Abstract
Phospholipases A2 (PLA2s) are known to mediate signaling cascades during plant growth and development, as well as biotic and abiotic stress responses. In this context, the present study provides extensive characterization of specific PLA2s in durum wheat, and assesses their involvement in durum wheat response to drought stress. In durum wheat leaves, four full-length expressed sequences encoding putative PLA2s were isolated and characterized as belonging to the class of secretory PLA2s (sPLA2s): TdsPLA2I, TdsPLA2II, TdsPLA2III and TdsPLA2IV. PLA2 activity was also detected, the characteristics of which resemble those of previously characterized plant sPLA2s: strong preference for phospholipids; requirement for millimolar Ca2+ concentrations; optimal activity at basic pH; heat stability; and inhibition by the reducing agent dithiothreitol. With drought stress imposed at both the vegetative and reproductive stages, accumulation of TdsPLA2I and TdsPLA2III transcripts, and to a lesser extent of TdsPLA2IV transcript, paralleled increased PLA2 activity; both transcript levels and enzymatic activity decreased as a consequence of stress recovery. Consistently, free fatty acid analysis of drought-stressed leaves revealed increased linoleate, linolenate and palmitate contents, which were reversed by plant re-watering. Overall, these findings strongly suggest that there are inducible sPLA2 isoforms in durum wheat that have roles in orchestrating the plant response to drought stress.
1. Introduction
To adapt growth and metabolism to variations in environmental conditions, plants have developed complex signaling networks to perceive environmental stimuli and then transduce the information across the plasma membrane into the cell, where it activates a specific signaling cascade. In this context, the production of lipid mediators triggered by phospholipases through the hydrolysis of membrane phospholipids has a pivotal role in plant response to environmental stress [1].
In plants, as in other organisms, there are three major classes of phospholipases that are distinguished based on their substrate cleavage site: phospholipase C (PLC), phospholipase D (PLD) and phospholipases A (PLA) [2]. While the roles of both PLC and PLD as important signaling enzymes under stress conditions have been extensively investigated [1], information about the deacylating enzymes remains very limited.
PLA2 (phosphatide 2-acylhydrolase, EC 3.1.1.4) is a deacylating enzyme that specifically hydrolyses glycerophospholipids at the sn-2 position to yield free fatty acids (FFAs) and lysophospholipids (LPLs). Both of these reaction products represent precursors for signaling molecules that are biologically active in a wide range of physiological and pathological processes. Currently, the PLA2 superfamily consists of several groups of enzymes that are classified into five different types on the basis of their amino acid sequence, molecular weight, Ca2+-dependence, and cell localization: the low-molecular-weight, secretory PLA2s (sPLA2s); the cytosolic, Ca2+-dependent PLA2s (cPLA2s); the cytosolic, Ca2+-independent PLA2s (iPLA2s); the platelet-activating factor acylhydrolases; and the lysosomal PLA2s [3].
In plants, to the best of our knowledge, the only specific PLA2s discovered belong to the type of sPLA2s. Less specific patatin-like PLAs have been described that show low positional specificity for the sn-1 and sn-2 C atom in their phospholipid substrates, and the ability to also hydrolyze galactolipids [4]. Even after sequencing the entire genomes of various plant species, no cPLA2-like genes have yet been found.
As far as the specific sPLA2s are concerned, these were first purified from elm seeds [5] and rice shoots [6], and more recently from castor bean leaves [7]. The cDNA for putative sPLA2s from rice shoots and carnation flowers were the first ones to be cloned [6,8], followed by the four sPLA2s of A. thaliana[9] and, very recently, by the five sPLA2s of G. max[10]. With progress in genome sequencing, the number of plant sPLA2s identified is steadily increasing. As for their animal counterparts, the plant sPLA2s are characterized by low molecular weights (13–18 kDa) and contain conserved regions, including twelve Cys residues that form six intramolecular disulfide bridges, a His residue in the catalytic site, a Ca2+-binding loop and a signal peptide for secretion [9]. According to the criteria established for animal sPLA2s, plant sPLA2s are classified as a separate group, group XI, that is divided into two clusters, XIA and XIB, on the basis of the amino acid sequence identities [3].
Plant PLA2s are known to be involved in a number of important physiological and pathological processes [11]. Nevertheless, in most cases, the molecular identities of the enzymes that contribute to these processes remain unknown. Thus, activities not assigned to a specific class of the PLA2s have been shown to have roles in plant responses to auxin, wounding, pathogen attack and elicitors [11], programmed cell death [12] and cold acclimation [13]. In other cases, this information relates to non-specific lipid acyl hydrolases, such as the patatin-like PLAs, that have been shown to have roles in cellulose deposition and cell elongation [14–16], as well as in signaling cascades triggered by wounding, pathogens and elicitors, and in abiotic stress responses [4].
With regard to specific sPLA2s, numerous lines of evidence have demonstrated roles for this class of enzymes in flowering [8], auxin-mediated cell elongation and shoot gravitropism [17], light signal transduction in guard cells [18], PIN protein trafficking [19], pollen development and germination [20]. Only recently has the first evidence been reported about the involvement of this class of PLA2s in plant responses to both biotic and abiotic stresses. sPLA2s have been found to be involved in plant response to both bacterial [21] and yeast [22] infections. Moreover, Ryu and co-workers demonstrated that over-expression of the AtsPLA2α and AtsPLA2β genes in transgenic plants of A. thaliana and N. tabacum increases their resistance not only to a variety of pathogen infections, but also to salt stress [23]. In line with these findings, Singh and co-workers reported the up-regulation of the OssPLA2α gene in rice seedlings exposed to drought stress [24].
Among the environmental stresses, water deficit represents one of the major factors that limit plant growth and productivity. Durum wheat cultivation faces drought conditions very often in various arid and semi-arid regions, such as the Mediterranean regions that produce some 75% of the world’s durum grain [25]. Thus, in order to sustain the yields and yield stability of this important crop species, adaptation to drought stress represents an important breeding target. In this context, there is the need for the identification of the mechanisms involved in durum wheat responses to water deficit. Therefore, the aim of the present study was to investigate the existence, in durum wheat, of specific sPLA2s and to establish their roles in durum wheat adaptation to drought stress.
2. Results
2.1. Isolation and Characterization of Durum Wheat Full-Length sPLA2 cDNAs and Gene Expression Analysis in Different Tissues
The full-length sPLA2 transcripts were isolated after amplification of cDNA from the youngest fully expanded leaves of durum wheat plants at the tillering stage. Four expressed sequences were isolated that ranged between 420 and 513 bp (from start to stop codon), and they were designated as TdsPLA2I, TdsPLA2II, TdsPLA2III and TdsPLA2IV (GenBank: JX021445, JX021446, JX021447 and JX021448, respectively) on the basis of their similarities with the coding sequences deduced from the Os02g0831700 (OssPLA2I), Os03g0261100 (OssPLA2II), Os03g0708000 (OssPLA2III) and Os11g0546600 (OssPLA2IV) genes.
Alignment of the deduced amino acid sequences of the four TdsPLA2s with some other known plant sPLA2s (Figure 1A) revealed that, analogous to the corresponding isoforms in rice [26], TdsPLA2I can be assigned to the cluster XIA, whereas the three other isoforms belong to the cluster XIB. As shown in Figure 1B, TdsPLA2I, TdsPLA2II, TdsPLA2III and TdsPLA2IV sequence lengths were 141, 157, 162 and 170 amino acids, respectively, with the molecular masses ranging from 15 to 17 kDa. All of the deduced proteins have typical features that have already been identified in other sPLA2s from both animal and plant sources (Figure 1B,C) [9]: (i) the signal peptide for secretion in the N-terminal region; (ii) the PLA2 signature domain that includes the Ca2+-binding loop and the catalytic site; and (iii) twelve Cys residues that have the potential to form six intramolecular disulfide bridges. Sequence alignment also revealed that, paralleling the OssPLA2 isoforms [26], the Asp residue of the highly conserved His/Asp catalytic dyad of the animal counterpart is replaced by a His residue in the durum wheat sPLA2 isoform I, and by an Asn residue in all of the other durum wheat sPLA2 isoforms. The intra-TdsPLA2 sequence comparisons yielded between 48.0% and 64.8% identity at the transcript level and between 24.7% and 49.7% identity at the protein level, with the TdsPLA2I isoform showing the lowest identity with all of the other isoforms at both transcript and amino acid level. This is expected, as this is the only durum wheat sPLA2 isoform that belongs to the cluster XIA. In spite of the low identity shared by these overall amino acid sequences, the conserved regions corresponding to the Ca2+-binding loop and the catalytic site were found to share 54.5% and 72.7% identities, respectively, among the four durum wheat sPLA2 isoforms.
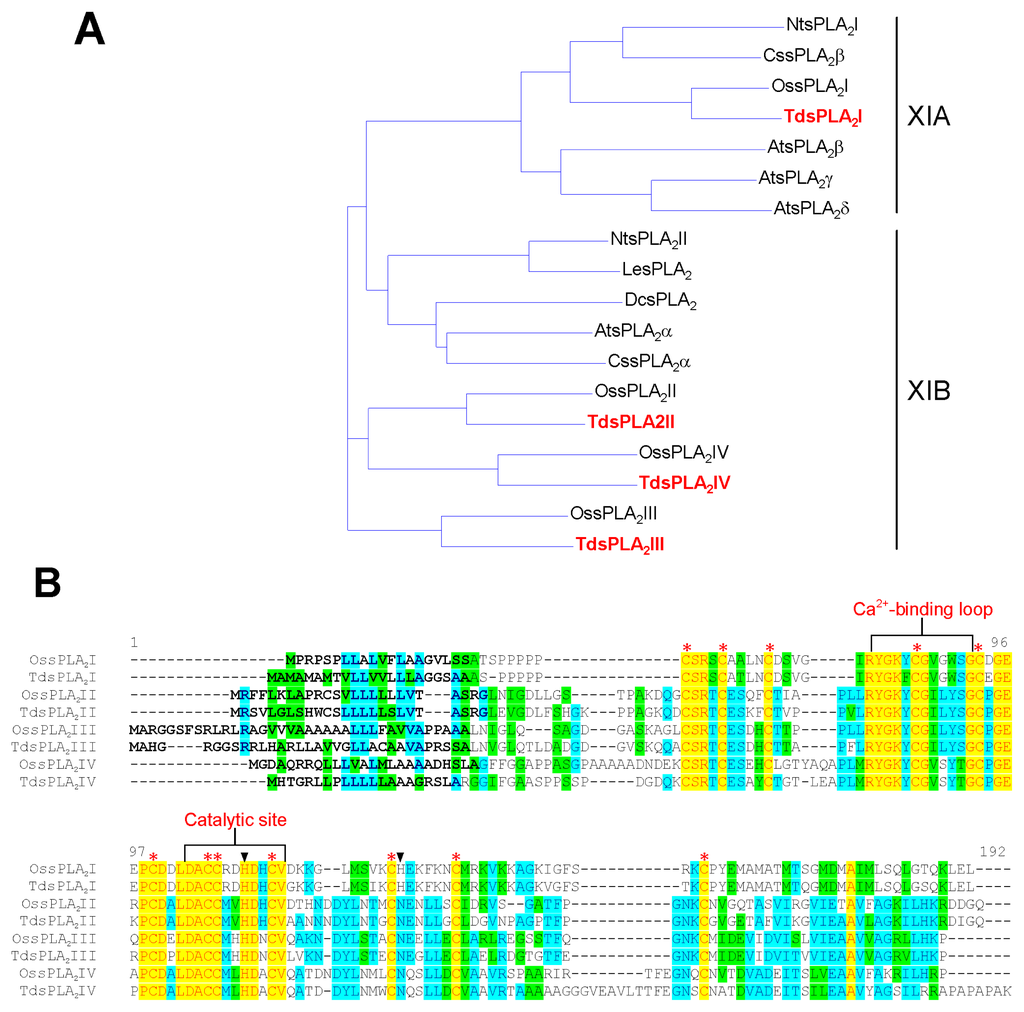
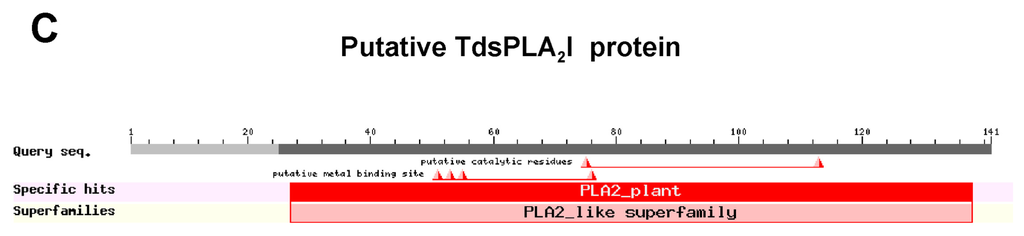
Figure 1.
