Optimization of Supercritical CO2 Extraction of Fish Oil from Viscera of African Catfish (Clarias gariepinus)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of SFE Parameters on the Oil Yield
2.2. Fitting the Response Surface Models
2.3. Analysis of Response Surface
3. Experimental Section
3.1. Materials
3.2. Sample Preparation for Experiments
3.3. Moisture Content Determination
3.4. Soxhlet Extraction
3.5. Apparatus and Procedure of Supercritical Fluid Extraction
3.6. Experimental Design
4. Conclusions
References
- De Graaf, G.; Janssen, H. Artificial Reproduction and Pond Rearing of the African Catfish Clarias Gariepinus in Sub-Saharan Africa—A handbook. In FAO Fisheries Technical Paper; FAO: Rome, Italy, 1996; p. 73. [Google Scholar]
- Bureau, D.P.; de laNoüe, J. Effect of dietary incorporation of crop residues on growth, mortality and feed conversion ratio of the African catfish, Clarias gariepinus (Burchell). Aquac. Res 1995, 26, 351–360. [Google Scholar]
- Fagbenro, O.A.; Davies, S.J. Use of soybean flour (dehulled, solvent-extracted soybean) as fish meal substitute in practical diets for African catfish, Clarias gariepinus (Burchell 1822): Growth, feed utilization and digestibility. J. Appl. Ichthyol 2001, 17, 64–69. [Google Scholar]
- Osibona, A.O.; Kusemiju, K.; Akande, G.R. Proximate composition and fatty acids profile of the African Catfish (Clarias gariepinus). Acta Satech 2009, 3, 85–89. [Google Scholar]
- Weaver, B.J.; Holub, B.J. Health effects and metabolism of dietary eicosapentaenoic acid. Prog. Food Nutr. Sci 1988, 12, 111–150. [Google Scholar]
- Conner, W.E. The beneficial effects of omega-3 fatty acids: Cadrdiovascular disease and neurodevelopment. Curr. Opin. Lipidol 1997, 8, 1–3. [Google Scholar]
- Kristinsson, H.G.; Rasco, B.A. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolysed with various alkaline proteases. J. Agric. Food Chem 2000, 48, 657–666. [Google Scholar]
- Gildberg, A. Utilization of male Arctic capelin and Atlantic cod intestines for fish sauce production—Evaluation of fermentation conditions. Biores. Technol 2001, 76, 119–123. [Google Scholar]
