Neuroprotective Effects of Germinated Brown Rice against Hydrogen Peroxide Induced Cell Death in Human SH-SY5Y Cells
Abstract
:1. Introduction
2. Results and Discussion
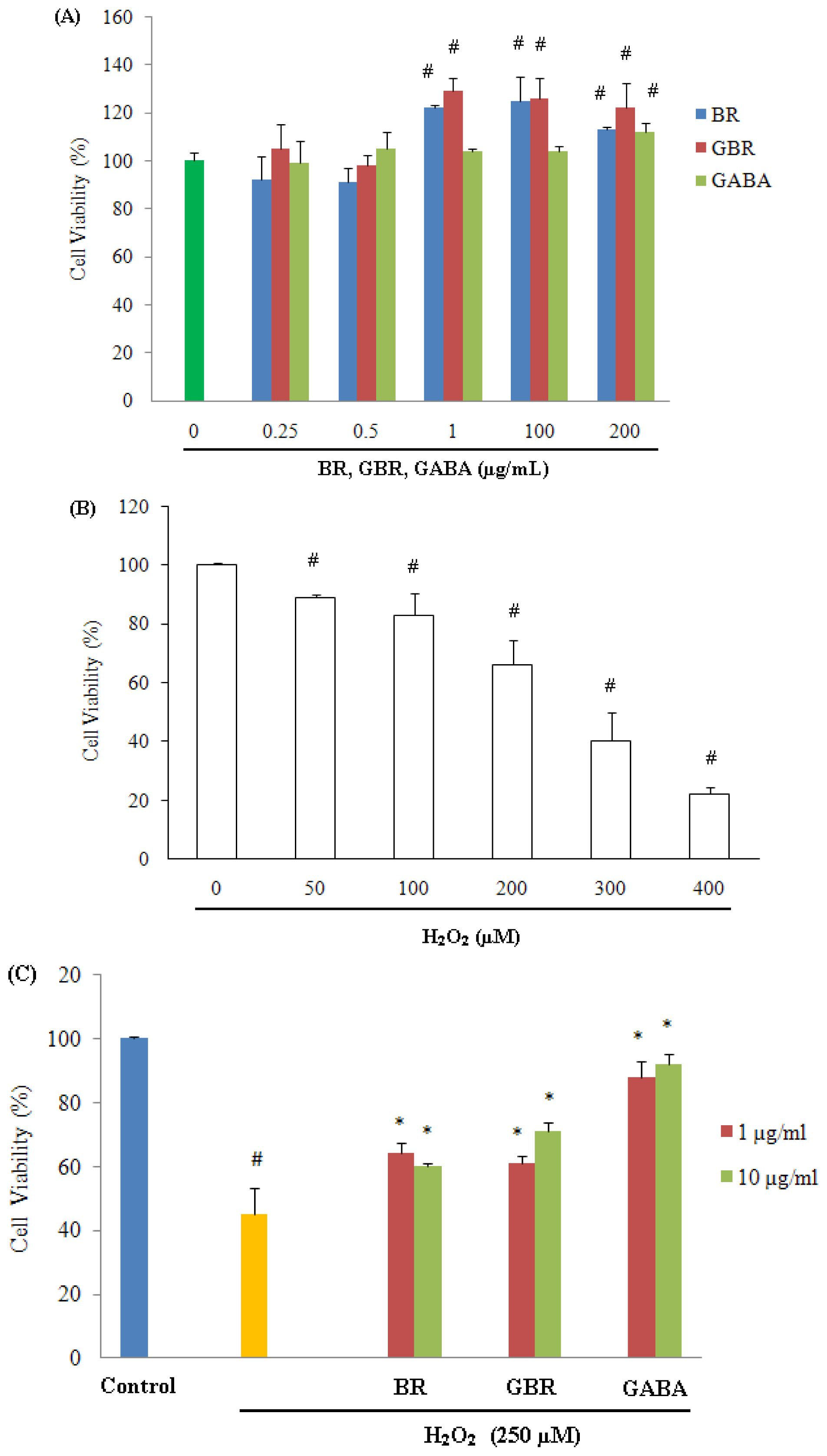
2.1. GABA, GBR and BR Preserved SH-SY5Y Cells against H2O2-Induced Cytotoxicity
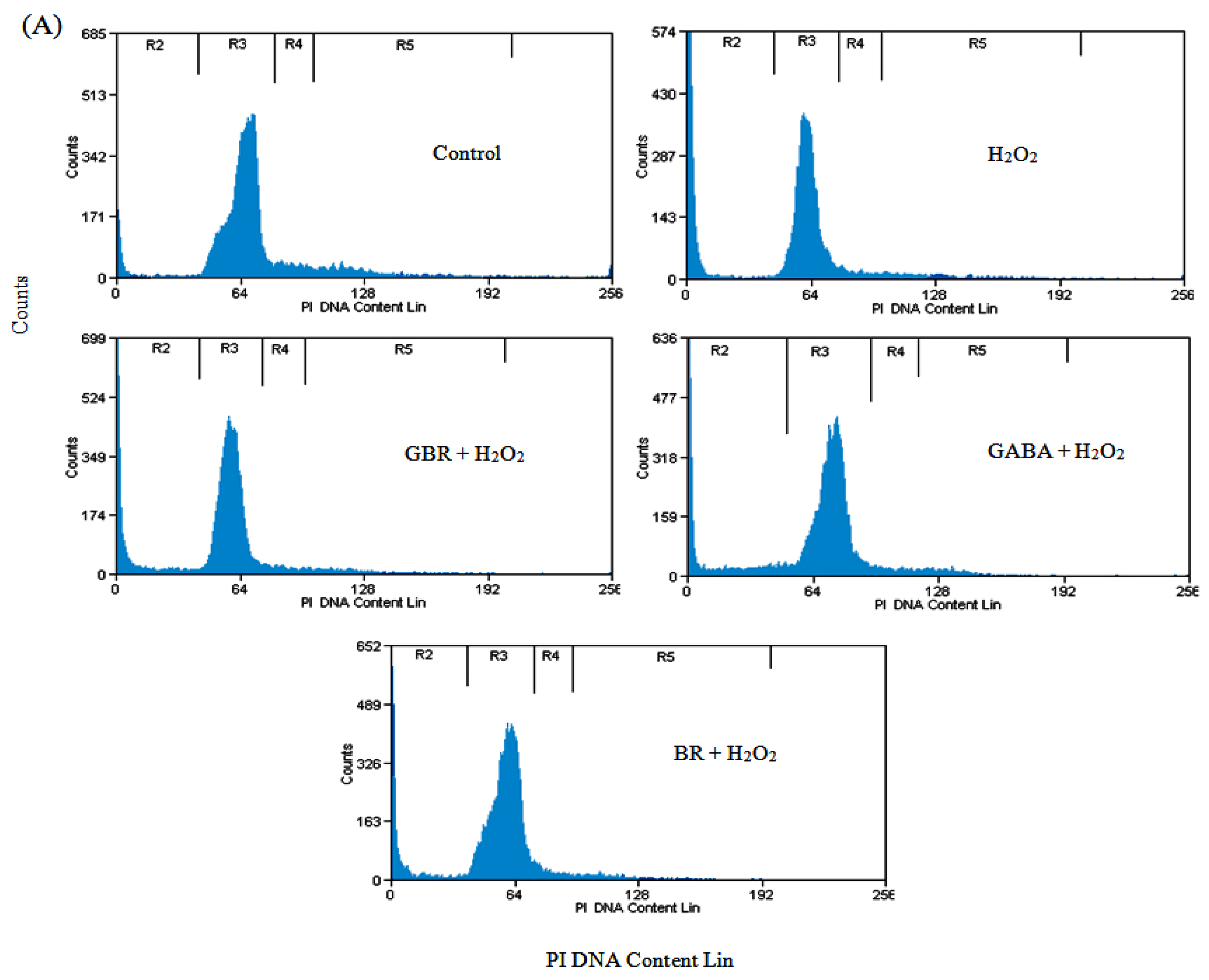
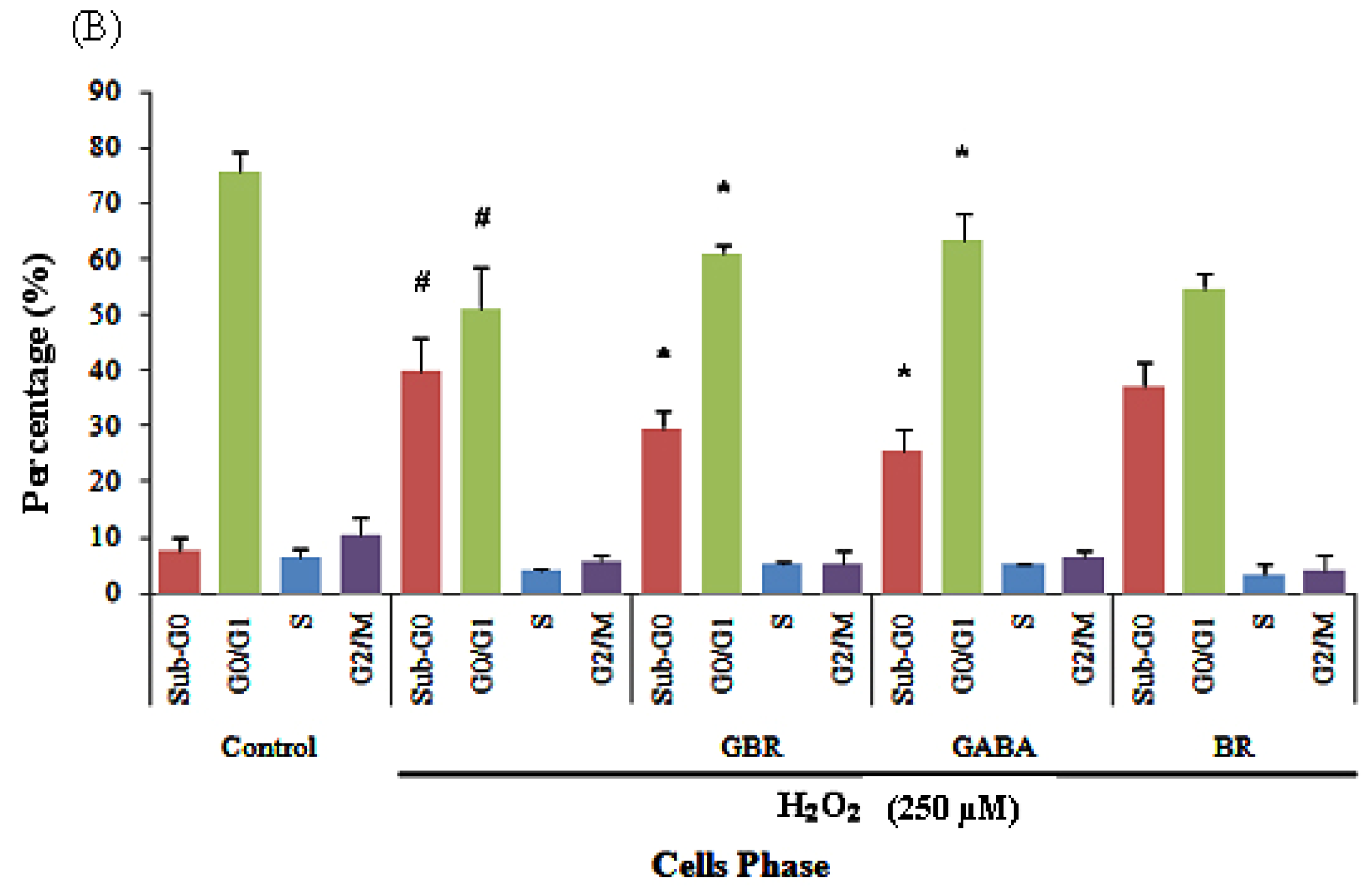
2.2. GBR and GABA Arrested SH-SY5Y Cells at G0/G1 Phase
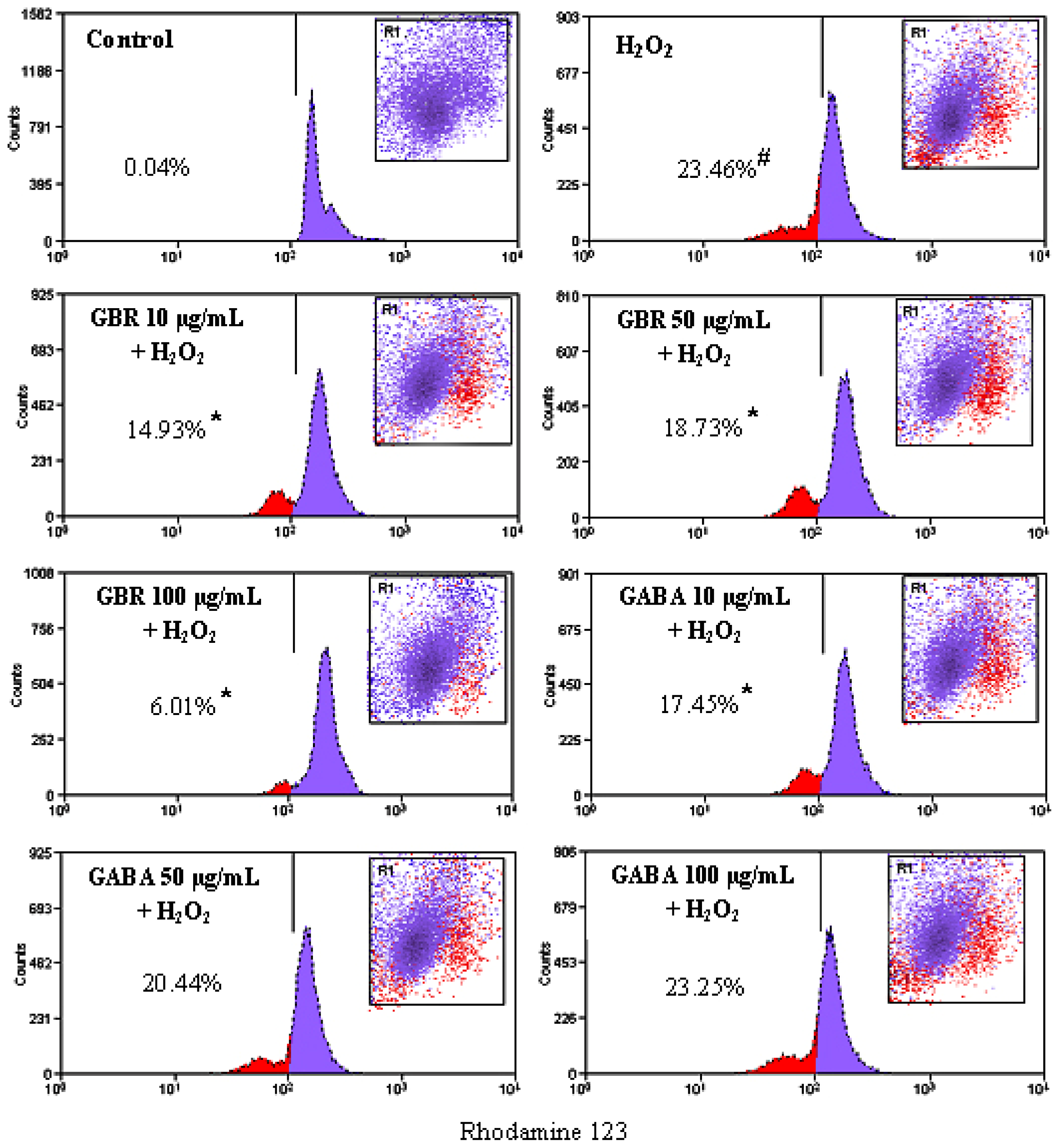
2.3. GBR and GABA Prevented H2O2-Induced Reduction of Mitochondrial Membrane Potential (MMP) in SH-SY5Y Cells
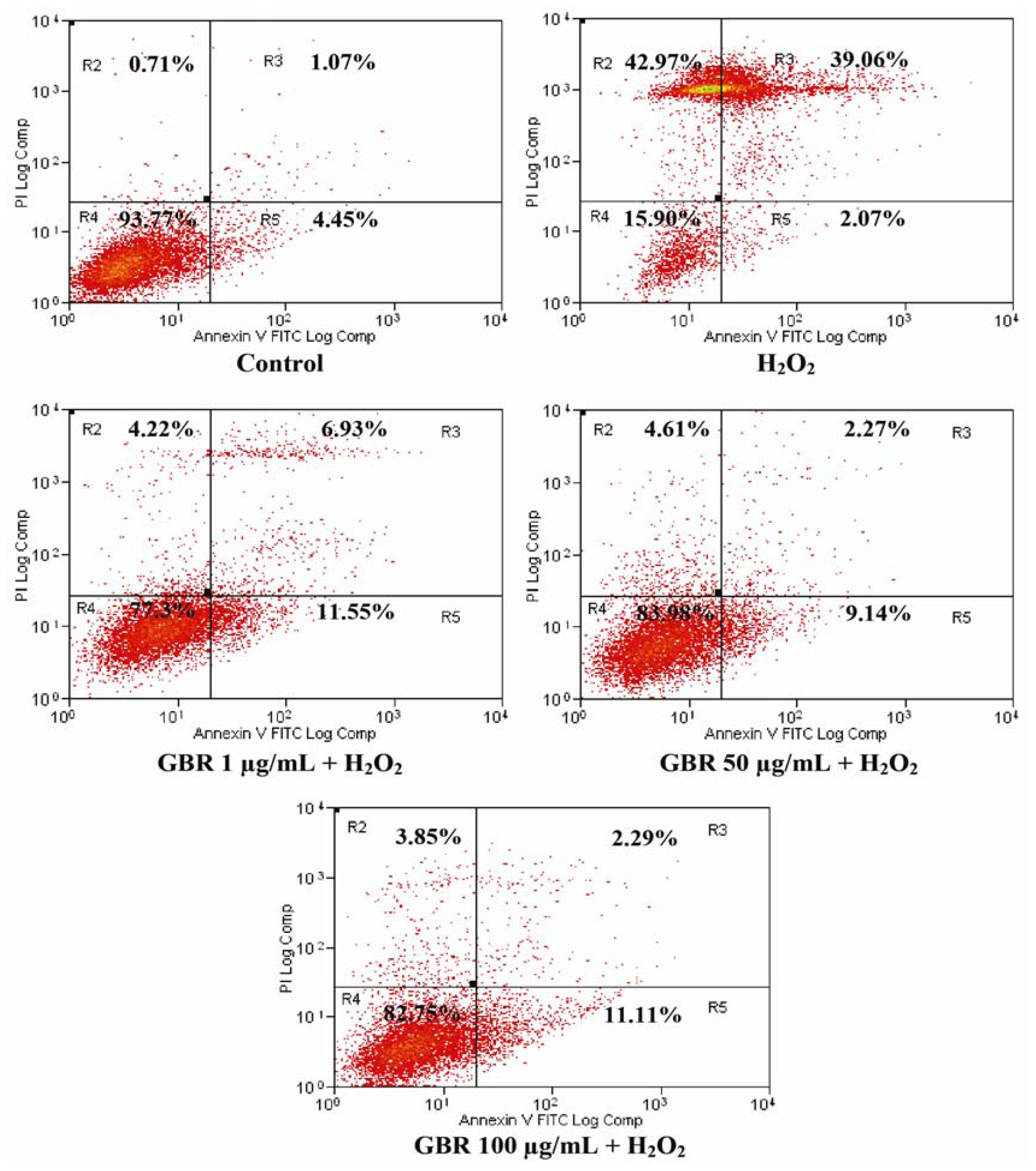
2.4. GBR Prevented H2O2-Induced Apoptosis and Necrosis in SH-SY5Y Cells
2.5. Antioxidant Activities of GBR, BR and GABA
3. Experimental Section
3.1. Materials
3.2. Production of GBR
3.3. Extraction of GBR and BR
3.4. Determination of GABA Content in GBR and BR
3.5. Cell Culture
3.6. MTT Assay
3.7. Cell Cycle Analyses
3.8. Measurement of Mitochondrial Membrane Potential (MMP)
3.9. Annexin V-FITC and Propidium Iodide Staining Assay
3.10. DPPH Radical Scavenging Assay
3.11. ABTS Radical Cation Scavenging Assay
3.12. Ferrous Ion Chelating Ability Assay