Analysis of the deduced amino acid sequences of sPLA2s from durum wheat leaves. (A) Phylogenetic tree of the deduced amino acid sequences of the four TdsPLA2s and some other plant sPLA2s. The GenBank accession numbers are as follows: A. thaliana isoform α (AtsPLA2α), β (AtsPLA2β), γ (AtsPLA2γ) and δ (AtsPLA2δ), At2g06925, At2g19690, At4g29460 and At4g29470, respectively; carnation (DcsPLA2), AF064732; durum wheat isoform I (TdsPLA2I), II (TdsPLA2II), III (TdsPLA2III) and IV (TdsPLA2IV), JX021445, JX021446, JX021447 and JX021448, respectively; orange isoform α (CssPLA2α) and β (CssPLA2β), GU075396 and GU075398, respectively; rice isoform I (OssPLA2I), II (OssPLA2II), III (OssPLA2III) and IV (OssPLA2IV), Os02g0831700, Os03g0261100, Os03g0708000 and Os11g0546600, respectively; tobacco isoform I (NtsPLA2I) and II (NtsPLA2II), AB190177 and AB190178, respectively; tomato (LesPLA2), AI487873. (B) Alignments between the deduced amino acid sequences of rice and durum wheat sPLA2s. The conserved domains with homology to the Ca2+-binding loop and the active site motifs of other known plant sPLA2s are indicated. Each of the twelve conserved Cys residues is marked with an asterisk. Triangles indicate the catalytic dyad. The signal peptides are highlighted in bold. The program used to produce phylogenetic tree and sequence alignment was the Vector NTI Suite software (version 9.0; Life Technology, Carlsbad, CA, USA). (C) Typical domains in TdsPLA2I as identified by the NCBI Conserved Domain Search Database [27]. The same result was obtained by using as query the amino acid sequences deduced from the other three TdsPLA2 isoforms.
As shown in Figure 2, the four TdsPLA2 genes were expressed in durum wheat tissues, although at different levels and with different tissue specificities. TdsPLA2I was expressed in all of the plant organs analyzed, with the highest levels in root and culm. TdsPLA2II was expressed in root and culm, and at very high levels in leaf and glume; in contrast, the expression levels of the TdsPLA2II gene were very low, and even absent, in awn and seed. Transcripts corresponding to TdsPLA2III were more abundant in root, but were also present in culm, glume and seed, and to a lesser extent, in leaf and awn. TdsPLA2IV was expressed at high levels in roots, glume and awn, whereas there were low TdsPLA2IV transcript levels detected in culm and seed.
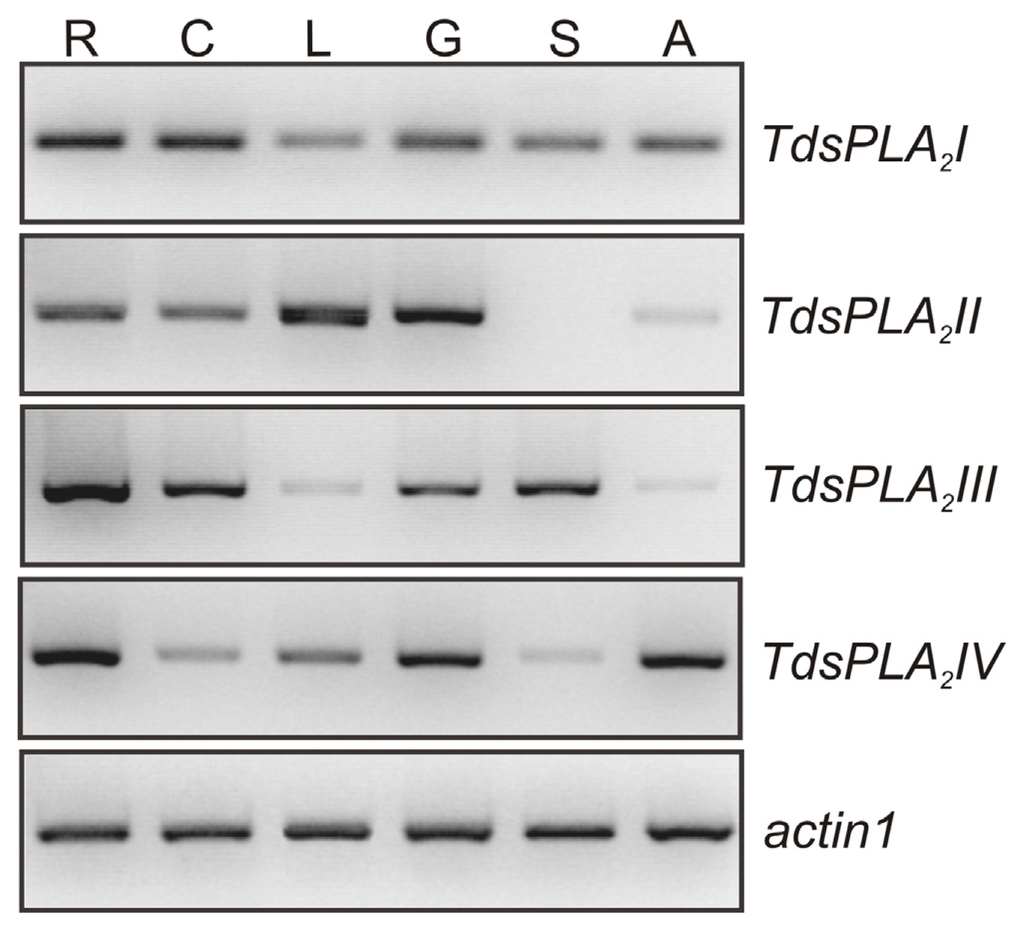
Figure 2.
Tissue-specific expression of the TdsPLA2 genes. Representative expression analysis of TdsPLA2I, TdsPLA2II, TdsPLA2III, TdsPLA2IV and actin1 genes carried out by using the specific primer pairs reported in Table 1. The figures of gel separation are presented in inverted colors. R, root; C, culm; L, leaf; G, glume; S, seed; A, awn.
2.2. Identification and Biochemical Characterization of a Ca2+-Dependent PLA2 Activity in Durum Wheat Leaves
In the light of the results obtained at the molecular level, an investigation was carried out to evaluate the existence, in durum wheat leaves, of a PLA2 activity resembling the characteristics typical of known sPLA2s. The enzymatic assays were carried out on crude extracts from the fully expanded leaves of durum wheat plants at the tillering stage.
As shown in Figure 3A, the addition of crude leaf extract (0.1 mg) to a reaction mixture containing 2 mM CaCl2, 4 enzymatic units (E.U.) lipoxygenase (LOX) and 1.5 mM 1-palmitoyl-2-linoleoyl-sn-glycero- 3-phosphocholine (PCLIN) induced an absorbance increase in the UV region with a maximum of 234 nm. This was due to the generation of free linoleate and its conversion into the corresponding hydroperoxide containing a conjugated diene. Further investigation was carried out by measuring continuously at 234 nm the hydroperoxidation of the linoleate released from the PCLIN. As shown in Figure 3B, an absorbance increase was observed as a consequence of the sequential additions of 4 E.U. LOX and 0.1 mg crude leaf extract to a reaction mixture containing 2 mM CaCl2 and 1.5 mM PCLIN. A PLA2 activity equivalent to 1.55 E.U. g−1 dry weight was determined.
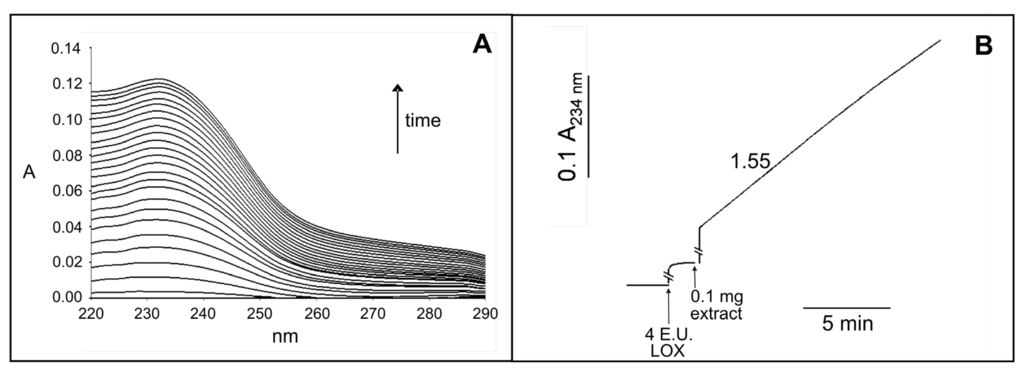
Figure 3.
Assay of PLA2 activity in the crude extract from durum wheat leaves. (A) PLA2 activity monitored as appearance of the typical hydroperoxide spectrum. The reaction mixture contained 2 mM CaCl2, 1.5 mM PCLIN and 4 E.U. LOX in 2 mL 50 mM Na borate buffer, pH 9.0; the reaction was started by the addition of 0.1 mg of crude leaf extract. The absorption spectra were recorded every 20 s; (B) PLA2 activity monitored as time course at 234 nm. The reaction mixture contained 2 mM CaCl2 and 1.5 mM PCLIN in 2 mL 50 mM Na borate buffer pH 9.0; 4 E.U. LOX and 0.1 mg of crude leaf extract were added at the time indicated. The number on the trace refers to E.U. per gram of dry weight.