- Rustad, T. Utilisation of marine byproducts. Electron. J. Environ. Agric. Food Chem 2003, 2, 458–463. [Google Scholar]
- Bhaskar, N.; Benila, T.; Radha, C.; Lalitha, R.G. Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Biores. Technol 2008, 99, 335–343. [Google Scholar]
- Arnesen, J.A.; Gildberg, A. Extraction and characterization of gelatine from Atlantic salmon (Salmo salar) skin. Biores. Technol 2007, 98, 53–57. [Google Scholar]
- Bhaskar, N.; Sathisha, A.D.; Sachindra, N.M.; Sakhare, P.Z.; Mahendrakar, N.S. Effect of acid ensiling on the stability of visceral waste proteases of Indian major carp Labeo rohita. J. Aquat. Food Prod. Technol 2007, 16, 73–86. [Google Scholar]
- Bhaskar, N.; Sudeepa, E.S.; Rashmi, H.N.; Tamil Selvi, A. Partial purification and characterization of protease of Bacillus proteolyticus CFR3001 isolated from fish processing waste and its antibacterial activities. Biores. Technol 2007, 98, 2758–2764. [Google Scholar]
- Gao, M.T.; Hirata, M.; Toorisaka, E.; Hano, T. Acid hydrolysis of fish wastes for lactic acid fermentation. Biores. Technol 2006, 97, 2414–2420. [Google Scholar]
- Babbit, K.J. Intrinsic Quality Species of North Pacific Fish in Making Profits out of Seafood Wastes; Proceedings of the International Conference on Fish by Products, Anchorage, AK, USA, 25–27 April 1990, Keller, S., Ed.; University of Alaska Sea Grant: Fairbanks, AK, USA, 1990; pp. 39–43. [Google Scholar]
- Hultin, H.O. Oxidation of Lipids in Seafoods. In Seafoods: Chemistry, Processing Technology and Quality; Shahidi, F., Botta, J.R., Eds.; Chapman & Hall: London, UK, 1994; pp. 49–74. [Google Scholar]
- Staby, A.; Mollerup, J. Separation of constituents of fish oil using supercritical fluids: A review of experimental solubility, extraction, and chromatographic data. Fluid Phase Equilibr 1993, 91, 349–386. [Google Scholar]
- Esquível, M.M.; Bandarra, N.M.; Fontan, I.; Bernardo-Gil, M.G.; Batista, I.; Nunes, M.L.; Empis, J.A. Supercritical carbon dioxide extraction of sardine Sardina pilchardus oil. Lebensm. Wiss. Technol 1997, 30, 715–720. [Google Scholar]