3.13. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: Role of ERK1/2 in H2O2-induced cell death. Eur. J. Clin. Pharmacol 2004, 483, 163–173. [Google Scholar]
- Fordel, E.; Thijs, L.; Martinet, W.; Lenjou, M.; Lauf, T.; Bockstaele, D.V.; Moens, L.; Dewild, S. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci. Lett 2006, 410, 146–151. [Google Scholar]
- Kwon, S.H.; Kim, J.A.; Hong, S.I.; Jung, Y.H.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem. Int 2011, 58, 533–541. [Google Scholar]
- King, K.L.; Cidlowski, J.A. Cell cycle and apoptosis: Common pathways to life and death. J. Cell Biochem 1995, 58, 175–180. [Google Scholar]
- Sherr, C.J. G1 phase progression: Cycling on cue. Cell 1994, 79, 551–555. [Google Scholar]
- Finney, P.L. Potential for the Use of Germinated Wheat and Soybeans to Enhance Human Nutrition. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 1978; pp. 681–701. [Google Scholar]
- Tkachuk, R. Free amino acids in germinated wheat. J. Sci. Food Agric 1979, 30, 53–58. [Google Scholar]
- Saikusa, T.; Horino, T.; Mori, Y. Distribution of free amino acids in the rice kernel and kernel fractions and the effect of water soaking on the distribution. J. Agric. Food Chem 1994, 42, 1122–1126. [Google Scholar]