A set of experiments was carried out to make a first biochemical characterization of the PLA2 activity. For the pH profile (Figure 4A), the activity of the crude extract showed two peaks, the smaller at pH 5.0, and the larger at pH 9.0. The two peaks showed different behaviors with respect to Ca2+ requirements and heat inactivation. Indeed, the addition to the reaction mixture of 10 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), a Ca2+ chelator, resulted in the disappearance of the peak at pH 9.0, whereas the peak at pH 5.0 was not affected. In contrast, the denaturation of the crude leaf extract (15 min at 100 °C) completely abolished the peak at pH 5.0, whereas that at pH 9.0 was only slightly affected.
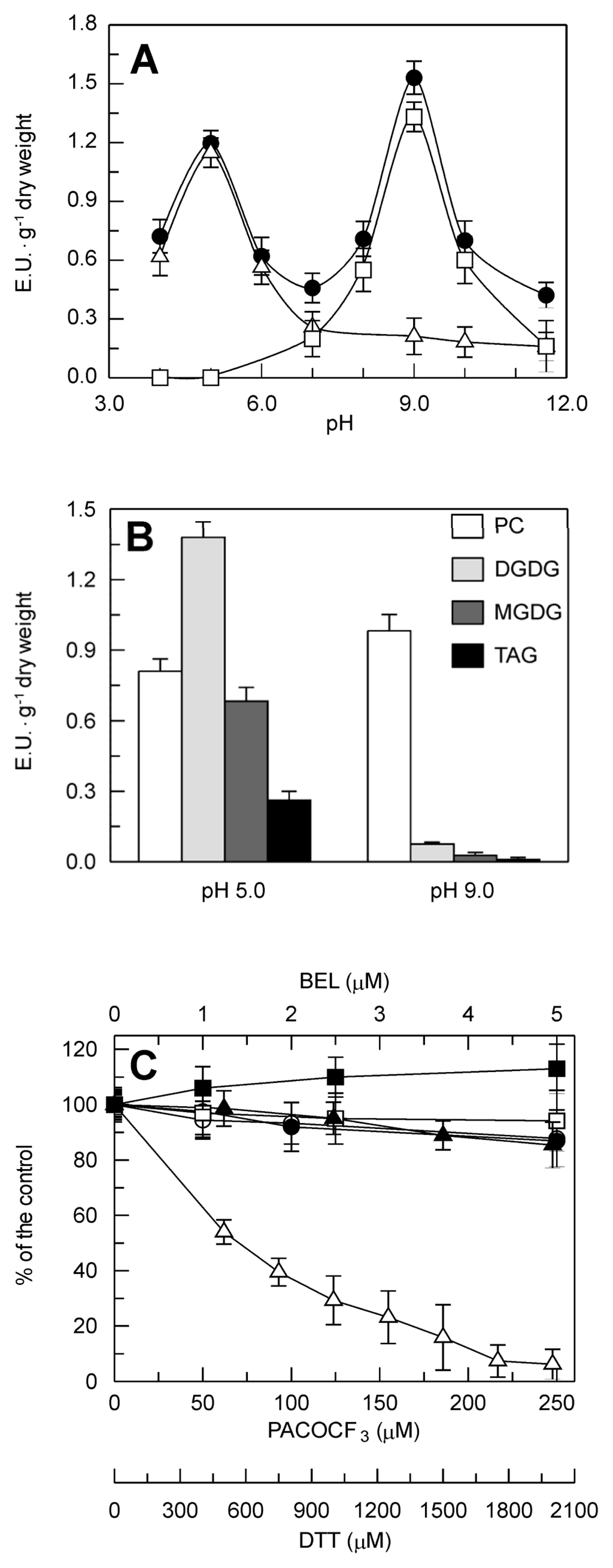
Figure 4.
Biochemical characterization of the PLA2 activity detected in the crude extract from durum wheat leaves. (A) Effect of pH, Ca2+ and heat inactivation. Measurements were carried out at 25 °C in the presence of 2 mM CaCl2 (●), 10 mM EGTA (Δ) or denatured (15 min at 100 °C) crude leaf extract (□). (B) Substrate preference. PC, phosphatidylcholine; DGDG, digalactosyldiacylglycerol; MGDG, monogalactosyldiacylglycerol; TAG, triacylglycerol. (C) Sensitivity to inhibitors. Open symbols refer to the PLA2 activity detected at pH 9.0, closed symbols refer to the PLA2 activity detected at pH 5.0. (■,□) BEL, bromoenol lactone, iPLA2 inhibitor; (●, ○) PACOCF3, palmityl trifluoromethyl ketone, cPLA2 and iPLA2 inhibitor; (▲, Δ) DTT, dithiothreitol, sPLA2 inhibitor. Data are means ± SD (n = 3).
To provide further insight into the nature of the activities detected at both of these pH optima, an investigation was carried out to assess: (i) the preference for different lipid substrates, and (ii) the sensitivity to specific PLA2 inhibitors. As shown in Figure 4B, all the four lipids tested were substrates of the crude leaf extract activity at pH 5.0, with the highest rate for digalactosyldiacylglycerol (DGDG), followed by phosphatidylcholine (PC), monogalactosyldiacylglycerol (MGDG) and triacylglycerol (TAG). The activity at pH 5.0 was insensitive to Ca2+, whatever the substrate used (data not shown). At pH 9.0, the highest activity was seen with PC, whereas very low rates were observed with all of the other substrates, probably ascribable to the residual activity, at this pH value, of the enzyme/s responsible for the peak at pH 5.0; consistently, while the activity detected at pH 9.0 using PC as substrate was almost completely abolished by 10 mM EGTA (see Figure 4A), the activity toward all of these other substrates was not affected by the presence of the Ca2+ chelator (data not shown).
The effects of the following specific PLA2 inhibitors were also tested on the activities detected at pH 5.0 and pH 9.0 (Figure 4C): the disulfide bond-reducing agent dithiothreitol (DTT), an inhibitor of animal sPLA2s; palmityl trifluoromethyl ketone (PACOCF3), an inhibitor of animal cPLA2s and iPLA2s; bromoenol lactone (BEL), an inhibitor of animal iPLA2s [28]. The crude leaf extract activity measured at pH 9.0 was unaffected by increasing concentrations of PACOCF3 and BEL; in contrast, at pH 9.0, the crude leaf extract activity was inhibited in a dose-dependent manner by DTT, which decreased to almost zero at 2 mM DTT. For the activity detected in durum wheat leaves at acidic pH range, none of these PLA2s inhibitors had any effects.
Altogether, these findings suggest that the activity detected in durum wheat leaves at basic pH values could be ascribable to the presence in this tissue of one or more specific sPLA2 isoforms, as the properties of this activity resemble those of other known plant sPLA2s: preference for phospholipid substrate, optimum at basic pH, Ca2+-dependence, heat stability, and inhibition by the disulfide bond-reducing agent DTT. In contrast, the activity that peaked at the acidic pH of 5.0 is probably due to one or more generic acyl hydrolases. Consequently, to better characterize the specific Ca2+-dependent PLA2 activity detected at basic pH, which is hereafter referred to as the durum-wheat-leaves-PLA2 (DWL-PLA2), all of the experiments reported below were carried out at pH 9.0 in the presence of 2 mM CaCl2; moreover, to give a measure exclusive of the Ca2+-dependent PLA2 activity, the residual activity measured in the presence of 10 mM EGTA was consistently subtracted.
The DWL-PLA2 activity showed a marked preference for PC and phosphatidylethanolamine (PE), as compared to phosphatidylinositol (PI), phosphatidylglycerol (PG) and phosphatidylserine (PS) (Figure 5A). In light of this, PCLIN was used to evaluate the dependence of DWL-PLA2 on substrate concentration. As shown in Figure 5B, the reaction rate showed a sigmoidal dependence on substrate concentration. The K0.5 (substrate concentration which gives half maximal rate with sigmoidal kinetics) and the Vmax were 430 ± 37 μM (SD) and 1.43 ± 0.038 E.U. g−1 dry weight, respectively. The Hill plot (Figure 5B, inset) gave a coefficient of 3.29. The sigmoidal dependence of the DWL-PLA2 rate might depend on the presence in the crude leaf extract of more than one PLA2 isoform, each of which would have different Km values. Furthermore, it should be noted that interfacial effects between substrate and enzyme are important in PLA2 catalysis [29], and this might also, at least in part, explain these unusual kinetics.
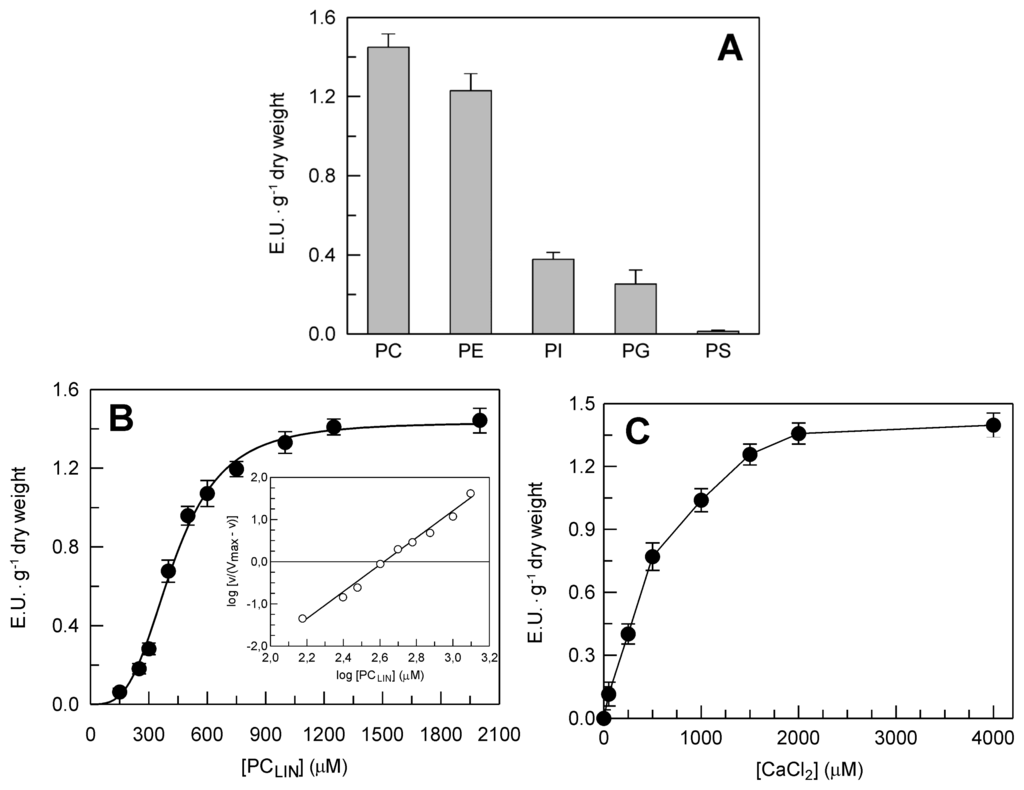
Figure 5.
