Buckwheat (Fagopyrum esculentum M.) Sprout Treated with Methyl Jasmonate (MeJA) Improved Anti-Adipogenic Activity Associated with the Oxidative Stress System in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic Compounds and Antioxidant Capacity of Buckwheat Sprouts Extracts
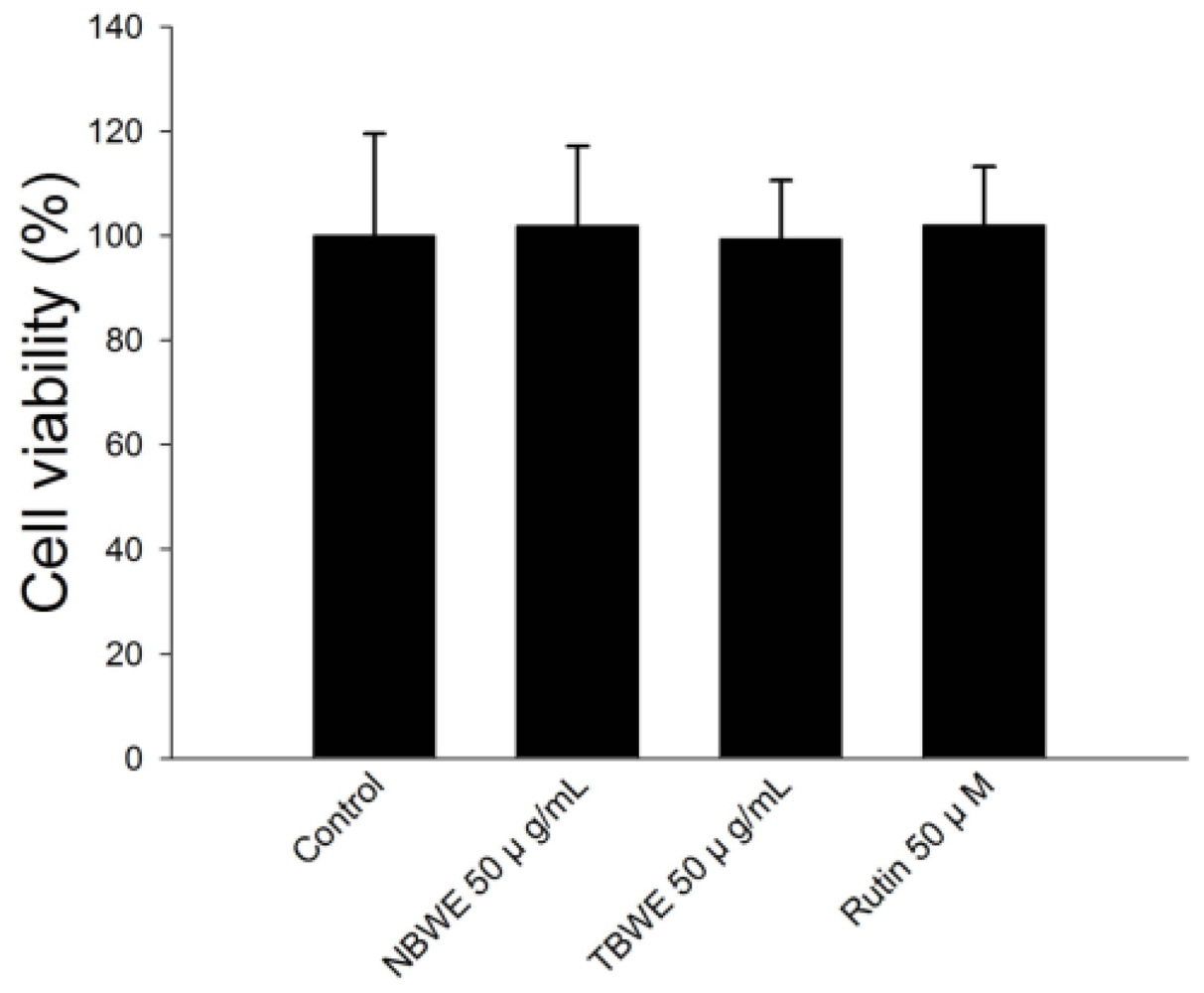
2.2. Cell Viability of Buckwheat Sprouts Extracts in 3T3-L1 Preadipocytes
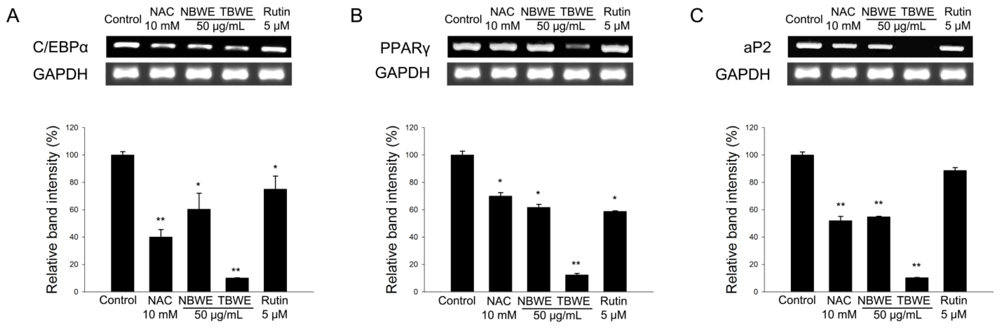
2.3. TBWE Suppresses the Expression of Adipocyte Markers
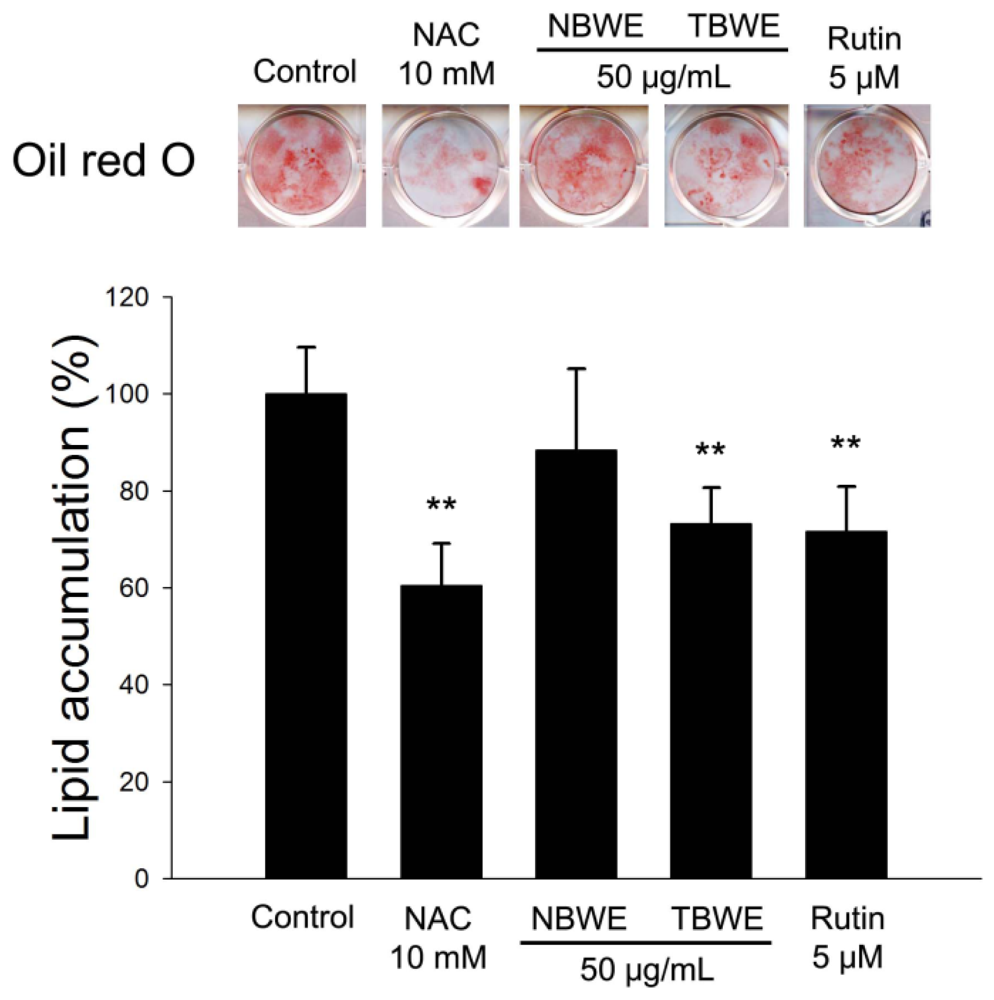
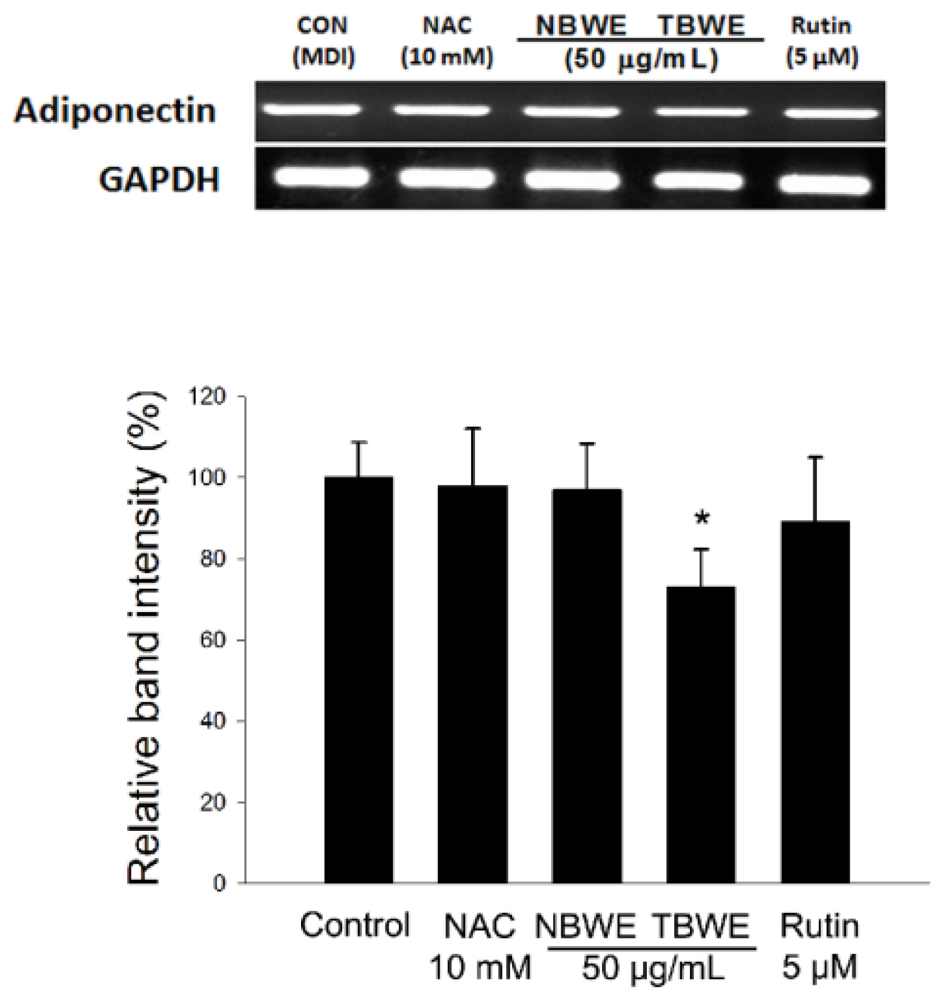
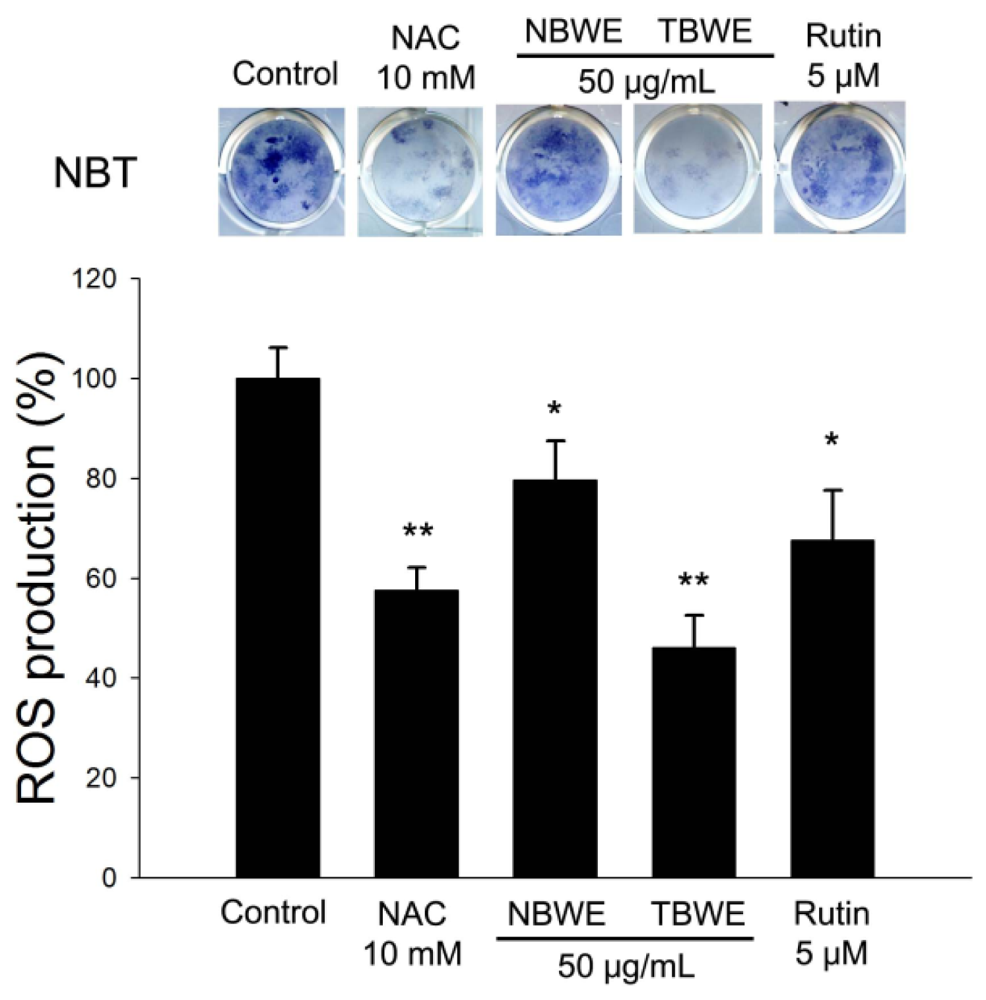
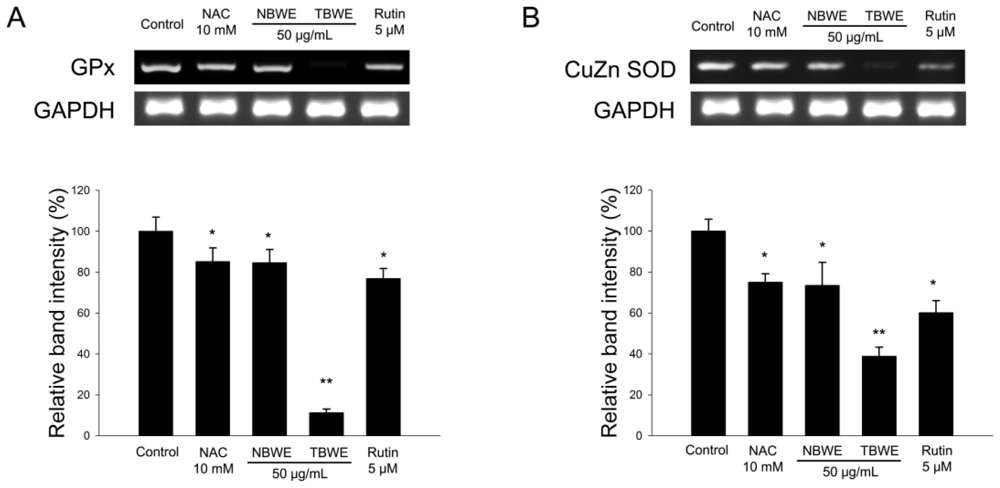
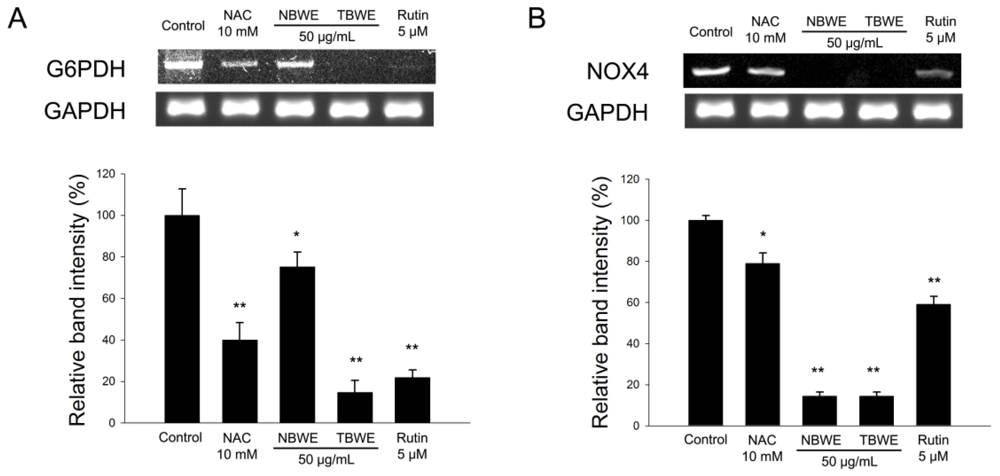
2.4. The Effect of TBWE on Lipid Accumulation and ROS Production in 3T3-L1 Cells
2.5. Discussion
3. Experimental Section
3.1. Materials
3.2. Determination of Total Content of Phenolic Compounds
3.3. The Ferric Reducing Power (FRAP) Assay
3.4. The Oxygen Radical Absorbance Capacity (ORAC) Assay
3.5. Cultivation and Extraction of Buckwheat Sprout Treated with 0.1 mM MeJA
3.6. Cell Culture
3.7. Cell Viability Assay
3.8. Determination of Lipid Accumulation
3.9. ROS Production in 3T3-L1 Preadipocyte