- Kayahara, H.; Tsukahara, K. Flavor, Health and Nutritional Quality of Pre-Spruted Brown Rice. Proceedings of International Chemical Congress of Pacific Basin Societies, Honolulu, HI, USA, 14–19 December 2000.
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice and germinated brown rice. J. Agric. Food Chem 2004, 52, 4808–4813. [Google Scholar]
- Komatsuzaki, N.; Tsukahara, K.; Toyoshima, H.; Suzuki, T.; Shimizu, N.; Kimura, T. Effect of soaking and gaseous treatment on GABA content in germinated brown rice. J. Food Eng 2007, 58, 556–560. [Google Scholar]
- Usuki, S.; Ito, Y.; Morikawa, K.; Kise, M.; Ariga, T.; Rivner, M.; Yu, R.K. Effect of pre-germinated brown rice intake on diabetic neuropathy in streptozotocin-induced diabetic rats. Nutr. Metab 2007, 4. [Google Scholar] [CrossRef]
- Esa, N.M.; Kadir, K.K.A.; Amom, Z.; Azlan, A. Improving the lipid profile in hypercholesterolemia-induced rabbit by supplementation of germinated brown rice. J. Agric. Food Chem 2011, 59, 7985–7991. [Google Scholar]
- Norazalina, S.; Norhaizan, M.E.; Hairuszah, I.; Nurul-Husna, S. Optimization of optimum condition for phytic acid extraction from rice bran. Afr. J. Plant Sci 2011, 5, 168–176. [Google Scholar]
