27 | — | — | — | — | — | 55.00 |
| 5-28 | — | — | — | — | — | 42.35 |
| 5-29 | — | — | — | — | — | 42.31 |
| 5-30 | — | — | — | — | — | 47.44 |
| Ampicillin | 25 (+++) | 26 (+++) | 20 (+++) | 18 (++) | 25 (+++) | / |
| Azoxystrobin | / | / | / | / | / | 100.00 |
| Compounds | MICs (μg/mL) | EC50 (μg/mL) | ||||
|---|---|---|---|---|---|---|
| B. cereus | B. subtilis | S. aureus | E. coli | P. aeruginosa | B. cinerea | |
| 5-02 | 100 | 100 | 100 | >100 | >100 | 21.07 |
| 5-07 | 25.0 | 12.5 | 25.0 | 50.0 | 100.0 | 17.62 |
| 5-12 | 100 | 100 | 100 | >100 | >100 | 10.68 |
| 5-14 | 100 | 100 | 100 | >100 | >100 | / |
| 5-15 | 50.0 | 50.0 | 100.0 | 100.0 | 100.0 | 19.75 |
| 5-17 | 100 | >100 | >100 | >100 | >100 | / |
| 5-19 | 6.25 | 12.5 | 12.5 | 50.0 | 100.0 | 14.23 |
| 5-20 | 100 | >100 | >100 | >100 | >100 | 17.33 |
| 5-21 | 100 | >100 | >100 | >100 | >100 | / |
| 5-22 | 100 | >100 | >100 | >100 | >100 | / |
| 5-25 | 100 | >100 | >100 | >100 | >100 | 16.39 |
| 5-26 | 100 | >100 | >100 | >100 | >100 | / |
| Ampicillin | 12.5 | 3.13 | 6.25 | 3.13 | 25.0 | / |
| Azoxystrobin | / | / | / | / | / | 1.53 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, N.; Yang, C.; Gan, X.; Wei, S.; Ji, Z. Synthesis of 1-isopropyl-3-acyl-5-methyl-benzimidazolone Derivatives and Their Antimicrobial Activity. Int. J. Mol. Sci. 2013, 14, 6790-6804. https://doi.org/10.3390/ijms14046790
Xu N, Yang C, Gan X, Wei S, Ji Z. Synthesis of 1-isopropyl-3-acyl-5-methyl-benzimidazolone Derivatives and Their Antimicrobial Activity. International Journal of Molecular Sciences. 2013; 14(4):6790-6804. https://doi.org/10.3390/ijms14046790
Chicago/Turabian StyleXu, Nan, Chunnan Yang, Xinqi Gan, Shaopeng Wei, and Zhiqin Ji. 2013. "Synthesis of 1-isopropyl-3-acyl-5-methyl-benzimidazolone Derivatives and Their Antimicrobial Activity" International Journal of Molecular Sciences 14, no. 4: 6790-6804. https://doi.org/10.3390/ijms14046790
APA StyleXu, N., Yang, C., Gan, X., Wei, S., & Ji, Z. (2013). Synthesis of 1-isopropyl-3-acyl-5-methyl-benzimidazolone Derivatives and Their Antimicrobial Activity. International Journal of Molecular Sciences, 14(4), 6790-6804. https://doi.org/10.3390/ijms14046790