Substrate and Ca2+ dependence of DWL-PLA2 detected at pH 9.0 in the crude extract from durum wheat leaves. (A) Headgroup selectivity. PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PG, phosphatidylglycerol; PS, phosphatidylserine; (B) Dependence on substrate concentration. The assays were carried out at different PCLIN concentrations ranging between 150 and 2000 μM; (C) Dependence on Ca2+ concentration. The assays were carried out at different CaCl2 concentrations ranging between 50 and 2000 μM. The point at zero Ca2+ concentration was obtained in the presence of 10 mM EGTA. Data are means ± SD (n = 3).
The DWL-PLA2 activity continuously increased with increasing Ca2+ concentrations, with a plateau at 2 to 4 mM Ca2+, even though 300 μM Ca2+ was sufficient to reach 50% of the maximal activity (Figure 5C).
2.3. Evaluation of TdsPLA2 Gene Expression and DWL-PLA2 Activity in Durum Wheat Leaves at Different Developmental Stages
A set of experiments was carried out to evaluate the transcript profile of the four TdsPLA2 genes and the levels of the DWL-PLA2 activity in the youngest fully expanded leaf of durum wheat plants at different stages of development. The results obtained are reported in Figure 6. All four of the TdsPLA2 genes decreased in their steady state transcript levels along with plant development (Figure 6A). In line with the overall expression pattern, DWL-PLA2 activity decreased with increasing plant age, with the exception of a small increase at the watery ripening stage (Figure 6B).
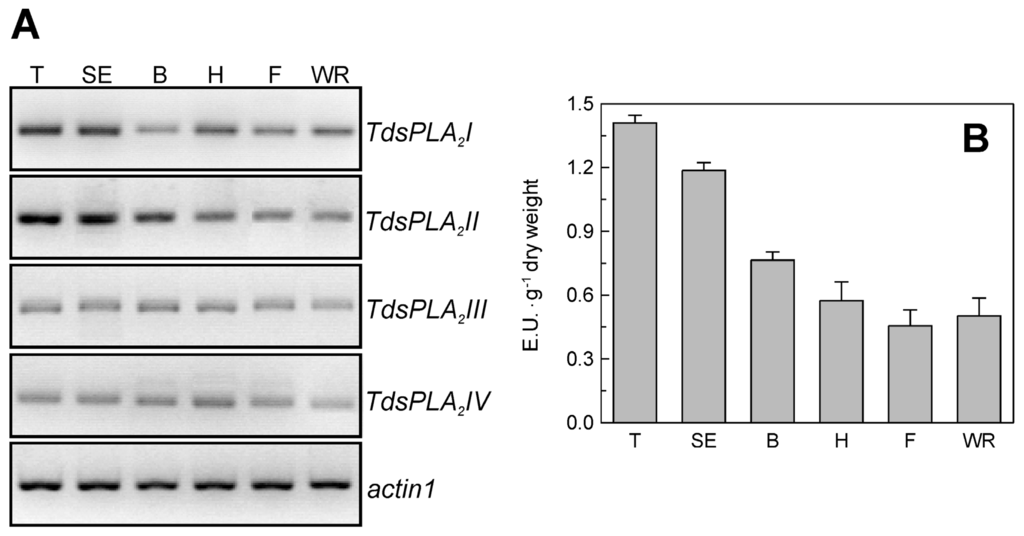
Figure 6.
Effect of growth stage on TdsPLA2 gene expression and DWL-PLA2 activity in durum wheat leaves. (A) Representative expression analysis of TdsPLA2I, TdsPLA2II, TdsPLA2III, TdsPLA2IV and actin 1 genes carried out by using the specific primer pairs reported in Table 1. The figures of gel separation are presented in inverted colors; (B) DWL-PLA2 activity. Data are means ± SD (n = 3). T: tillering, SE: stem elongation. B, booting; H, heading; F, flowering; WR, kernel watery ripening.
2.4. Evaluation of the Effect of the Drought Stress on TdsPLA2 Gene Expression and DWL-PLA2 Activity in Durum Wheat Leaves
The effect of dehydration imposed at the vegetative (stem elongation) and reproductive (kernel watery ripening) stages was evaluated on both the transcript levels of the four TdsPLA2 genes and the DWL-PLA2 activity in the fully expanded leaves (Figure 7).
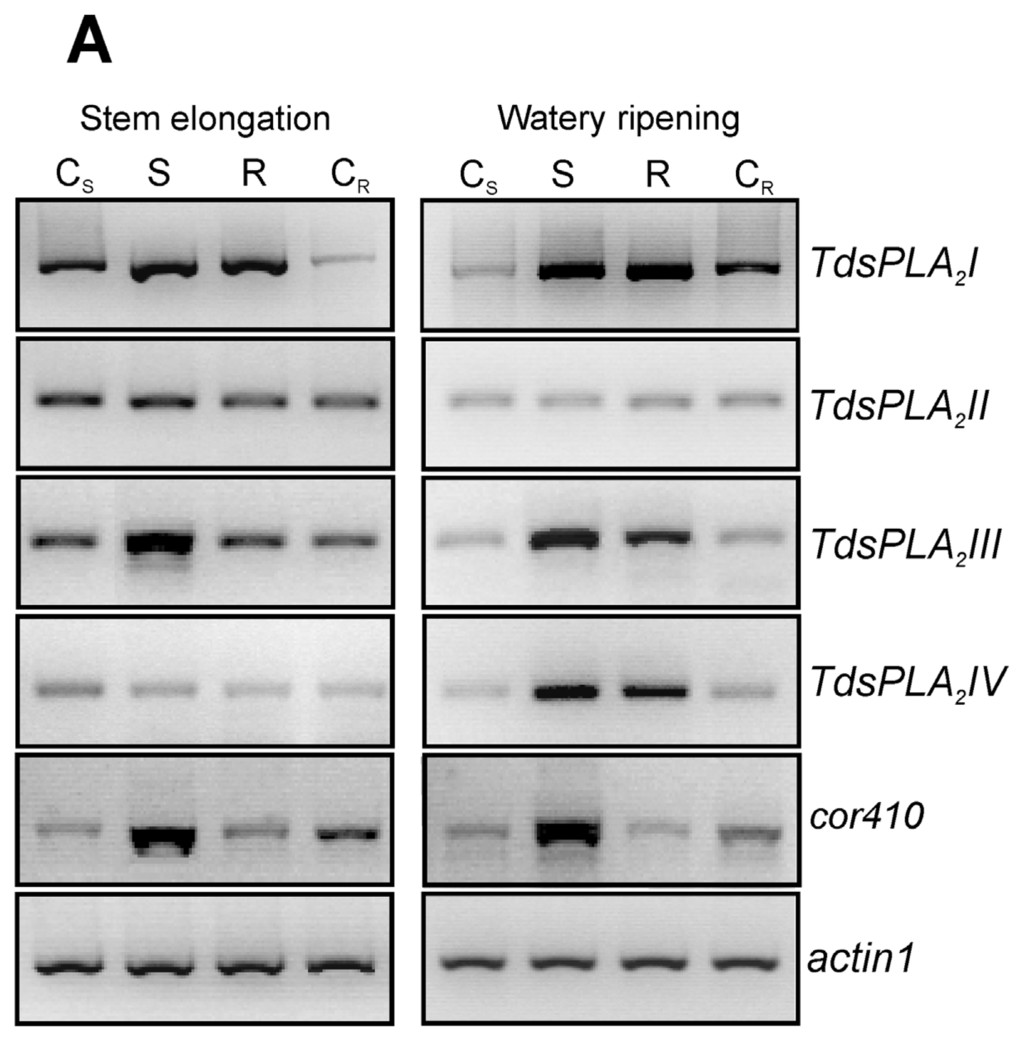
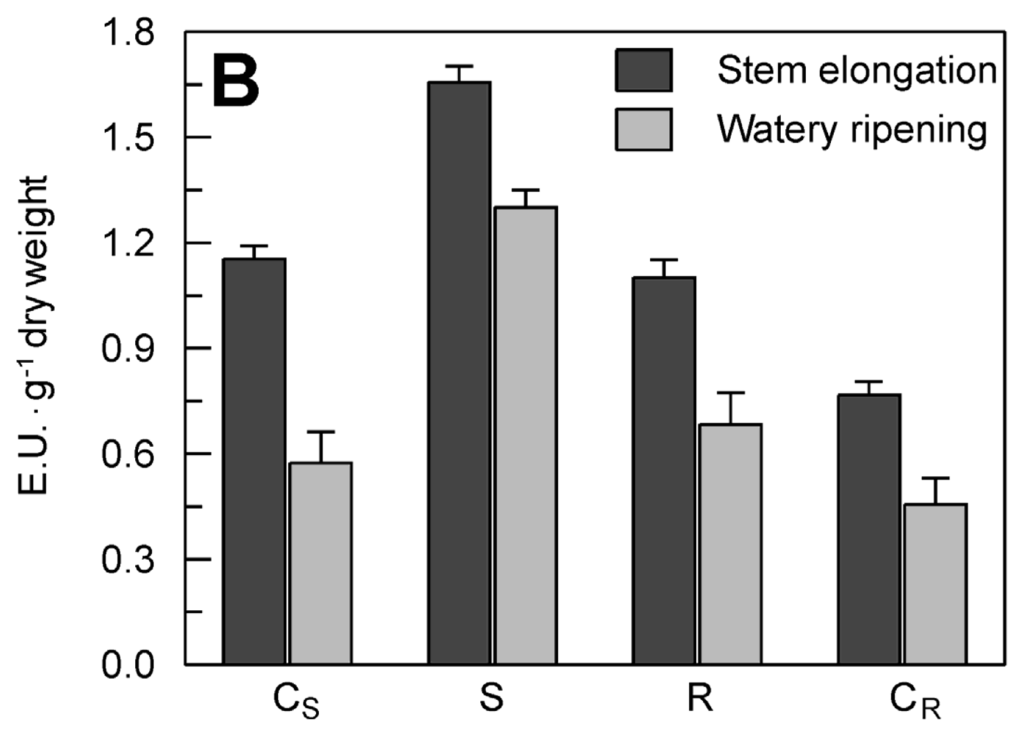
Figure 7.
Effect of drought stress on TdsPLA2 gene expression and DWL-PLA2 activity in durum wheat leaves. (A) Representative expression analysis of TdsPLA2I, TdsPLA2II, TdsPLA2III, TdsPLA2IV, cor410 and actin 1 genes carried out by using the specific primer pairs reported in Table 1. The figures of gel separation are presented in inverted colors; (B) DWL-PLA2 activity. Data are means ± SD (n = 3). CS: control of the stress, S: stress, R: recovery, CR: control of the recovery.
