Abstract
Novel hybrid xerogel materials were synthesized by a sol-gel procedure. n-octadecyltriethoxysilane was co-condensed with and without different cross-linkers using Q0 and T0 mono-functionalized organosilanes in the presence of n-hexadecylamine with different hydroxyl silica functional groups at the surface. These polymer networks have shown new properties, for example, a high degree of cross-linking and hydrolysis. Two different synthesis steps were carried out: simple self-assembly followed by sol-gel transition and precipitation of homogenous sols. Due to the lack of solubility of these materials, the compositions of the new materials were determined by infrared spectroscopy, 13C and 29Si CP/MAS NMR spectroscopy and scanning electron microscopy.
1. Introduction
Alkyl stationary phases are widely used in liquid chromatography (LC). Much effort has been made to prepare and describe the chromatographic properties [1]. In this field, the application of stationary phases based on silica gel is very popular. For the successful employment of silica, it is of great importance that the silica beads show a narrow particle size distribution and spherical shape. Most HPLC separations are carried out under reversed phase (RP) conditions [2]. The surface modification of well-defined silica beads with T-silyl functionalized organic systems is the major route to create phases for reversed-phase liquid chromatography (RPLC) [3–5]. All the RP prepared by the different conventional methods (solution or surface polymerized modification and monomeric synthesis) mentioned above do not exhibit complete cross-linked T and Q units. For long-term stability of stationary phases it is very important to prepare one with a high degree of cross-linked ligands. This may be achieved using the sol-gel process.
Sol-gel processing of polysiloxanes to prepare hybrid inorganic-organic materials (HIOM) [6–9] is quite a promising technique in field of chromatography [10,11]. It has been widely investigated to prepare potential matrices for reporter molecules in chemistry sensors [12]. More recently, such materials have also received attention as catalyst supports, enzymes and catalytically active transitions metal complexes [13].
Recently, many investigations were extended to nanostructure mesoporous silicas which are based on inorganic-organic hybrid polymers [14–19]. These materials possess extremely high surface areas and accessible pores. Moreover, the pore size can be tuned with different sizes in the nanometer range by choosing template systems or with a co-solvent [15]. For the generation of stationary phases, T-functionalized silanes of the type Fn-Si(OR)3 were sol-gel processed with and without co-condensation agents, which play an important role in controlling the density and the distance of the reactive centers; in general Fn represents either alkyl spacer alone or end by a metal complex [20]. These reactive centers are distributed across the entire carrier matrix and play an important role in catalysis [21–38].
In this work, hybrid materials were synthesized by a simple one-step self assembly followed by co-condensation of different silane cross-linkers with n-octadecyltrialkoxysilane as the chromatographic selector in the presence of n-hexadecylamine surfactant. Application of different alkoxysilane co-condensation agents, such as Si(OEt)4, Si(OMe)4 (Q0) and MeSi(OMe)3 (T0) during the sol-gel processes enable the building of polymer frameworks with different hydroxyl silica functional groups and new properties compared to surface modified silica gel.
2. Results and Discussion
2.1. Sol-Gel Processing
The properties of the sol-gel processed products strongly depend on the reaction conditions, such as type of solvent, temperature, time, catalysts, concentration of the monomers and type of the cross-linkers [9,10]. To ensure comparable results, uniform reaction conditions throughout hydrolysis and the sol-gel transition have been maintained. Alcohol is necessary to homogenize the reaction mixture. All poly-condensations were performed in a mixture of ethanol with a certain amount of water as catalyst for the sol-gel process (see Experimental section). Long alkyl chains commonly used for liquid chromatography were introduced by using the CH3(CH2)17Si(OEt)3, n-octadecyltriethoxysilane (X), in the presence of n-hexadecylamine as template, which concomitantly serves as material to allow mesoporous hybrid compounds. During the sol-gel process, a fixed ratio of X, template and co-condensation agents [1:9:9], respectively, were used. Several types of co-condensation agents used, such as Si(OEt)4 (TEOS), Si(OMe)4 (TMOS) and MeSi(OMe)3, are shown in Scheme 1.