- Riha, V.; Brunner, G. Separation of fish oil ethyl esters with supercritical carbon dioxide. J. Supercrit. Fluids 2000, 17, 55–64. [Google Scholar]
- Létisse, M.; Rozières, M.; Hiol, A.; Sargent, M.; Comeau, L. Enrichment of EPA and DHA from sardine by supercritical fluid extraction without organic modifier I. Optimization of extraction conditions. J. Supercrit. Fluids 2006, 38, 27–36. [Google Scholar]
- Perretti, G.; Motori, A.; Bravi, E.; Favati, F.; Montanari, L.; Fantozzi, P. Supercritical carbon dioxide fractionation of fish oil fatty acid ethyl esters. J. Supercrit. Fluids 2007, 40, 349–353. [Google Scholar]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Yazid, A.M.; Khatib, A.; Norulaini, N.A.N. Fatty acid compositions of fish oil extracted from different parts of Indian mackerel (Rastrelliger kanagurta) using various techniques of supercritical CO2 extraction. Food Chem 2010, 120, 879–885. [Google Scholar]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Jahurul, M.H.A.; Khatib, A.; Norulaini, N.A.N. Extraction of fish oil from the skin of Indian mackerel using supercritical fluids. J. Food Eng 2010, 99, 63–69. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem 2006, 98, 136–148. [Google Scholar]
- Norulaini, N.A.N.; Setianto, W.B.; Zaidul, I.S.M.; Nawi, A.H.; Azizi, C.Y.M.; Mohd Omar, A.K. Effects of supercritical carbon dioxide extraction parameters on virgin coconut oil yield and medium-chain triglyceride content. Food Chem 2009, 116, 193–197. [Google Scholar]
- Zaidul, I.S.M.; Norulaini, N.N.A.; Omar, A.K.M.; Smith, R.L., Jr. Supercritical carbon dioxide (SC-CO2) extraction and fractionation of palm kernel oil from palm kernel as cocoa butter replacers blend. J. Food Eng. 2006, 73, 210–216. [Google Scholar]
- Zaidul, I.S.M.; Norulaini, N.N.A.; Omar, A.K.M.; Smith, R.L., Jr. Supercritical carbon dioxide (SC-CO2) extraction of palm kernel oil from palm kernel. J. Food Eng. 2007, 79, 1007–1014. [Google Scholar]