- Zhang, R.; Lu, H.; Tian, S.; Yin, J.; Chen, Q.; Ma, L.; Cui, S.; Niu, Y. Protective effects of pre-germinated brown rice diet on low levels of Pb-induced learning and memory deficits in developing rat. Chem. Biol. Interact 2010, 184, 484–491. [Google Scholar]
- Mamiya, T; Kise, M.; Morikawa, K.; Aoto, H.; Ukai, M.; Noda, Y. Effects of pre-germinated brown rice on depression-like behavior in mice. Pharmacol. Biochem. Behav. 2007, 86, 62–67. [Google Scholar]
- Mamiya, T.; Asanuma, T.; Kise, M.; Ito, Y.; Mizukuchi, A.; Aoto, H.; Ukai, M. Effects of pre-germinated brown rice on β-amyloid protein-induced learning and memory deficits in mice. Biol. Pharm. Bull. 2004, 27, 1041–1045. [Google Scholar]
- Soi-Ampornkul, R.; Junnu, S.; Kanyok, S.; Liammongkolkul, S.; Katanyoo, W.; Umpornsirirat, S. Antioxidative and neuroprotective activities of the pre-germinated brown rice extract. Food Nutr. Sci 2012, 3, 135–140. [Google Scholar]
- Jung, C.H.; Hong, M.H.; Kim, J.H.; Lee, J.Y.; Ko, S.G.; Cho, K.; Seog, H.M. Protective effect of a phenolic-rich fraction from Schisandra chinensis against H2O2-induced apoptosis in SH-SY5Y cells. J. Pharm. Pharmacol 2007, 59, 455–462. [Google Scholar]
