High Throughput Mining and Characterization of Microsatellites from Common Carp Genome
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microsatellite Statistics in Common Carp Genome
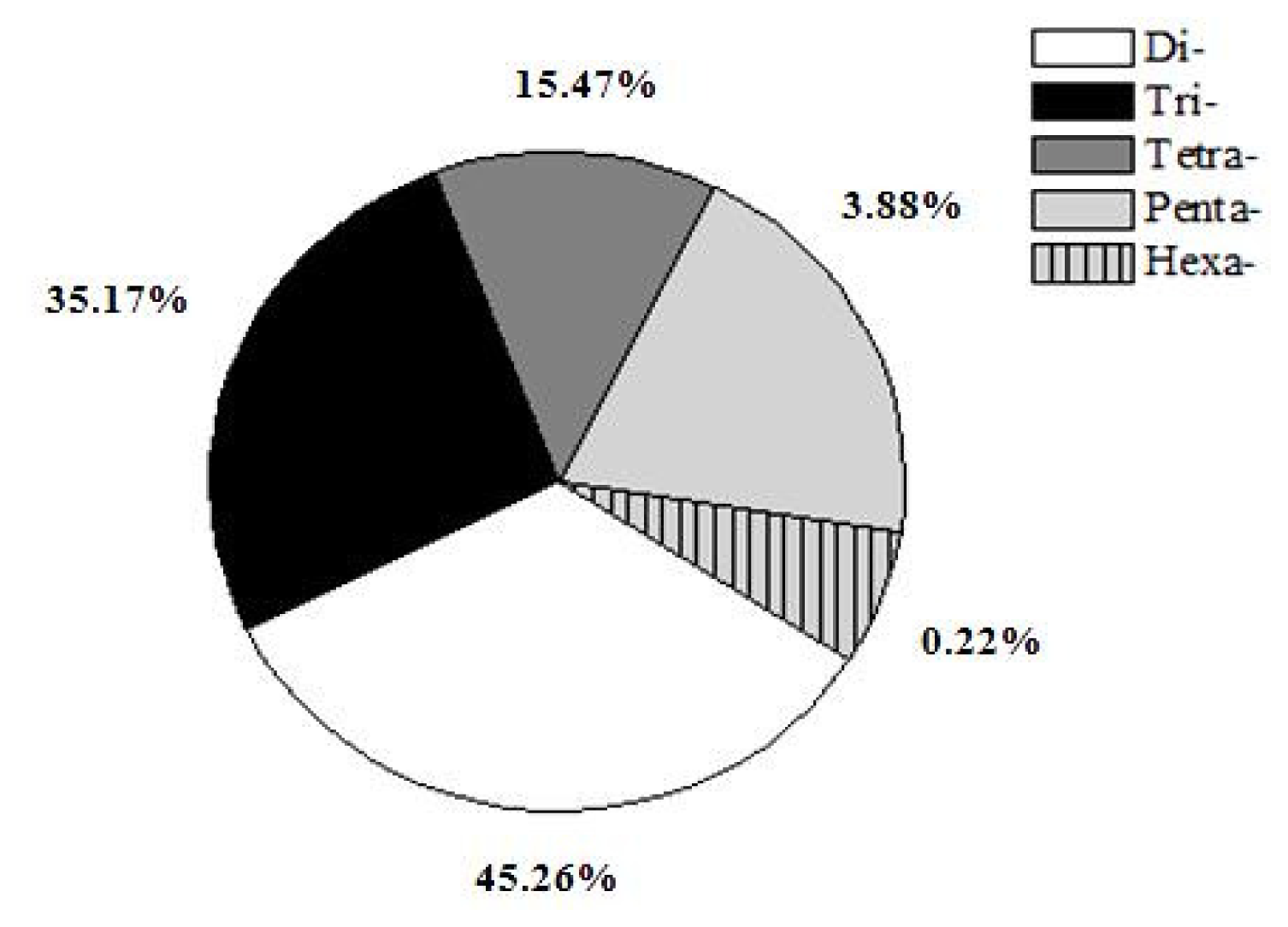
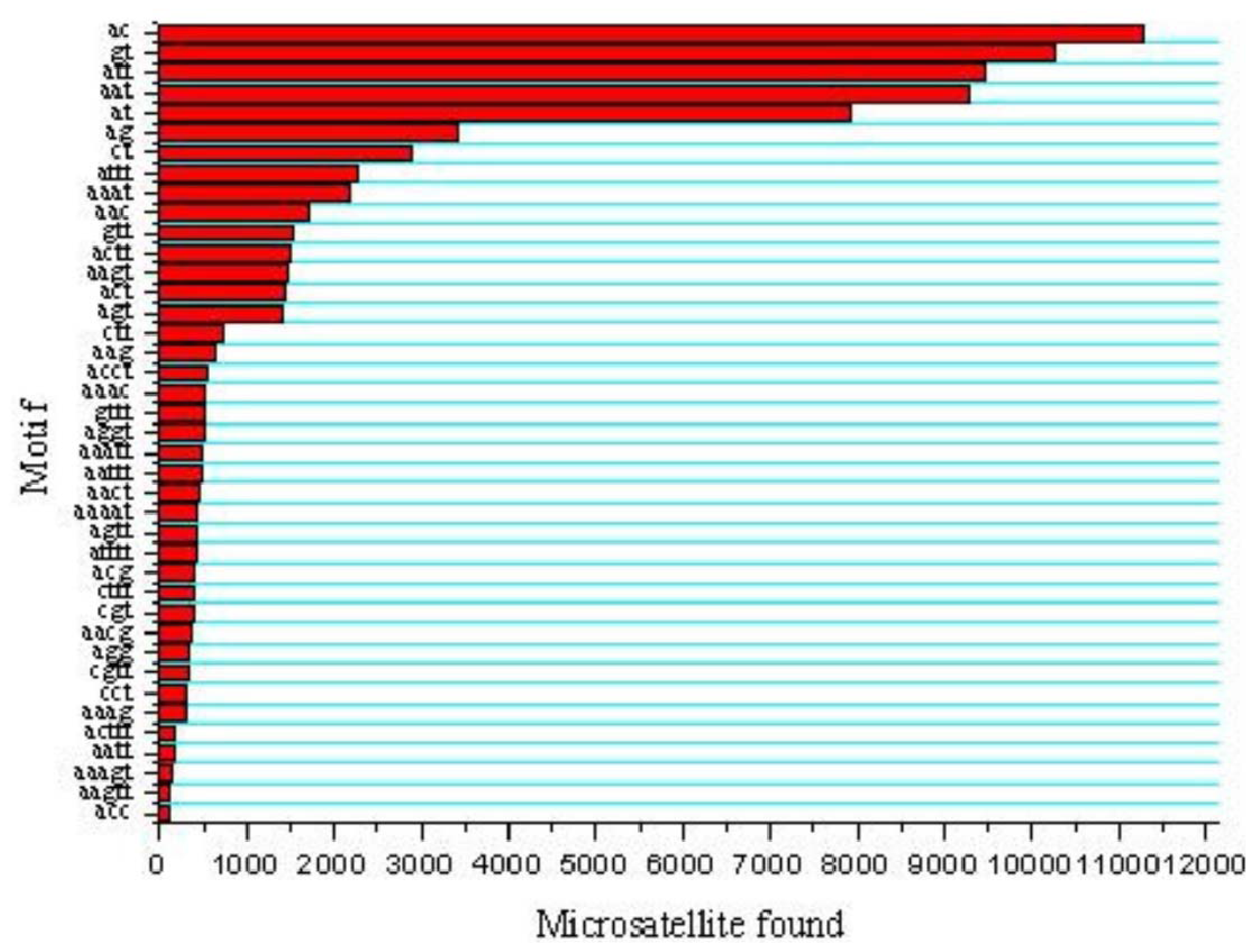
2.2. Characterization of Microsatellites
2.3. Database Construction for the Microsatellites
2.4. Microsatellite Loci Validation
3. Experimental Section
3.1. Whole Genome Shotgun Sequence Assembly
3.2. BAC End Sequences
3.3. Microsatellite Identification
3.4. Microsatellite Statistics and Characterization
3.5. Microsatellite Database Construction
3.6. Microsatellite Marker Validation and Genotyping
4. Conclusions
Acknowledgments
References
- FAO. Available online: http://www.fao.org/fishery/culturedspecies/Cyprinus_carpio/en accessed on 12 March 2012.