- Zaidul, I.S.M.; Norulaini, N.N.A.; Omar, A.K.M.; Smith, R.L., Jr. Blending of supercritical carbon dioxide (SC-CO2) extracted palm kernel oil fractions and palm oil to obtain cocoa butter replacers. J. Food Eng. 2007, 78, 1397–1409. [Google Scholar]
- Zaidul, I.S.M.; Norulaini, N.A.N.; Omar, A.K.M.; Sato, Y.; Smith, R.L., Jr. Separation of palm kernel oil from palm kernel with supercritical carbon dioxid0e using pressure swing technique. J. Food Eng. 2007, 81, 248–419. [Google Scholar]
- Temelli, F.; Güçlü-Üstündağ, Ő. upercritical Fluid Technology for Fats and Oils Processing. In Bailey’s Industrial Oil and Fat Products, 6th ed; Shahidi, F., Ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2005; Volume 5, Chapter 10; pp. 397–432. [Google Scholar]
- Temelli, F.; Saldana, M.D.A.; Moquin, P.H.L.; Sun, M. Supercritical Fluid Extraction of Specialty Oils. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; Martinez, J.L., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 51–101, Chapter 3. [Google Scholar]
- Temelli, F. Perspectives on supercritical fluid processing of fats and oils. J. Supercrit. Fluids 2009, 47, 583–590. [Google Scholar]
- Joglekar, A.M.; May, A.T. Product excellence through design of experiments. Cereal Foods World 1987, 32, 857–868. [Google Scholar]
- Xu, X.; Gao, Y.X.; Liu, G.M.; Wang, Q.; Zhao, J. Optimization of supercritical carbon dioxide extraction of sea buckthorn (Hippophae thamnoides L.) oil using response surface methodology. Food Sci. Technol 2008, 41, 1223–1231. [Google Scholar]
- Hawthorne, S.B.; Miller, D.J.; Langefeld, J.J. Advances in Analytical Supercritical Fluid Extraction. In Hyphenated Techniques in Supercritical Fluid Chromatography and Extraction; Jinno, K., Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 1992; Volume 53, pp. 225–254. [Google Scholar]
- Wei, Z.-J.; Liao, A.-M.; Zhang, H.-X.; Liu, J.; Jiang, S.-T. Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Biores. Technol 2009, 100, 4214–4219. [Google Scholar]