The up-regulation of the dehydrin gene cor410 confirmed the correct imposition of the drought stress (Figure 7A). Among the four TdsPLA2 genes, both TdsPLA2I and TdsPLA2III were up-regulated under drought stress at both developmental stages. Induction was also observed in the expression of the TdsPLA2IV gene when the drought stress was imposed at the kernel watery ripening stage, while no variations were seen in the response to dehydration at transcript level for the TdsPLA2II gene. Stress recovery resulted in decreased transcript levels of the TdsPLA2III gene, which was more evident at stem elongation, when it reached values comparable to those of the control, than at kernel watery ripening, when it remained slightly higher compared to the corresponding control. Rehydration of the plants at kernel watery ripening also induced a slight decrease in the transcript levels of the TdsPLA2IV gene. In contrast, the stress recovery at both of these developmental stages did not affect the transcript levels of the TdsPLA2I gene, which remained comparable to those observed under stress.
In line with the expression profile, a strong increase was also observed in DWL-PLA2 activity as a consequence of the water stress imposition at both developmental stages (Figure 7B): by about 44% at stem elongation, and by 140% at kernel watery ripening. Stress recovery led to a decrease in the DWL-PLA2 activity, which, however, remained higher than that measured in the corresponding controls at both developmental stages.
As far as the PLA2 activity detected at pH 5.0, it was found to be not affected by stress imposition at both developmental stages (data not shown).
2.5. Evaluation of the Effect of the Drought Stress on FFA Content in Durum Wheat Leaves
In the light of the stress-induced increases in both the TdsPLA2 transcript levels and the DWL-PLA2 activity, an investigation was carried out to assess the effect of dehydration on the FFA content of the durum wheat leaves. As shown in Figure 8, the relative amounts of the FFAs were increased as a consequence of the stress imposition. Under stress, the levels of free linoleate and linolenate showed greater increases, of about 3.5-fold and 2.8-fold, respectively, at stem elongation, and about 3.6-fold and 2.5-fold, respectively, at watery ripening. A less evident stress-induced increase was observed for free palmitate: about 1.8-fold and 1.4-fold at stem elongation and watery ripening, respectively. Stress recovery caused a strong decrease in the FFA levels, although they remained higher when compared with the corresponding control, in particular at stem elongation.
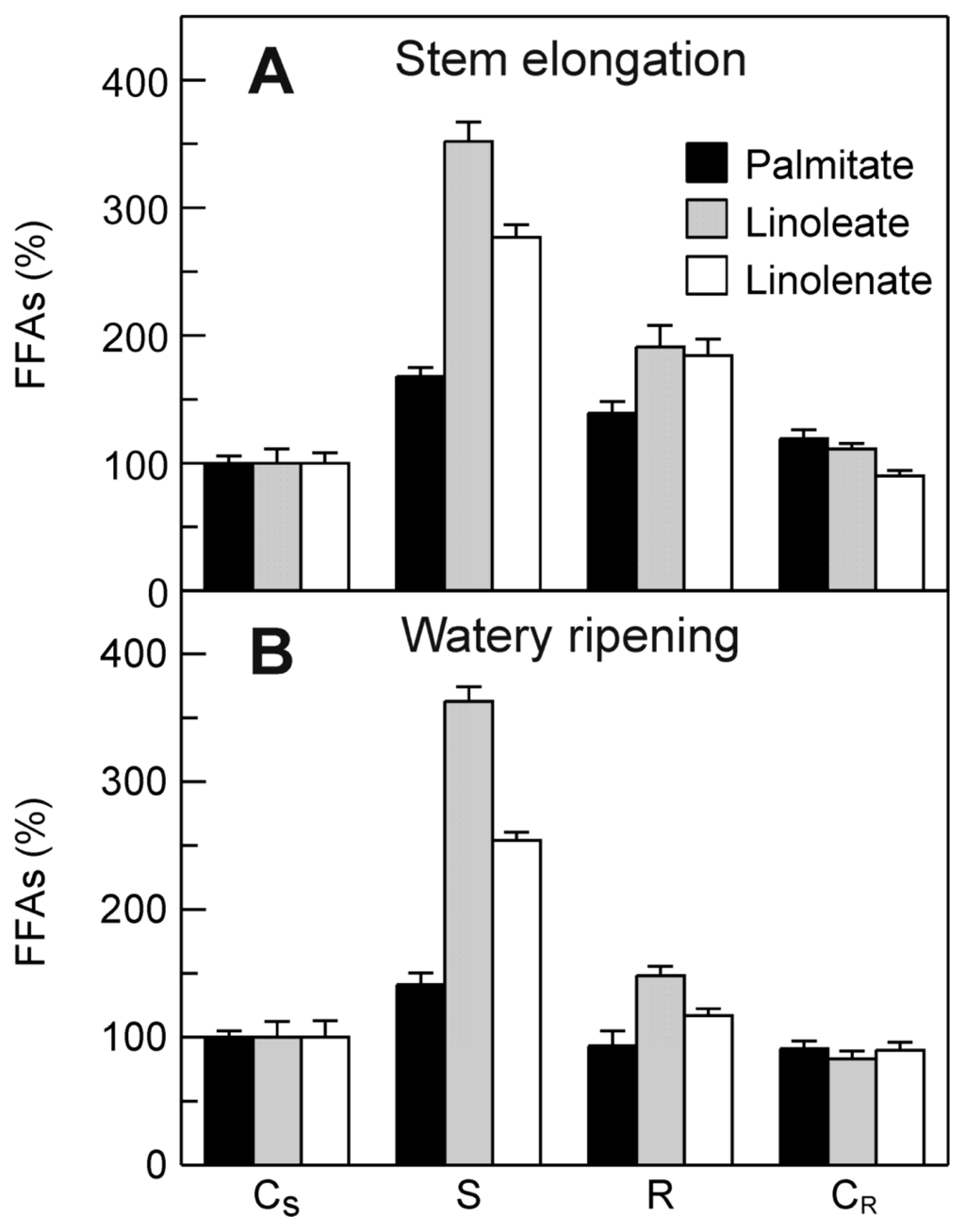
Figure 8.
Effect of drought stress on FFA content in durum wheat leaves. Measurements were carried out at stem elongation (A) and watery ripening (B). CS: control of the stress; S: stress; R: recovery; CR: control of the recovery. Relative amounts of each FFA are expressed as percentage of the amount obtained for the CS taken as 100%. Data are means ± SD (n = 3).
3. Discussion
3.1. Identification and Characterization of the sPLA2 Genes and the DWL-PLA2 Activity in Durum Wheat
On the basis of the molecular analysis, we propose that a gene family exists in durum wheat encoding four putative sPLA2 isoforms. This statement is also supported by a BLAST search at the Wheat Sequence Repository [30] where three genomic contigs for each sPLA2 cDNA sequence, one on each genome of the hexaploid wheat, have been identified. TdsPLA2I matched genomic sequences on chromosomes 2AL, 2BL and 2DL; TdsPLA2II on chromosomes 4AS, 4BL and 4DL; TdsPLA2III on chromosomes 4AL, 4BS and 4DS, and TdsPLA2IV on chromosomes 7AL, 7BL and 7DL. Two copies for each TdsPLA2 gene should, therefore, be present in the durum wheat genome, and our results show that one copy is expressed, while the other one either is not expressed or its transcript is undistinguishable to the one cloned due to the high level of sequence identity shared by the two copies in their coding regions. As a consequence, the expression levels observed for each TdsPLA2 isoform are the result of the transcript abundance of both the two copies, as the primer pairs employed do not discriminate between them; regardless, estimation of the relative contribution of each copy is not relevant for the purpose of the present study, since the two copies should give rise to putative protein products with almost identical sequence and structure and, consequently, with similar functional features.
Similar to other known plant sPLA2s [26], TdsPLA2s can be sorted into the two clusters, XIA and XIB, contain twelve conserved Cys residues and a signal peptide at the N-terminus. On the basis of an in silico-based analysis, TdsPLA2I, TdsPLA2II and TdsPLA2IV are predicted to be secreted into the extracellular space. Transient expression in onion epidermal cells revealed that AtsPLA2β and AtsPLA2γ are indeed secreted into the extracellular space [9]. As far as TdsPLA2III, the bioinformatic tools predict TdsPLA2III to be targeted to mitochondria with a probability ranging between 0.4 and 0.9. At this regard, a great deal of evidence has been reported about the existence in mammalian mitochondria of both secretory and Ca2+-independent PLA2s [31]; as far as plant mitochondria, only recently has an acyl hydrolase activity resembling the characteristics of a PLA2 activity been detected in mitochondria purified from durum wheat seedlings [32]. Analysis of the transcript levels revealed that, with the exception of the TdsPLA2II transcript in seeds, the four TdsPLA2 genes are expressed in all of the tissues of durum wheat plants examined. Similar results have been reported for the AtsPLA2α, AtsPLA2β and AtsPLA2γ genes, and the Nt1PLA2 and Nt2PLA2 genes, the transcripts of which have been detected in several tissues [9,33]. This is expected, considering the involvement of this class of enzyme in a variety of cellular processes in plants [9].
The PLA2 enzymatic activity detected in durum wheat leaves at pH 9.0, which is referred to as the DWL-PLA2 activity, presents biochemical characteristics that resemble those of previously characterized sPLA2s from plants and animals. Firstly, the enzyme(s) responsible for this DWM-PLA2 activity function as PLA2s, rather than as non-specific acyl hydrolases, as the hydrolytic activity is exerted exclusively against phospholipids, instead of galactolipids and triglycerides, with strong preference for PC and PE compared to PI, PG and PS. In contrast to animal sPLA2s that are selective for anionic phospholipids, most of the plant sPLA2s prefer zwitterionic phospholipids, such as PC and PE [9,34,35]. It has been assumed that the substrate preference of plant sPLA2s represents an adaptation to the difference in the natural phospholipid compositions between animal and plant membranes [26], with the latter containing mainly PC and PE [36]. Moreover, in addition to the use of a phospholipid as substrate, sPLA2s require Ca2+ as a cofactor for catalysis. Similarly, DWL-PLA2 activity was completely suppressed in the presence of EGTA and required millimolar Ca2+ concentrations to reach maximal activity, thus resembling the behavior of the animal counterpart [3] and of most of the sPLA2s identified in plants [5,8,9,34,35].
Two other typical features of known plant sPLA2s are also shown by DWL-PLA2: (i) the optimum at alkaline pH, which has already been reported for purified [5] and recombinant [9,35,37] sPLA2s from different plant sources; and (ii) the structural stability, as demonstrated by the resistance of DWL-PLA2 activity to high temperatures (87% of the activity detected at pH 9.0 was retained after treatment of the crude leaf extract at 100 °C for 15 min). AtsPLA2α and AtsPLA2β retained 80% to 95% of their enzyme activities following 5 min treatment in boiling H2O [9], and a similar result was obtained for sPLA2 purified from seeds of elm [5]. Previous findings with animal sPLA2s indicate that the structural stability of this class of PLA2s might be ascribable to the high number of disulfide bonds [9]. Consistent with this, our results clearly show that the DWL-PLA2 activity is inhibited in a dose-dependent manner by DTT, thus suggesting that one or more intramolecular disulfide bridges will be present in the enzyme(s) responsible for DWL-PLA2 activity. Overall, the biochemical characterization of the DWL-PLA2 activity strongly suggests that it is ascribable to one or more isoforms encoded by the TdsPLA2 genes. This hypothesis is strengthened by the observation that plant senescence, as well as drought stress, causes fluctuations in the enzyme activity that are comparable to those in the overall expression levels of these TdsPLA2 genes.