- Olivieri, G.; Otten, U.; Meier, F.; Baysang, G.; Dimitriades-Schmutz, B.; Muller-Spahn, F.; Savaskan, E. Amyloid modulates tyrosine kinase B receptor expression in SHSY5Y neuroblastoma cells: Influence of the antioxidant melatonin. Neuroscience 2003, 120, 659–665. [Google Scholar]
- Ismail, N.; Ismail, M.; Latiff, L.; Mazlan, M.; Mariod, A. Black cumin seed (Nigella sativa Linn.) oil and its fractions protect against beta amyloid peptide-induced toxicity in primary cerebellar granule neurons. J. Food Lipids 2008, 15, 519–533. [Google Scholar]
- Jeffrey, A.K.; Susan, L.A. Oxidative stress, cell cycle, and neurodegeneration. J. Clin. Invest 2003, 111, 785–793. [Google Scholar]
- Kruman, I.I. Why do neurons enter the cell cycle? Cell Cycle 2004, 3, 769–773. [Google Scholar]
- Folch, J.; Junyent, F.; Verdaguer, E.; Auladell, C.; Pizarro, J.G.; Beas-Zarate, C.; Pallàs, M.; Camins, A. Role of cell cycle re-rntry in neurons: A common apoptotic mechanism of neuronal cell death. Neurotox. Res 2011. [Google Scholar] [CrossRef]
- Schwartz, E.I.; Smilenov, L.B.; Price, M.A.; Osredkar, T.; Baker, R.A.; Ghosh, S.; Shi, F.D.; Vollmer, T.L.; Lencinas, A.; Stearns, D.M.; et al. Cell cycle activation in postmitotic neurons is essential for DNA repair. Cell Cycle 2007, 6, 318–329. [Google Scholar]