- Wang, L.; Weller, C.L.; Schlegel, V.L.; Carr, T.P.; Cuppett, S.L. Supercritical CO2 extraction of lipids from grain sorghum dried distillers grains with soluble. Biores. Technol 2008, 99, 1337–1382. [Google Scholar]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng 2012, 109, 238–248. [Google Scholar]
- Association of Analytical Chemists, Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Arlington, TX, USA, 1994; Volume I & II, p. 1298.
- Cossuta, D.; Simandi, B.; Vagi, E.; Hohmann, J.; Prechl, A.; Lemberkovics, E.; Kery, A.; Keve, T. Supercritical fluid extraction of Vitex agnus castus fruit. J. Supercrit. Fluids 2008, 47, 188–194. [Google Scholar]
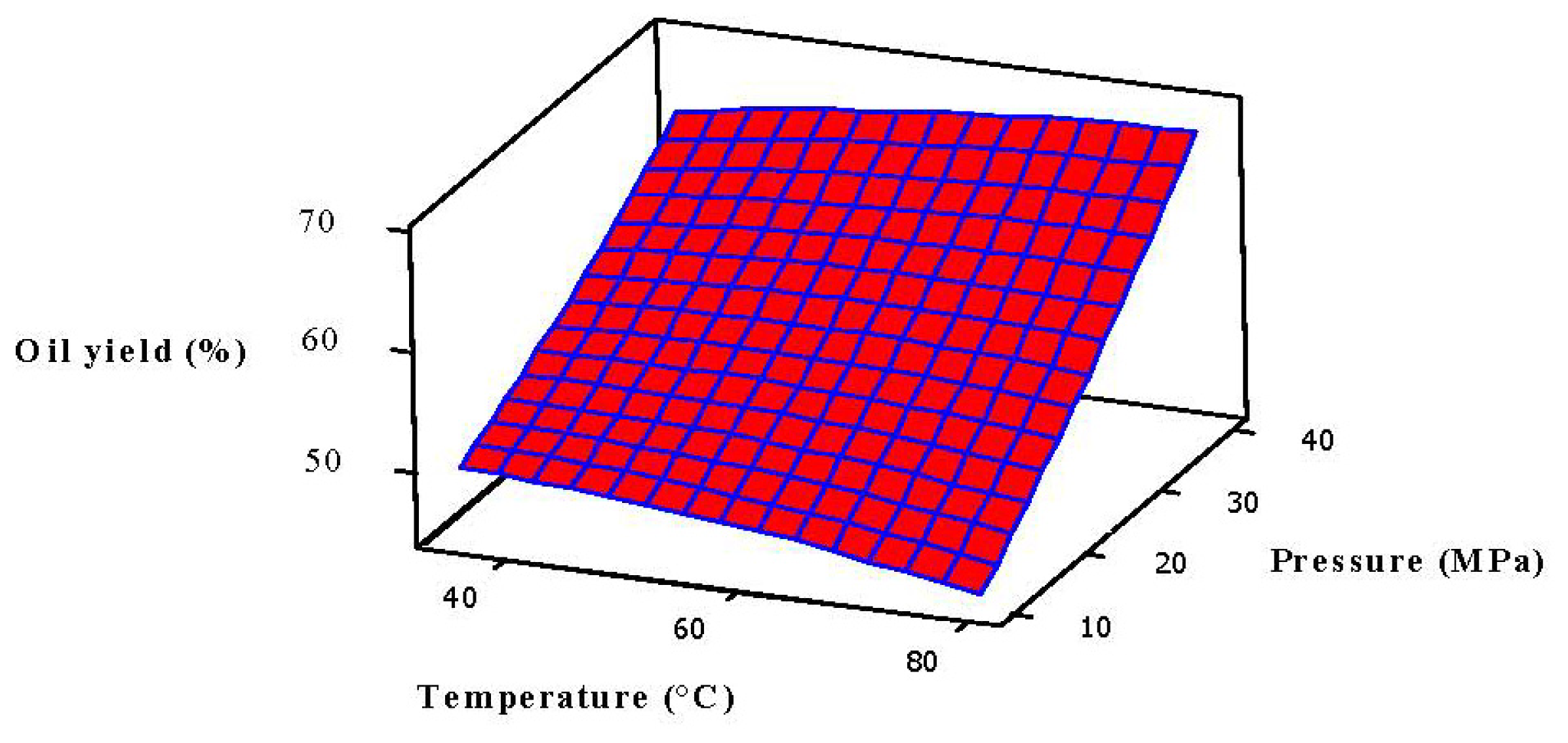

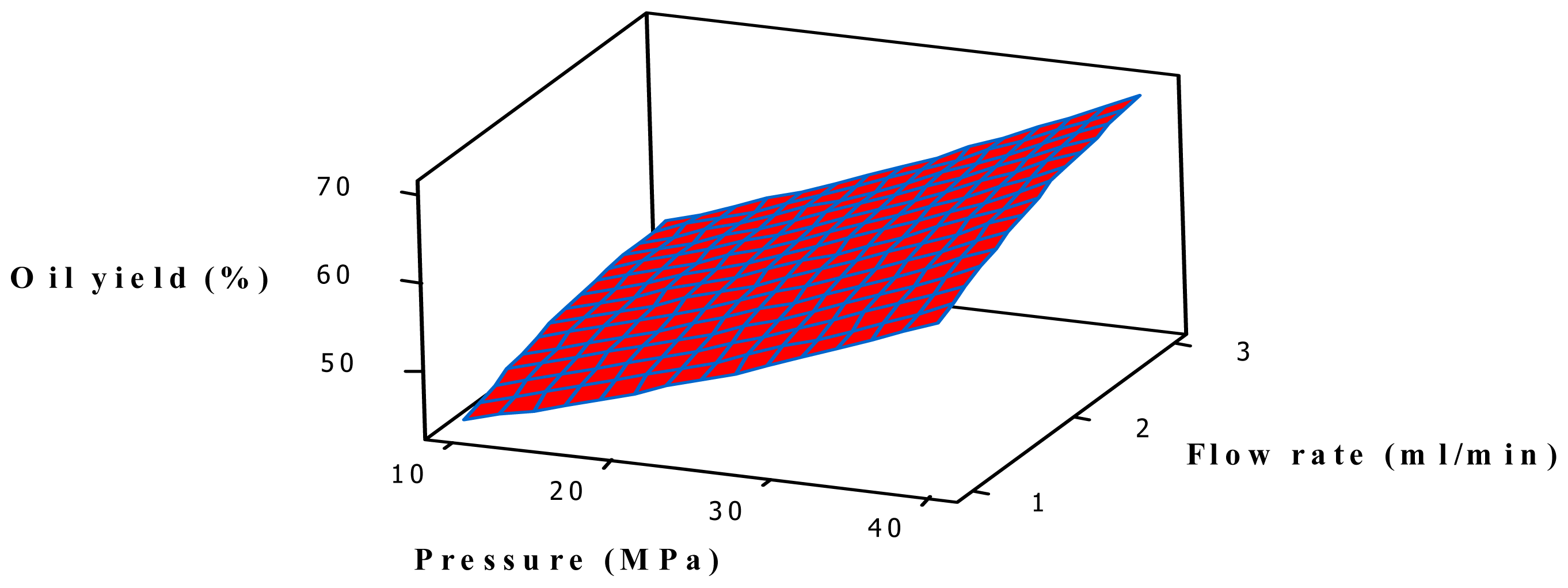
| Run Order | Blocks | Temperature (°C) | Pressure (MPa) | Flow rate (mL/min) | Soaking time (h) | Oil yield (%) | Predicted yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 57.50 | 25.0 | 2.0 | 4.00 | 58.2 ± 0.34 | 58.33 |
| 2 | 3 | 35.00 | 25.0 | 2.0 | 2.50 | 55.9 ± 0.43 | 56.00 |
| 3 | 3 | 57.50 | 25.0 | 3.0 | 2.50 | 59.0 ± 0.40 | 58.92 |
| 4 | 3 | 57.50 | 25.0 | 2.0 | 2.50 | 56.5 ± 0.22 | 56.57 |
| 5 | 3 | 57.50 | 25.0 | 1.0 | 2.50 | 51.0 ± 0.38 | 51.62 |
| 6 | 3 | 57.50 | 10.0 | 2.0 | 2.50 | 47.5 ± 0.39 | 48.22 |
| 7 | 3 | 80.00 | 25.0 | 2.0 | 2.50 | 55.2 ± 0.25 | 55.63 |
| 8 | 3 | 57.50 | 25.0 | 2.0 | 1.00 | 56.2 ± 0.39 | 56.60 |
| 9 | 3 | 57.50 | 25.0 | 2.0 | 2.50 | 56.6 ± 0.37 | 56.57 |
| 10 | 3 | 57.50 | 40.0 | 2.0 | 2.50 | 67.0 ± 0.17 | 66.82 |
| 11 | 1 | 68.75 | 32.5 | 1.5 | 3.25 | 59.8 ± 0.21 | 59.93 |
| 12 | 1 | 68.75 | 17.5 | 1.5 | 1.75 | 49.0 ± 0.42 | 48.87 |
| 13 | 1 | 46.25 | 32.5 | 2.5 | 3.25 | 62.9 ± 0.26 | 62.87 |
| 14 | 1 | 68.75 | 17.5 | 2.5 | 3.25 | 53.5 ± 0.41 | 53.43 |
| 15 | 1 | 57.50 | 25.0 | 2.0 | 2.50 | 56.5 ± 0.47 | 56.57 |
| 16 | 1 | 57.50 | 25.0 | 2.0 | 2.50 | 56.6 ± 0.37 | 56.57 |
| 17 | 1 | 46.25 | 17.5 | 2.5 | 1.75 | 53.9 ± 0.35 | 53.60 |
| 18 | 1 | 46.25 | 17.5 | 1.5 | 3.25 | 51.6 ± 0.27 | 51.57 |
| 19 | 1 | 68.75 | 32.5 | 2.5 | 1.75 | 63.6 ± 0.20 | 63.47 |
| 20 | 1 | 46.25 | 32.5 | 1.5 | 1.75 | 58.5 ± 0.32 | 58.40 |
| 21 | 2 | 46.25 | 32.5 | 1.5 | 3.25 | 59.7 ± 0.22 | 59.57 |
| 22 | 2 | 46.25 | 17.5 | 1.5 | 1.75 | 51.3 ± 0.29 | 50.85 |