- David, L.; Rothbard, S.; Rubinstein, I.; Katzman, H.; Hulata, G.; Hillel, J.; Lavi, U. Aspects of red and black color inheritance in the Japanese ornamental (Koi) carp (Cyprinus carpio L.). Aquaculture 2004, 233, 129–147. [Google Scholar]
- Sun, X.; Liang, L. A genetic linkage map of common carp (Cyprinus carpio L.) and mapping of a locus associated with cold tolerance. Aquaculture 2004, 238, 165–172. [Google Scholar]
- Zhou, J.; Wu, Q.; Wang, Z.; Ye, Y. Genetic variation analysis within and among six varieties of common carp (Cyprinus carpio L.) in China using microsatellite markers. Genetika 2004, 40, 1389–1393. [Google Scholar]
- Zhang, Y.; Liang, L.; Jiang, P.; Li, D.; Lu, C.; Sun, X. Genome evolution trend of common carp (Cyprinus carpio L.) as revealed by the analysis of microsatellite loci in a gynogentic family. J. Genet. Genomics 2008, 35, 97–103. [Google Scholar]
- Cheng, L.; Liu, L.; Yu, X.; Wang, D.; Tong, J. A linkage map of common carp (Cyprinus carpio) based on AFLP and microsatellite markers. Anim. Genet 2010, 41, 191–198. [Google Scholar]
- Kongchum, P.; Palti, Y.; Hallerman, E.M.; Hulata, G.; David, L. SNP discovery and development of genetic markers for mapping innate immune response genes in common carp (Cyprinus carpio). Fish Shellfish Immunol 2010, 29, 356–361. [Google Scholar]
- Kohlmann, K.; Kersten, P.; Flajshans, M. Microsatellite-based genetic variability and differentiation of domesticated, wild and feral common carp (Cyprinus carpio L.) populations. Aquaculture 2005, 247, 253–266. [Google Scholar]
- Yue, G.; David, L.; Orban, L. Mutation rate and pattern of microsatellites in common carp (Cyprinus carpio L.). Genetica 2007, 129, 329–331. [Google Scholar]
- Hulak, M.; Kaspar, V.; Kohlmann, K.; Coward, K.; Tesitel, J.; Rodina, M.; Gela, D.; Kocour, M.; Linhart, O. Microsatellite-based genetic diversity and differentiation of foreign common carp (Cyprinus carpio) strains farmed in the Czech Republic. Aquaculture 2010, 298, 194–201. [Google Scholar]
- Ludanny, R.; Chrisanfova, G.; Prizenko, V.; Bogeruk, A.; Semyenova, S. Polymorphism of microsatellite markers in Russian common carp (Cyprinus carpio L.) breeds. Russ. J. Genet 2010, 46, 572–577. [Google Scholar]
- Zheng, X.; Kuang, Y.; Zhang, X.; Lu, C.; Cao, D.; Li, C.; Sun, X. A genetic linkage map and comparative genome analysis of common carp (Cyprinus carpio L.) using microsatellites and SNPs. Mol. Genet. Genomics 2011, 286, 261–277. [Google Scholar]
- Zhang, Y.; Liang, L.Q.; Chang, Y.M.; Hou, N.; Lu, C.Y.; Sun, X.W. Mapping and genetic effect analysis of quantitative trait loci related to body size in common carp (Cyprinus carpio L.). Yi Chuan 2007, 29, 1243–1248. [Google Scholar]
- Mao, R.X.; Liu, F.J.; Zhang, X.F.; Zhang, Y.; Cao, D.C.; Lu, C.Y.; Liang, L.Q.; Sun, X.W. Studies on quantitative trait loci related to activity of lactate dehydrogenase in common carp (Cyprinus carpio). Yi Chuan 2009, 31, 407–411. [Google Scholar]
- Zhang, Y.; Xu, P.; Lu, C.; Kuang, Y.; Zhang, X.; Cao, D.; Li, C.; Chang, Y.; Hou, N.; Li, H.; et al. Genetic linkage mapping and analysis of muscle fiber-related QTLs in common carp (Cyprinus carpio L.). Mar. Biotechnol 2011, 13, 376–392. [Google Scholar]
- Li, Y.; Xu, P.; Zhao, Z.; Wang, J.; Zhang, Y.; Sun, X.W. Construction and characterization of the bac library for common carp (Cyprinus carpio L.) and establishment of microsynteny with zebrafish. Danio Rerio Mar. Biotechnol 2011, 13, 706–712. [Google Scholar]
- Xu, P.; Li, J.; Li, Y.; Cui, R.; Wang, J.; Wang, J.; Zhang, Y.; Zhao, Z.; Sun, X. Genomic insight into the common carp (Cyprinus carpio) genome by sequencing analysis of BAC-end sequences. BMC Genomics 2011, 12, 188. [Google Scholar]
- Xu, P.; Wang, J.; Wang, J.; Cui, R.; Li, Y.; Zhao, Z.; Ji, P.; Zhang, Y.; Li, J.; Sun, X. Generation of the first BAC-based physical map of the common carp genome. BMC Genomics 2011, 12, 537. [Google Scholar]