In durum wheat leaves, we also detected a generic acyl hydrolase activity that shows its optimum at acidic pH. A class of acyl hydrolases typical of the plant kingdom is seen in the patatin-related PLAs [4]. Similar to the activity detected in durum wheat leaves, patatin-related PLAs purified from other plant sources show Ca2+-independent and heat-sensitive activity. However, in contrast to the activity detected in durum wheat leaves, this class of enzymes is active on phospholipids and galactolipids and inactive towards diacylglycerol and triacylglycerol [11]; moreover, it displays a pH optimum at 7–8 and is strongly inhibited by several known PLA2 inhibitors [14,38,39]. Therefore, the possibility that the activity detected in durum wheat leaves at acidic pH is a generic acyl hydrolase different from the patatin-related PLAs cannot be excluded. This question merits further investigation in view of the important role played by different members of the acyl hydrolase family in membrane degradation and signaling in plants [11].
3.2. Effect of Drought Stress on TdsPLA2 Gene Expression, DWL-PLA2 Activity and FFA Release
Previous studies carried out on durum wheat have shown that water shortage during both the vegetative and reproductive stages can strongly affect growth and productivity of this crop species [40,41]. In light of this, stem elongation and kernel watery ripening were chosen as the vegetative and reproductive stages, respectively, at which to evaluate the involvement of sPLA2s in durum wheat response to water deficit, both at the molecular and biochemical levels.
Our results show that, in durum wheat leaves, DWM-PLA2 activity is modulated by water supply; contrarily, no effect was detected on the activity of the generic acyl hydrolases responsible for the activity detected at pH 5.0. The variations of the DWL-PLA2 activity observed under drought stress and recovery followed the overall expression pattern of the TdsPLA2 genes. Such a closely coupled relationship strongly suggests that the up-regulation of the TdsPLA2I, TdsPLA2III and TdsPLA2IV genes and the consequent increase in the de novo synthesis of the corresponding sPLA2 isoforms represents the molecular mechanism behind this increase in DWL-PLA2 activity under stress. However, in addition to the regulation of gene expression, post-translational modifications that can affect the functionality of the TdsPLA2 isoforms cannot also be excluded. One possible mechanism might be the activation of the sPLA2 enzyme(s) due to activator factors, such as Ca2+ and pH, which are known to have key roles in plant defense responses against stress [42]. The resting Ca2+ concentration in the extracellular environment and in the intracellular stores ranges between 1 and 10 mM, whereas in the cytoplasm it is maintained in the nanomolar range [43]; thus, it is reasonable to assume that the TdsPLA2 isoforms responsible for DWL-PLA2 might be only partially activated depending on the specific free Ca2+ levels, and that transient elevation of Ca2+ levels due to stress signals [43] could represent a way to regulate them. The same goes for the pH. TdsPLA2 isoforms responsible for DWL-PLA2 require, to reach the maximal activity, pH values higher than those normally present in the extra- and intra-cellular compartments (around 5.5 and 7.5, respectively, [42]); it is thus plausible that they may be activated by alkalinization, that is known to occur under biotic and abiotic stresses in different compartments from several plant species [42,44] including cereals [45].
In terms of the physiological meaning of these data, they are consistent with the information available in the literature concerning the involvement of lipid acyl hydrolases in plant response to environmental stresses, in general, and to water stress, in particular. With respect to the latter, several evidences have been reported about the role of patatin-related PLAs in plant response to drought [46–48]. Our results show that, in durum wheat, specific sPLA2s rather than generic acyl hydrolases play the major role in response to drought stress. In line with this observation, evidence exist in the literature that sPLA2 genes, CssPLA2α and CssPLA2β, are involved in post-harvest peel pitting, a water stress-related disorder in citrus fruit [49]. Interestingly, these two genes are orthologous with the TdsPLA2III and TdsPLA2I genes, respectively, that are up-regulated in durum wheat plants under water deficit. Similar to our findings, these authors reported that the variations in the total PLA2 activity in the pitted areas of the peel (with respect to the healthy ones) were comparable to the changes in the expression levels of the CssPLA2 genes. A similar observation was reported in citrus seedlings exposed to blue light/dark cycles, in which the total PLA2 activity showed the same rhythmicity as CssPLA2α gene expression [50]. In line with our findings, there are also the observations of Ryu and co-workers, who reported that the overexpression of the AtsPLA2β gene, which is orthologous to the TdsPLA2I gene, enhances tolerance of A. thaliana plants to salt stress [23].
As far as cereals, the only observation reported to date in the literature concerns up-regulation of the OssPLA2α gene observed in drought-stressed rice seedlings [24]. Thus, the results reported in the present study further confirm the involvement of specific sPLA2 genes in the adaptation of important crops to water stress conditions. Moreover, stress-dependent induction of TdsPLA2 expression was related to the increase in the total PLA2 activity and to the amounts of FFAs observed under the same conditions. This latter finding is consistent with previous observations that in leaves of durum wheat plants exposed to water stress, there is a decrease in membrane phospholipids that is followed by a concomitant increase in FFA content [51,52]. Linoleate and linolenate are the major fatty acids in plant membranes, and they are located preferentially at the sn-2 position of phospholipids. For this reason, it is feasible that their release is regulated by specific acyl hydrolases, such as the sPLA2s. For the slight increase in free palmitate, which is mainly esterified at the sn-1 position of plant phospholipids, this cannot be ascribed to the PLA1 activity of a generic acyl hydrolase, as the generic acyl hydrolase activity detected in durum wheat leaves was not affected by water stress. However, a role of the specific TdsPLA2 in liberating the palmitate cannot be excluded. In this regard, Fujikawa and co-workers have very recently characterized the recombinant enzyme encoded by the Nt1PLA2 gene of N. tabacum, which was found to hydrolyze the ester bond at the sn-1 position of phospholipids, as well as at the sn-2 position [33]. The release of FFAs under stress conditions is consistent with their role as second messengers in the lipid signaling in plant response to adverse environmental stimuli. In particular, free linoleate and linolenate can be metabolized via the octadecanoic pathway to oxylipins, such as jasmonic acid and its methyl ester, which are essential components in the signaling pathway involved not only in plant response to pathogens and wounding, but also to adverse environmental conditions [53]. In particular, there is evidence that in barley, a species that is phylogenetically closely related to wheat, there is an increase in jasmonic acid levels in plants exposed to osmotic and salt stress [54,55].
4. Experimental Section
4.1. Plant Material and Growing Conditions
Seeds from durum wheat cultivar Ofanto were grown in a growth chamber. This genotype was chosen as it is a modern semi-dwarf cultivar well adapted to the rainfed conditions typical of the areas of the Mediterranean basin where it is widely cultivated [52]. After vernalization at 4 °C for 1 week, five seeds per pot were sown in 2.5 L plastic pots filled with soil, sand and peat (3:1:1). Twenty grams of ammonium nitrate fertilizer were applied to each pot at sowing, and a mix of mineral superphosphate (1.2 g/pot), ammonium nitrate (2.0 g/pot) and potassium sulfate (0.1 g/pot) was applied at tillering. Growth conditions varied from 10 °C day/7 °C night, 60% relative humidity, 14 h light:10 h darkness, 500 mmol m−2·s−1 photon flux density at the third leaf stage, to 30 °C day/25 °C night, 35% relative humidity, 18 h light:6 h darkness, 500 mmol m−2·s−1 photon flux density at kernel physiological maturity. Under these conditions, about 4 months were needed to achieve maturity. To evaluate the plant water status, the youngest fully expanded leaves were selected at random for each treatment, and measurements of leaf water potential (ψI) were carried out using a pressure chamber (PMS Instruments Co., Corvallis, OR, USA).
As far as water treatments, pots were watered to exceed field capacity (i.e., the maximum amount of water that a soil can hold) and left to drain until a constant weight was reached. In order to calculate the soil water content (SWC) at field capacity, the weight of soil of three extra pots at both water-saturated and completely dried state was measured, and the mean value obtained for the SWC at field capacity was used to calculate the amount of water to be applied to each pot. The SWC was controlled by weighing each pot every day and then, on the basis of the weight of the soil and of the empty pot determined before sowing, the amount of water needed to maintain SWC close to field capacity (33%) was added until plants reached the developmental stages chosen to impose the drought treatment: stem elongation and kernel watery ripening. At these two stages, the control plants continued to be watered so as to maintain 33% SWC, while the watering of plants assigned to the drought treatment was stopped in order to allow the soil to dry up to reach 18% SWC. The stress condition was maintained for several days by adding the amount of water needed to maintain 18% SWC until the ψI of the water-stressed plants reached −3.05 ± 0.215 MPa; control plants at the same time showed a ψI of −0.95 ± 0.096 MPa. To allow recovery of the water status, water-stressed plants were re-watered by adding the amount of water needed to restore the 33% SWC and reach a ψI of 1.05 ± 0.112 MPa, comparable to that measured in the control plants at the same time: 1.00 ± 0.095 MPa. A completely randomized design was adopted, with three replications for each treatment, as well-watered and water-stressed. For each treatment, tissue from the youngest fully expanded leaves of three randomly selected plants for replication was used for gene expression, enzyme activity, and FFA content determinations. In addition, gene expression analysis was carried out in tissues from roots, culms, glumes, seeds and awns.
4.2. Isolation and Sequence Analysis of the Full-Length sPLA2 Transcripts
A BLAST search was carried in the TIGR wheat and barley EST database and in the GenBank database, using as query the full-length transcript sequences deduced from the four genes encoding putative sPLA2s in Oryza sativa deposited in the Rice Annotation Project Database [56]: Os02g0831700 (OssPLA2I) Os03g0261100 (OssPLA2II), Os03g0708000 (OssPLA2III) and Os11g0546600 (OssPLA2IV).
The BLAST search allowed the identification in wheat of two full-length expressed sequences, CV769583 and TC381660, showing, respectively, the highest identity with OssPLA2II and OssPLA2III, and in barley of two full-length expressed sequences TC224841 and AK358216.1, showing, respectively, the highest identity with OssPLA2I and OssPLA2IV.
Total RNA was isolated from the youngest fully expanded leaves at the tillering stage using the Trizol reagent (Invitrogen), following the manufacturer instructions. The single-stranded cDNA was synthesized using 200 E.U. SuperScript™ II RNase H-reverse transcriptase (Invitrogen) and a poly(T) primer, on 1 μg total RNA, following the manufacturer’s instructions. The first strand cDNA was used as template for the amplification of the full-length sPLA2 transcripts using the primer pairs reported in Table 1 and high-fidelity “Phusion” Taq DNA polymerase (Finnzymes). The PCR conditions were as follows: preheating at 98 °C for 30 s, then 35 cycles of denaturation at 98 °C for 10 s, annealing at 58 °C to 62 °C for 30 s and extension at 72 °C for 30 s, followed by final extension at 72 °C for 5 min. All the PCR products were visualized on agarose gels, cloned and sequenced on both strands to confirm their identity.

Table 1.
Primer pairs used to amplify TdsPLA2, actin1 and cor410 transcripts, annealing temperature and PCR product sizes.