- Wang, W.; Sun, F.; An, Y.; Ai, H.; Zhang, L.; Huang, W.; Li, L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharmacol 2009, 613, 19–23. [Google Scholar]
- Wang, W.; Huang, W.T.; Li, L.; Ai, H.X.; Sun, F.L.; Liu, C.; An, Y. Morroniside prevents peroxide-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells. Cell Mol. Neurobiol 2008, 28, 293–305. [Google Scholar]
- Wettasinghe, M.; Shahidi, F. Scavenging of reactive-oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chem. 2000, 70, 17–26. [Google Scholar]
- Netzel, M.; Strass, G.; Bitsch, I.; Könitz, R.; Christmann, M.; Bitsch, R. Effect of grape processing on selected antioxidant phenolics in red wine. J. Food Eng 2003, 56, 223–228. [Google Scholar]
- Miller, D.M. Minerals, 3rd ed; Marcel Dekker: New York, NY, USA, 1996; pp. 618–647. [Google Scholar]
- Thompson, M.; Williams, C.R. Stability of flavonoid complexes of copper (II) and flavonoid antioxidant activity. Anal. Chim. Acta 1976, 85, 375–381. [Google Scholar]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice and germinated brown rice. J. Agric. Food Chem 2004, 52, 4808–4813. [Google Scholar]