3.10. RNA Extraction and Semi-Quantitative RT-PCR
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Larsson, B.; Svardsudd, K.; Welin, L.; Wilhelmsen, L.; Bjorntorp, P.; Tibblin, G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br. Med. J 1984, 288, 1401–1404. [Google Scholar]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar]
- Herberg, L.; Doppen, W.; Major, E.; Gries, F.A. Dietary-induced hypertrophic—Hyperplastic obesity in mice. J. Lipid Res 1974, 15, 580–585. [Google Scholar]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem 1995, 64, 345–373. [Google Scholar]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar]
- Lee, O.H.; Seo, M.J.; Choi, H.S.; Lee, B.Y. Pycnogenol(R) inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother. Res 2011, 26, 403–411. [Google Scholar]
- Sun, X.; Morris, K.L.; Zemel, M.B. Role of calcitriol and cortisol on human adipocyte proliferation and oxidative and inflammatory stress: A microarray study. J. Nutrigenet. Nutrigenomics 2008, 1, 30–48. [Google Scholar]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol 2010, 635, 1–8. [Google Scholar]
- Yuliana, N.D.; Iqbal, M.; Jahangir, M.; Wijaya, C.H.; Korthout, H.; Kottenhage, M.; Kim, H.K.; Verpoorte, R. Screening of selected Asian spices for anti obesity-related bioactivities. Food Chem 2011, 126, 1724–1729. [Google Scholar]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci 2010, 86, 791–797. [Google Scholar]
- Hsu, C.L.; Yen, G.C. Induction of cell apoptosis in 3T3-L1 pre-adipocytes by flavonoids is associated with their antioxidant activity. Mol. Nutr. Food Res 2006, 50, 1072–1079. [Google Scholar]
- Choi, I.; Park, Y.; Choi, H.; Lee, E.H. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet. Biofactors 2006, 26, 273–281. [Google Scholar]
- Andersen, C.; Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and adipogenesis. Biofactors 2010, 36, 415–422. [Google Scholar]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem 2006, 98, 508–512. [Google Scholar]
- Kim, S.J.; Zaidul, I.S.M.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem 2008, 110, 814–820. [Google Scholar]
- Li, S.Q.; Zhang, Q.H. Advances in the development of functional foods from buckwheat. Crit. Rev. Food Sci. Nutr 2001, 41, 451–464. [Google Scholar]
- Chen, Z.Y.; Jiao, R.; Ma, K.Y. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem 2008, 56, 8761–8773. [Google Scholar]