The deduced protein sequences were subjected to bioinformatic analysis. Alignments were carried out using the Vector NTI Suite software (version 9.0; Invitrogen). The physico-chemical parameters of the amino acid sequences were estimated using the ProtParam tool, which is available at the ExPASy molecular biology server [57]. The conserved domains were determined using the NCBI Conserved Domain Database [27]. The putative localization was predicted by the application of the predictive tools iPSORT [58], TargetP [59] and Predotar [60].
4.3. Semi-Quantitative RT-PCR Analysis
Total RNA was isolated from different plant tissues using Trizol reagent (Invitrogen), following the manufacturer’s instructions. To avoid starch contamination, the seeds were ground under liquid nitrogen and the powder obtained was treated with 50 mM Tris-HCl buffer, pH 9.0, 200 mM NaCl, 1% sarcosil, 20 mM ethylenediaminetetraacetic acid, and 5 mM DTT, and subjected to phenol-chloroform extraction. The purified samples were then used for Trizol extraction. A DNase treatment step was performed at the end of the extraction to ensure the removal of genomic DNA from the total RNA extracted.
First strand cDNA was used as a template for the amplification of fragments corresponding to the TdsPLA2-expressed sequences. Normalization of the Reverse-Transcription-PCR (RT-PCR) reactions was performed by amplifying the wheat TC264064, which was 99% identical to the amino acid level of the rice actin1 gene. A dehydrin gene, cor410, was amplified to monitor correct drought stress imposition.
Amplifications of fragments were performed using the specific primer pairs reported in Table 1 and Go-Taq DNA polymerase (Promega), under the following amplification conditions: preheating at 94 °C for 5 min, then 30 cycles of denaturation at 94 °C for 1 min, annealing at 58 to 67 °C for 30 s, and extension at 72 °C for 1 min, followed by a final extension of 5 min.
4.4. Assay of PLA2 Activity in the Crude Leaf Extract
PLA2 activity was assayed on extracts obtained by homogenization of the leaf tissue under liquid nitrogen. One gram of the powder obtained was suspended in 5 mL of cold-grinding 50 mM Na phosphate buffer, pH 7.0, and the suspension was centrifuged (twice) at 35,000 × g at 4 °C for 15 min. The supernatant was stored in an ice-water bath and used daily. Total protein content of the crude leaf extract was determined by the Lowry’s method as modified by Harris [61], using bovine serum albumin as the standard.
Lipid solutions (50 mM) were prepared by dissolving the appropriate amount of PCLIN, PC, PE, PI, PS, PG, MGDG, DGDG and TAG in ethanol containing 15% (v/v) Tween 20. PACOCF3 and BEL were dissolved in dimethyl sulfoxide. All of the solutions were stored in an ice-water bath and used daily.
PLA2 activity was evaluated using a continuous spectrophotometric method based on the PLA2/LOX coupled reactions, as reported by Trono et al.[32]. Unless otherwise specified, the assay was carried out in 2 mL of 50 mM Na borate buffer (pH 9.0) containing 4 E.U. LOX, 1.5 mM PCLIN and 2 mM CaCl2 at 25 °C. The reaction was started with the addition of 0.1 mg crude leaf extract, and the generation of free linoleate due to PLA2 hydrolysis was monitored following the absorbance increase at 234 nm caused by its conversion into conjugated diene hydroperoxide catalyzed by the coupled LOX reaction (ɛ234 = 31 mM−1·cm−1, [62]). Control was always performed to verify that the overall rate of the reaction was not limited by LOX activity. Specific activities were expressed as E.U. per gram of fresh weight of the leaf tissue and were reported as mean value ± SD of three independent experiments.
In the experiment of the pH profile (Figure 4A) the buffers were as follows: 50 mM Na acetate, pH 4.0 and 5.0; 50 mM Na phosphate, pH 6.0–8.0; 50 mM Na borate, pH 9.0–11.5. In the experiment reported in Figure 4B, a substrate concentration of 500 μM was used, as at higher concentrations TAG caused turbidity in the reaction mixture and did not allow an accurate determination of the reaction rate.
4.5. Determination of FFA Content in Durum Wheat Leaves
Total lipids were extracted from 40 mg dried leaf tissue added with 1.5 mL diethyl ether:hexane (1:1, v/v), to which a known amount of heptadecanoic acid (17:0) was previously added as the internal standard. The resulting mixture was vigorously shaken and subsequently centrifuged at 1700× g for 10 min. The extraction was repeated three times and the organic phases were pooled in a 5 mL calibrated flask, and taken to volume with diethyl ether–hexane 1:1.
Separation of FFAs from bound fatty acids present in the lipidic extract was achieved by solid-phase extraction (SPE) procedure according to Gambacorta et al.[63], with some modifications. STRATA NH2 cartridges pre-packed with 500 mg of amminopropyl–silica gel (Phenomenex, Torrance, CA, USA) were used. FFAs were recovered by elution with 5 mL of diethyl ether–formic acid (2%) solution, dried under vacuum and resuspended in 200 μL diethyl ether.
Quantitative analysis of linoleate, linolenate and palmitate in the SPE fraction was carried out using gas chromatography coupled to mass spectrometry (GC-MS). A 6890N series gas chromatograph (Agilent Technologies) equipped with a ZB-FFAP capillary column (30 m × 0.32 mm i.d.; 50 μm film thickness; Restek, Bellerofonte, PA, USA) and an Agilent 5973 mass selective detector was used. The identification of FFAs was achieved by comparing mass spectra with those of the data system library (NIST 98, p > 95%) and retention indices with standards. Calibration graphs were prepared by the GC-MS analysis of diethyl ether solutions containing known amounts of the standards covering the range for the FFAs assayed. The linear correlation coefficient (r2) of 0.996 suggests that the method effectively addressed the quantification of FFAs. Recovery of the SPE process was determined by the analysis of the solution containing known amounts of the standards before and after the SPE extraction. The percentage recovery was >99% for all the compounds investigated.
5. Conclusions
Overall, our data indicate the existence in durum wheat of four genes encoding putative sPLA2 isoforms. Each TdsPLA2 gene is differently expressed in the different tissues, and shows differently modulated expression during leaf senescence and under drought stress. This suggests a different role for each of these sPLA2 isoforms in both physiological metabolism and stress-induced signaling of durum wheat plants. In light of this, further studies aimed at characterizing the biochemical features and clarifying the role of each of these isoforms are needed. The characterization of purified and/or recombinant TdsPLA2s, together with the use of mutants lacking or overexpressing the TdsPLA2 genes, will be helpful to distinguish the roles of this class of enzymes under stress and also under normal conditions.
Acknowledgments
This work was supported by grants from the Italian Ministry of Education, University and Research (MIUR) project “AGROGEN.” The authors wish to thank Aldo Di Luccia and Sandra Pati of the University of Foggia (Italy) for placing their GC-MS at our disposal for the determination of FFAs, and Christopher Berrie for scientific English language editorial assistance.
Conflict of Interest
The authors declare no conflict of interest.
References
- Munnik, T.; Testerink, C. Plant phospholipid signaling: “In a nutshell”. J. Lipid Res 2009, 50, S260–S265. [Google Scholar]
- Wang, G.; Ryu, S.; Wang, X. Plant phospholipases: An overview. Methods Mol. Biol 2012, 861, 123–137. [Google Scholar]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 2006, 1761, 1246–1259. [Google Scholar]
- Scherer, G.F.E.; Ryu, S.B.; Wang, X.; Matos, A.R.; Heitz, T. Patatin-related phospholipase A: Nomenclature, subfamilies and functions in plants. Trends Plant Sci 2010, 15, 693–700. [Google Scholar]
- Ståhl, U.; Ek, B.; Stymne, S. Purification and characterization of a low-molecular-weight phospholipase A2 from developing seeds of elm. Plant Physiol 1998, 117, 197–205. [Google Scholar]
- Ståhl, U.; Lee, M.; Sjodahl, S.; Archer, D.; Cellini, F.; Ek, B.; Iannacone, N.; MacKenzie, D.; Semeraro, L.; Tramontano, E.; et al. Plant low-molecular-weight phospholipase A2s (PLA2s) are structurally related to the animal secretory PLA2s and are present as a family of isoforms in rice (Oryza sativa). Plant Mol. Biol 1999, 41, 481–490. [Google Scholar]
- Domingues, S.J.S.; Souza, T.F.; Soares, A.M.S.; Jacinto, T.; Machado, O.L.T. Activation of phospholipase PLA2 activity in Ricinus communis leaves in response to mechanical wounding. Braz. J. Plant Physiol 2007, 19, 35–42. [Google Scholar]
- Kim, J.Y.; Chung, Y.S.; Ok, S.H.; Lee, S.G.; Chung, W.I.; Kim, I.Y.; Shin, J.S. Characterization of the full-length sequences of phospholipase A2 induced during flower development. Biochim. Biophys. Acta 1999, 1489, 389–392. [Google Scholar]
- Lee, H.Y.; Bahn, S.C.; Shin, J.S.; Hwang, I.; Back, K.; Doelling, J.H.; Ryu, S.B. Multiple forms of secretory phospholipase A2 in plants. Prog. Lipid Res 2005, 44, 52–67. [Google Scholar]
- Mariani, M.E.; Villarreal, M.A.; Cheung, F.; Leiva, E.P.; Madoery, R.R.; Fidelio, G.D. In silico and in vitro characterization of phospholipase A2 isoforms from soybean (Glycine max). Biochimie 2012, 94, 2608–2619. [Google Scholar]
- Matos, A.R.; Pham-Thi, A.-T. Lipid deacylating enzymes in plants: Old activities, new genes. Plant Physiol. Biochem 2009, 47, 491–503. [Google Scholar]
- Reina-Pinto, J.J.; Voisin, D.; Kurdyukov, S.; Faust, A.; Haslam, R.P.; Michaelson, L.V.; Efremova, N.; Franke, B.; Schreiber, L.; Napier, J.A.; et al. Misexpression of FATTY ACID ELONGATION1 in the Arabidopsis epidermis induces cell death and suggests a critical role for phospholipase A2 in this process. Plant Cell 2009, 21, 1252–1272. [Google Scholar]