- Xu, Q.; Kanthasamy, A.G.; Reddy, M.B. Phytic acid protects against 6-hydroxydopamine-induced dopaminergic neuron apoptosis in normal and iron excess conditions in a cell culture model. Park. Dis 2011. [Google Scholar] [CrossRef]
- Mamiya, T; Kise, M; Morikawa, K. Ferulic acid attenuated cognitive deficits and increase in carbonyl proteins induced by buthionine-sulfoximine in mice. Neurosci. Lett. 2008, 430, 115–118. [Google Scholar]
- Guizani, N.; Waly, M.I.; Ali, A.; Al-Saidi, G.; Singh, V.; Bhatt, N.; Rahman, M.S. Papaya epicarp extract protects against hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuronal cells. Exp. Bio. Med 2011, 236, 1205–1210. [Google Scholar]
- Fukui, K.; Takatsu, H.; Koike, T.; Urano, S. Hydrogen peroxide induces neurite degeneration: Prevention by tocotrienols. Free Radic. Res 2011, 45, 681–691. [Google Scholar]
| Samples | GABA content (μg/mL) | DPPH (mg TE/g extract) | ABTS (mg TE/g extract) | Ferrous ion chelating (mg EDTAE/g extract) |
|---|---|---|---|---|
| GBR | 50 ± 1.22 a | 5.66 ± 0.04 a | 13.97 ± 0.12 a | 4.55 ± 0.09 a |
| BR | 9 ± 0.54 b | 4.32 ± 0.39 b | 11.82 ± 0.50 b | 4.26 ± 0.24 a |
| GABA | NA | 1.77 ± 0.46 c | 0.96 ± 0.29 c | 0.27 ± 0.18 b |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ismail, N.; Ismail, M.; Farhana Fathy, S.; Asma Musa, S.N.; Umar