- Kim, H.J.; Park, K.J.; Lim, J.H. Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. J. Agric. Food Chem 2011, 59, 5707–5713. [Google Scholar]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L). J. Agric. Food Chem 2005, 53, 1750–1756. [Google Scholar]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem 2001, 49, 5165–5170. [Google Scholar]
- Hsu, C.L.; Yen, G.C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem 2007, 55, 8404–8410. [Google Scholar]
- Christy, R.J.; Yang, V.W.; Ntambi, J.M.; Geiman, D.E.; Landschulz, W.H.; Friedman, A.D.; Nakabeppu, Y.; Kelly, T.J.; Lane, M.D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev 1989, 3, 1323–1335. [Google Scholar]
- Guan, H.P.; Ishizuka, T.; Chui, P.C.; Lehrke, M.; Lazar, M.A. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev 2005, 19, 453–461. [Google Scholar]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev 2000, 14, 1293–1307. [Google Scholar]
- Baehner, R.L.; Boxer, L.A.; Davis, J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood 1976, 48, 309–313. [Google Scholar]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev 2002, 16, 22–26. [Google Scholar]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun 2008, 375, 602–607. [Google Scholar] [Green Version]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol 2010, 101, 3751–3754. [Google Scholar]
- Pajvani, U.B.; Du, X.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J. Biol. Chem 2003, 278, 9073–9085. [Google Scholar]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res 2005, 46, 1369–1379. [Google Scholar]
- Alia, M.; Mateos, R.; Ramos, S.; Lecumberri, E.; Bravo, L.; Goya, L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur. J. Nutr 2006, 45, 19–28. [Google Scholar]
- Tsuda, K. Roles of adiponectin and oxidative stress in the regulation of membrane microviscosity of red blood cells in hypertensive men—An electron spin resonance study. J. Obesity 2011, 2011, 1–8. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem 1996, 239, 70–76. [Google Scholar]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med 1993, 14, 303–311. [Google Scholar]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest 2004, 114, 1752–1761. [Google Scholar]
- Schroder, K.; Wandzioch, K.; Helmcke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol 2009, 29, 239–245. [Google Scholar]