- Gustavsson, M.H.; Sommarin, M. Characterization of a plasma membrane-associated phospholipase A2 activity increased in response to cold acclimation. J. Plant Physiol 2002, 159, 1219–1227. [Google Scholar]
- Holk, A.; Rietz, S.; Zahn, M.; Quader, H.; Scherer, G.F.E. Molecular identification of cytosolic, patatin-related phospholipases A from Arabidopsis with potential functions in plant signal transduction. Plant Physiol 2002, 130, 90–101. [Google Scholar]
- Rietz, S.; Dermendjiev, G.; Oppermann, E.; Tafesse, F.G.; Effendi, Y.; Holk, A.; Parkera, J.E.; Teige, M.; Scherer, G.F.E. Roles of Arabidopsis patatin-related phospholipases A in root development are related to auxin responses and phosphate deficiency. Mol. Plant 2010, 3, 525–538. [Google Scholar]
- Li, M.; Bahn, S.C.; Guo, L.; Musgrave, W.; Berg, H.; Welti, R.; Wang, X. Patatin-related phospholipase pPLAIIIβ-induced changes in lipid metabolism alter cellulose content and cell elongation in Arabidopsis. Plant Cell 2011, 23, 1107–1123. [Google Scholar]
- Lee, H.Y.; Bahn, S.C.; Kang, Y.-M.; Lee, K.H.; Kim, H.J.; Noh, E.K.; Palta, J.P.; Shin, J.S.; Ryu, S.B. Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell 2003, 15, 1990–2002. [Google Scholar]
- Seo, J.; Lee, H.Y.; Choi, H.; Choi, Y.; Lee, Y.; Kim, Y.-W.; Ryu, S.B.; Lee, Y. Phospholipase A2β mediates light-induced stomatal opening in Arabidopsis. J. Exp. Bot 2008, 59, 3587–3594. [Google Scholar]
- Lee, O.R.; Kim, S.J.; Kim, H.J.; Hong, J.K.; Ryu, S.B.; Lee, S.H.; Ganguli, A.; Cho, H.-T. Phospholipase A2 is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell 2010, 22, 1812–1825. [Google Scholar]
- Kim, H.J.; Ok, S.H.; Bahn, S.C.; Jang, J.; Oh, S.A.; Park, S.K.; Twell, D.; Ryu, S.B.; Shin, J.S. Endoplasmic reticulum- and Golgi-localized phospholipase A2 plays critical roles in Arabidopsis pollen development and germination. Plant Cell 2011, 23, 94–110. [Google Scholar]
- Jung, J.; Kumar, K.; Lee, H.Y.; Park, Y., II; Cho, H.-T.; Ryu, S.B. Translocation of phospholipase A2α to apoplasts is modulated by developmental stages and bacterial infection in. Arabidopsis. Front. Plant Sci 2012. [Google Scholar] [CrossRef]
- Heinze, M.; Herre, M.; Massalski, C.; Hermann, I.; Conrad, U.; Roos, W. Signal transfer in the plant plasma membrane: Phospholipase A2 is regulated via an inhibitory Gα protein and a cyclophilin. Biochem. J. 2012. [Google Scholar] [CrossRef]
- Ryu, S.B.; Lee, H.Y.; Hwang, I.W.; Jiwan, P.P. Transgenic plants with increased resistance to biotic and abiotic stresses and accelerated flowering time due to overexpression of a secretory phospholipase A2 (sPLA2). PCT Int. Appl. Patent US2010/0100984A1, 22 April 2010. [Google Scholar]
- Singh, A.; Baranwal, V.; Shankar, A.; Kanwar, P.; Ranjan, R.; Yadav, S.; Pandey, A.; Kapoor, S.; Pandey, G.K. Rice phospholipase A superfamily: Organization, phylogenetic and expression analysis during abiotic stresses and development. PLoS One 2012, 7, e30947. [Google Scholar]
- Elias, E.M.; Manthey, F.A. End products: present and future uses. In Durum Wheat Breeding: Current Approaches and Future Strategies; Royo, C., Nachit, M.M., di Fonzo, N., Araus, J.L., Pfeiffer, W.H., Slafer, G.A., Eds.; Food Products Press: New York, NY, USA, 2005; Volume 1, pp. 63–86. [Google Scholar]
- Mansfeld, J. Plant phospholipases A2: Perspectives on biotechnological applications. Biotechnol. Lett 2009, 31, 1373–1380. [Google Scholar]
- NCBI Conserved Domain Database. Available online: http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml accessed on 12 March 2012.
- Yang, H.-C.; Mosior, M.; Johnson, C.A.; Chen, Y.; Dennis, E.A. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal. Biochem 1999, 269, 278–288. [Google Scholar]
- Winget, J.M.; Pan, Y.H.; Bahnson, B.J. The interfacial binding surface of phospholipase A2s. Biochim. Biophys. Acta 2006, 1761, 1260–1269. [Google Scholar]
- URGI Wheat Sequence Repository. Available online: http://urgi.versailles.inra.fr/Species/Wheat/Sequence-Repository accessed on 10 September 2012.
- Ježek, J.; Jabůrek, M.; Zelenka, J.; Ježek, P. Mitochondrial phospholipase A2 activated by reactive oxygen species in heart mitochondria induces mild uncoupling. Physiol. Res 2010, 59, 737–747. [Google Scholar]
- Trono, D.; Soccio, M.; Laus, M.N.; Pastore, D. The existence of phospholipase A2 activity in plant mitochondria and its activation by hyperosmotic stress in durum wheat (Triticum durum Desf.). Plant Sci. 2013, 199–200, 91–102. [Google Scholar]
- Fujikawa, Y.; Fujikawa, R.; Iijima, N.; Esaka, M. Characterization of secretory phospholipase A2 with phospholipase A1 activity in tobacco, Nicotiana tabacum (L.). Lipids 2012, 47, 303–312. [Google Scholar]
- Mansfeld, J.; Ulbrich-Hofmann, R. Secretory phospholipase A2α from Arabidopsis thaliana: Functional parameters and substrate preference. Chem. Phys. Lipids 2007, 150, 156–166. [Google Scholar]
- Fujikawa, R.; Fujikawa, Y.; Iijima, N.; Esaka, M. Molecular cloning, expression, and characterization of secretory phospholipase A2 in tobacco. Lipids 2005, 40, 901–908. [Google Scholar]
- AOCS Lipid Library. Available online: http://lipidlibrary.aocs.org accessed on 25 October 2012.
- Bahn, S.C.; Lee, H.Y.; Kim, H.J.; Ryu, S.B.; Shin, J.S. Characterization of Arabidopsis secretory phospholipase A2-γ cDNA and its enzymatic properties. FEBS Lett 2003, 553, 113–118. [Google Scholar]
- Jung, K.M.; Kim, D.K. Purification and characterization of a membrane-associated 48-kilodalton phospholipase A2 in leaves of broad bean. Plant Physiol 2000, 123, 1057–1068. [Google Scholar]
- Rietz, S.; Holk, A.; Scherer, G.F.E. Expression of the patatin related phospholipase A gene AtPLA IIA in Arabidopsis thaliana is up-regulated by salicylic acid, wounding, ethylene, and iron and phosphate deficiency. Planta 2004, 219, 743–753. [Google Scholar]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar]
- Passioura, J.B.; Condon, A.G.; Richards, R.A. Water deficits, the development of leaf area and crop productivity. In Water Deficits Plant Responses from Cell to Community; Smith, J.A.C., Griffiths, H., Eds.; BIOS Scientific Publishers limited: Oxford, UK, 1993; pp. 253–264. [Google Scholar]
- Kader, M.A.; Lindberg, S. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal Behav 2010, 5, 233–238. [Google Scholar]
- Reddy, A.S.N. Calcium: Silver bullet in signaling. Plant Sci 2001, 160, 381–404. [Google Scholar]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signalling under drought. Trends Plant Sci 2008, 13, 281–287. [Google Scholar]
- Felle, H.H.; Herrmann, A.; Hückelhoven, R.; Kogel, K.-H. Root-to-shoot signalling: apoplastic alkalinization, a general stress response and defence factor in barley (Hordeum vulgare). Protoplasma 2005, 227, 17–24. [Google Scholar]
- Sahsah, Y.; Campos, P.; Gareil, M.; Zuily-Fodil, Y.; Pham-Thi, A.T. Enzymatic degradation of polar lipids in Vigna unguiculata leaves and influence of drought stress. Physiol. Plant 1998, 104, 577–586. [Google Scholar]
- Matos, A.R.; d’Arcy-Lameta, A.; França, M.; Pêtres, S.; Edelman, L.; Kader, J.; Zuily-Fodil, Y.; Pham-Thi, A.T. A novel patatin-like gene stimulated by drought stress encodes a galactolipid acyl hydrolase. FEBS Lett 2001, 491, 188–192. [Google Scholar]
- Matos, A.R.; Gigon, A.; Laffray, D.; Petrês, S.; Zuily-Fodil, Y.; Pham-Thi, A.T. Effects of progressive drought stress on the expression of patatin-like lipid acyl hydrolase genes in Arabidopsis leaves. Physiol. Plant 2008, 134, 110–120. [Google Scholar]
- Alferez, F.; Lluch, Y.; Burns, J.K. Phospholipase A2 and postharvest peel pitting in citrus fruit. Postharv. Biol. Technol 2008, 49, 69–76. [Google Scholar]
- Liao, H.-L.; Burns, J.K. Light controls phospholipase A2α and β gene expression in Citrus sinensis. J. Exp. Bot 2010, 61, 2469–2478. [Google Scholar]
- Navari-Izzo, F.; Milone, M.T.; Quartacci, M.F.; Pinzini, C. Metabolic changes in wheat plants subjected to a water-deficit stress programme. Plant Sci 1993, 92, 151–157. [Google Scholar]
- Quartacci, M.F.; Pinzino, C.; Sgherri, C.L.M.; Navari-Izzo, F. Lipid composition and protein dynamics in thylakoids of two wheat cultivars differently sensitive to drought. Plant Physiol 1995, 108, 191–197. [Google Scholar]
- Weber, H. Fatty acid-derived signals in plants. Trends Plant Sci 2002, 7, 217–224. [Google Scholar]
- Lehmann, J.; Atzorn, R.; Brückner, C.; Reinbothe, S.; Leopold, J.; Wasternack, C. Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta 1995, 1, 156–162. [Google Scholar]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Closem, T.J. Large-scale expression profiling and physiological characterization of jasmonic acid mediated adaptation of barley to salinity stress. Plant Cell Environ 2007, 30, 410–421. [Google Scholar]
- Rice Annotation Project Database. Available online: http://rapdb.dna.affrc.go.jp accessed on 10 May 2011.
- ExPASy ProtParam tool. Available online: http://web.expasy.org/protparam/ accessed on 21 February 2012.
- iPSORT. Available online: http://ipsort.hgc.jp accessed on 20 March 2012.
- TargetP. Available online: http://www.cbs.dtu.dk/services/TargetP accessed on 20 March 2012.
- Predotar. Available online: http://urgi.versailles.inra.fr/predotar/predotar.html accessed on 20 March 2012.
- Harris, D.A. Spectrophotometric assays. In Spectrophotometry and Spectrofluorimetry: A Practical Approach; Bashford, C.L., Harris, D.A., Eds.; IRL Press: Oxford, UK, 1987; pp. 59–61. [Google Scholar]
- Pastore, D.; Trono, D.; Padalino, L.; Simone, S.; Valenti, D.; Di Fonzo, N.; Passarella, S. Inhibition by α-tocopherol and L-ascorbate of linoleate hydroperoxidation and β-carotene bleaching activities in durum wheat semolina. J. Cereal Sci 2000, 31, 41–54. [Google Scholar]
- Gambacorta, G.; Sinigaglia, M.; Schena, A.; Baiano, A.; Lamacchia, C.; Pati, S.; La Notte, E. Changes in free fatty acid and diacylglycerol compounds in short-ripening dry-cured sausage. J. Food Lipids 2009, 16, 1–18. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).