| 23 | 2 | 68.75 | 17.5 | 1.5 | 3.25 | 50.4 ± 0.44 | 49.68 |
| 24 | 2 | 57.50 | 25.0 | 2.0 | 2.50 | 56.6 ± 0.37 | 56.57 |
| 25 | 2 | 57.50 | 25.0 | 2.0 | 2.50 | 56.6 ± 0.37 | 56.57 |
| 26 | 2 | 68.75 | 32.5 | 1.5 | 1.75 | 59.0 ± 0.45 | 58.67 |
| 27 | 2 | 46.25 | 17.5 | 2.5 | 3.25 | 54.1 ± 0.56 | 54.07 |
| 28 | 2 | 68.75 | 17.5 | 2.5 | 1.75 | 53.1 ± 0.59 | 52.87 |
| 29 | 2 | 68.75 | 32.5 | 2.5 | 3.25 | 64.4 ± 0.19 | 64.48 |
| 30 | 2 | 46.25 | 32.5 | 2.5 | 1.75 | 61.6 ± 0.26 | 61.95 |
| Factors | Codes | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Temperature (°C) | X1 | 35 | 57.5 | 80 |
| Pressure (MPa) | X2 | 10 | 25 | 40 |
| Flow rate (mL/min) | X3 | 1 | 2 | 3 |
| Soaking time (hr.) | X4 | 1 | 2.5 | 4 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sarker, M.Z.I.; Selamat, J.; Habib, A.S.M.A.; Ferdosh, S.; Akanda, M.J.H.; Jaffri, J.M. Optimization of Supercritical CO2 Extraction of Fish Oil from Viscera of African Catfish (Clarias gariepinus). Int. J. Mol. Sci. 2012, 13, 11312-11322. https://doi.org/10.3390/ijms130911312
Sarker MZI, Selamat J, Habib ASMA, Ferdosh S, Akanda MJH, Jaffri JM. Optimization of Supercritical CO2 Extraction of Fish Oil from Viscera of African Catfish (Clarias gariepinus). International Journal of Molecular Sciences. 2012; 13(9):11312-11322. https://doi.org/10.3390/ijms130911312
Chicago/Turabian StyleSarker, Mohamed Zaidul Islam, Jinap Selamat, Abu Sayem Md. Ahsan Habib, Sahena Ferdosh, Mohamed Jahurul Haque Akanda, and Juliana Mohamed Jaffri. 2012. "Optimization of Supercritical CO2 Extraction of Fish Oil from Viscera of African Catfish (Clarias gariepinus)" International Journal of Molecular Sciences 13, no. 9: 11312-11322. https://doi.org/10.3390/ijms130911312
APA StyleSarker, M. Z. I., Selamat, J., Habib, A. S. M. A., Ferdosh, S., Akanda, M. J. H., & Jaffri, J. M. (2012). Optimization of Supercritical CO2 Extraction of Fish Oil from Viscera of African Catfish (Clarias gariepinus). International Journal of Molecular Sciences, 13(9), 11312-11322. https://doi.org/10.3390/ijms130911312