| Total phenolic compound (mg/g) | Rutin (mg/g) | FRAP Assay (mM Fe2+/g of dried sample) | ORAC Values (μM trolox) | |||
|---|---|---|---|---|---|---|
| Hydrophilic | Lipophilic | Total | ||||
| NBWE | 14.4 ± 0.4 | 2.09 ± 0.27 | 0.09 ± 0.01 | 60.6 ± 3.8 | 104.0 ± 5.1 | 164.6 ± 6.3 |
| TBWE | 22.9 ± 0.6 ** | 2.99 ± 0.11 ** | 0.21 ± 0.02 ** | 103.3 ± 19.2 * | 102.5 ± 7.0 | 205.8 ± 25.8 |
| Primers | Sequences | |
|---|---|---|
| Forward | Reverse | |
| PPARγ | CCAGAGTCTGCTGATCTGCG | GCCACCTCTTTGCTCTGATC |
| CEBPα | GGTGCGCAAGAGCCGAGATAAAG | AGTTCACGGCTCAGCTGTTCCAC |
| aP2 | GACCTGGAAACTCGTCTCCA | CATGACACATTCCACCACCA |
| GPx | CTCGGTTTCCCGTGCAATCAG | GTGCAGCCAGTAATCACCAAG |
| Cu/Zn-SOD | CAGCATGGGTTCCACGTCCA | CACATTGGCCACACCGTCCT |
| Adiponectin | CATGACCAGGAAACCACGACT | TGAATGCTGAGCGGTAT |
| NOX4 | GAAGCCCATTTGAGGAGTCA | GGGTCCACAGCAGAAAACTC |
| G6PDH | CGATGGCAGAGCAGGT | GATCTGGTCCTCACG |
| GAPDH | AACTTTGGCATTGTGGAAGG | ACACATTGGGGGTAGGAACA |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, Y.-J.; Kim, K.-J.; Park, K.-J.; Yoon, B.-R.; Lim, J.-H.; Lee, O.-H. Buckwheat (Fagopyrum esculentum M.) Sprout Treated with Methyl Jasmonate (MeJA) Improved Anti-Adipogenic Activity Associated with the Oxidative Stress System in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2013, 14, 1428-1442. https://doi.org/10.3390/ijms14011428