- Tiersch, T.R.; Chandler, R.W.; Wachtel, S.S.; Elias, S. Reference standards for flow cytometry and application in comparative studies of nuclear DNA content. Cytometry 1989, 10, 706–710. [Google Scholar]
- Xu, P.; Wang, S.; Liu, L.; Peatman, E.; Somridhivej, B.; Thimmapuram, J.; Gong, G.; Liu, Z. Channel catfish BAC-end sequences for marker development and assessment of syntenic conservation with other fish species. Anim. Genet 2006, 37, 321–326. [Google Scholar]
- Somridhivej, B.; Wang, S.; Sha, Z.; Liu, H.; Quilang, J.; Xu, P.; Li, P.; Hu, Z.; Liu, Z. Characterization, polymorphism assessment & database construction for microsatellites from BAC end sequences of channel catfish (Ictalurus punctatus): A resource for integration of linkage and physical maps. Aquaculture 2008, 275, 76–80. [Google Scholar]
- Ji, P.; Liu, G.; Xu, J.; Wang, X.; Li, J.; Zhao, Z.; Zhang, X.; Zhang, Y.; Xu, P.; Sun, X. Characterization of common carp transcriptome: Sequencing, de novo assembly, annotation and comparative genomics. PLoS One 2012, 7, e35152. [Google Scholar]
- Soler, L.; Conte, M.A.; Katagiri, T.; Howe, A.E.; Lee, B.Y.; Amemiya, C.; Stuart, A.; Dossat, C.; Poulain, J.; Johnson, J.; et al. Comparative physical maps derived from BAC end sequences of tilapia (Oreochromis niloticus). BMC Genomics 2010, 11, 636. [Google Scholar]
- Gendrel, C.G.; Boulet, A.; Dutreix, M. (CA/GT)(n) microsatellites affect homologous recombination during yeast meiosis. Genes Dev. 2000, 14, 1261–1268. [Google Scholar]
- Tong, J.; Wang, Z.; Yu, X.; Wu, Q.; Chu, K.H. Cross-species amplification in silver carp and bighead carp with microsatellite primers of common carp. Mol. Ecol. Notes 2002, 2, 245–247. [Google Scholar]
- Yue, G.; Ho, M.Y.; Orban, L.; Komen, J. Microsatellites within genes and ESTs of common carp and their applicability in silver crucian carp. Aquaculture 2004, 234, 85–98. [Google Scholar]
- Thurston, M.I.; Field, D. Msatfinder: Detection and characterisation of microsatellites. Available online: http://www.genomics.ceh.ac.uk/msatfinder/ accessed on 12 April 2010.
- Neilan, B.A.; Wilton, A.N.; Jacobs, D. A universal procedure for primer labelling of amplicons. Nucleic Acids Res 1997, 25, 2938–2939. [Google Scholar]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol 2000, 18, 233–234. [Google Scholar]
| WGS | BES | |
|---|---|---|
| WGS sequences surveyed | 982,074 a | 65,720 b |
| Base pairs surveyed (bp) | 504,292,105 a | 42,522,168 b |
| Microsatellite found | 79,014 a | 13,581 b |
| Sequences harboring microsatellites | 68,827 a | 10,355 b |
| Sequences harboring only one microsatellites | 60,259 a | 8,069 b |
| Sequences harboring two microsatellites | 7,269 a | 1,682 b |
| Sequences harboring three or more microsatellites | 1,299 a | 604 b |
| Microsatellite sequences with sufficient flanking regions on both sides * | 24,898 a | 5,150 b |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, P.; Zhang, Y.; Li, C.; Zhao, Z.; Wang, J.; Li, J.; Xu, P.; Sun, X. High Throughput Mining and Characterization of Microsatellites from Common Carp Genome. Int. J. Mol. Sci. 2012, 13, 9798-9807. https://doi.org/10.3390/ijms13089798
Ji P, Zhang Y, Li C, Zhao Z, Wang J, Li J, Xu P, Sun X. High Throughput Mining and Characterization of Microsatellites from Common Carp Genome. International Journal of Molecular Sciences. 2012; 13(8):9798-9807. https://doi.org/10.3390/ijms13089798
Chicago/Turabian StyleJi, Peifeng, Yan Zhang, Chao Li, Zixia Zhao, Jian Wang, Jiongtang Li, Peng Xu, and Xiaowen Sun. 2012. "High Throughput Mining and Characterization of Microsatellites from Common Carp Genome" International Journal of Molecular Sciences 13, no. 8: 9798-9807. https://doi.org/10.3390/ijms13089798
APA StyleJi, P., Zhang, Y., Li, C., Zhao, Z., Wang, J., Li, J., Xu, P., & Sun, X. (2012). High Throughput Mining and Characterization of Microsatellites from Common Carp Genome. International Journal of Molecular Sciences, 13(8), 9798-9807. https://doi.org/10.3390/ijms13089798