Imam, M.; Foo, J.B.; Iqbal, S. Neuroprotective Effects of Germinated Brown Rice against Hydrogen Peroxide Induced Cell Death in Human SH-SY5Y Cells. Int. J. Mol. Sci. 2012, 13, 9692-9708. https://doi.org/10.3390/ijms13089692
Ismail N, Ismail M, Farhana Fathy S, Asma Musa SN, Umar Imam M, Foo JB, Iqbal S. Neuroprotective Effects of Germinated Brown Rice against Hydrogen Peroxide Induced Cell Death in Human SH-SY5Y Cells. International Journal of Molecular Sciences. 2012; 13(8):9692-9708. https://doi.org/10.3390/ijms13089692
Chicago/Turabian StyleIsmail, Norsharina, Maznah Ismail, Siti Farhana Fathy, Siti Nor Asma Musa, Mustapha Umar Imam, Jhi Biau Foo, and Shahid Iqbal. 2012. "Neuroprotective Effects of Germinated Brown Rice against Hydrogen Peroxide Induced Cell Death in Human SH-SY5Y Cells" International Journal of Molecular Sciences 13, no. 8: 9692-9708. https://doi.org/10.3390/ijms13089692
APA StyleIsmail, N., Ismail, M., Farhana Fathy, S., Asma Musa, S. N., Umar Imam, M., Foo, J. B., & Iqbal, S. (2012). Neuroprotective Effects of Germinated Brown Rice against Hydrogen Peroxide Induced Cell Death in Human SH-SY5Y Cells. International Journal of Molecular Sciences, 13(8), 9692-9708. https://doi.org/10.3390/ijms13089692