Lee Y-J, Kim K-J, Park K-J, Yoon B-R, Lim J-H, Lee O-H. Buckwheat (Fagopyrum esculentum M.) Sprout Treated with Methyl Jasmonate (MeJA) Improved Anti-Adipogenic Activity Associated with the Oxidative Stress System in 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2013; 14(1):1428-1442. https://doi.org/10.3390/ijms14011428
Chicago/Turabian StyleLee, Young-Jun, Kui-Jin Kim, Kee-Jai Park, Bo-Ra Yoon, Jeong-Ho Lim, and Ok-Hwan Lee. 2013. "Buckwheat (Fagopyrum esculentum M.) Sprout Treated with Methyl Jasmonate (MeJA) Improved Anti-Adipogenic Activity Associated with the Oxidative Stress System in 3T3-L1 Adipocytes" International Journal of Molecular Sciences 14, no. 1: 1428-1442. https://doi.org/10.3390/ijms14011428
APA StyleLee, Y.-J., Kim, K.-J., Park, K.-J., Yoon, B.-R., Lim, J.-H., & Lee, O.-H. (2013). Buckwheat (Fagopyrum esculentum M.) Sprout Treated with Methyl Jasmonate (MeJA) Improved Anti-Adipogenic Activity Associated with the Oxidative Stress System in 3T3-L1 Adipocytes. International Journal of Molecular Sciences, 14(1), 1428-1442. https://doi.org/10.3390/ijms14011428