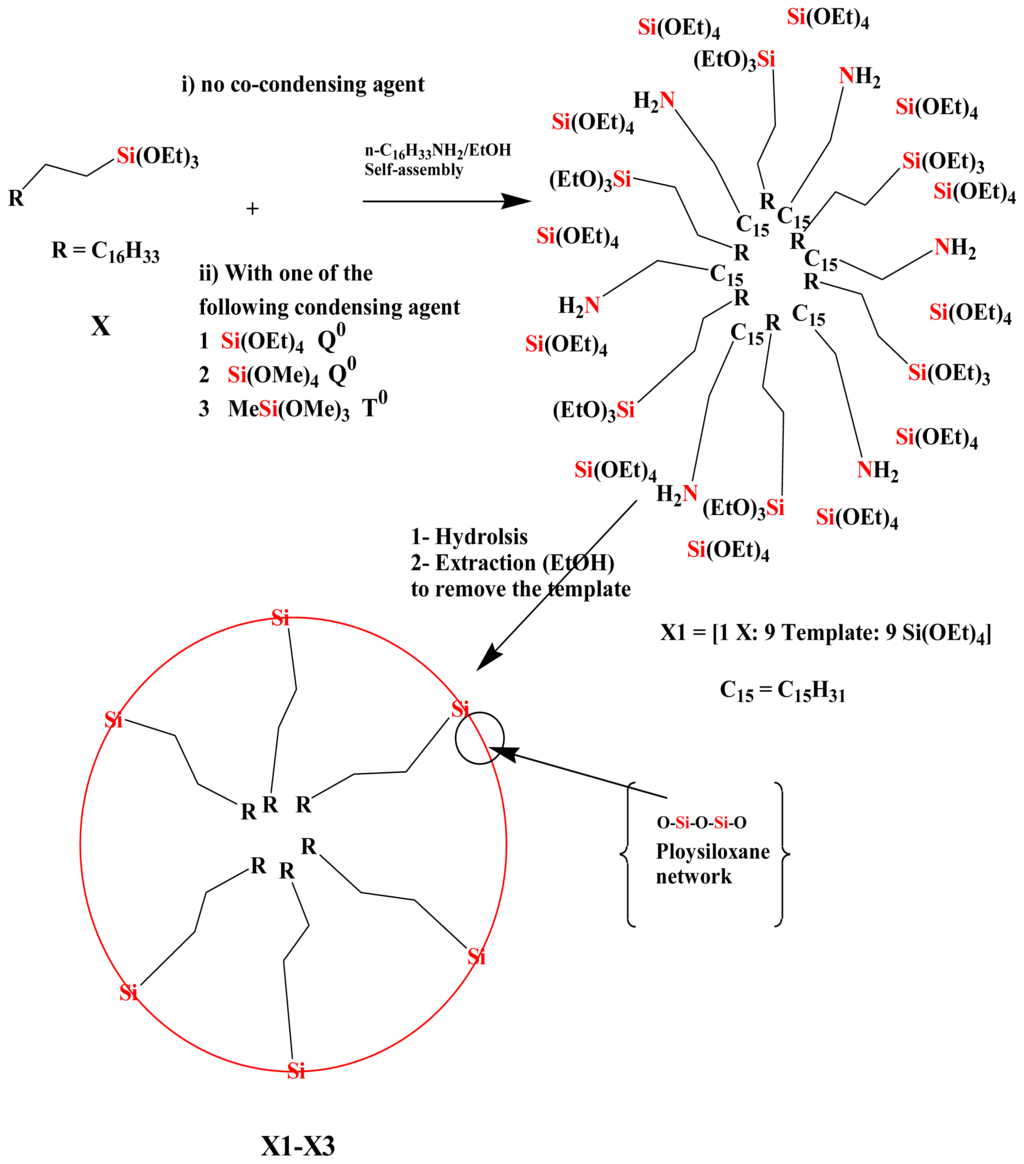
Scheme 1.
Synthesis of xerogels X0–X3: Self-assembly followed by sol-gel process at room temperature using several cross-linkers and the amine as template.
After sol-gel processing, the amine was removed from the mixture by Soxhlet extraction with ethanol. Four xerogels (X0–X3) were collected and are illustrated in Table 1.

Table 1.
Sol-gel processes, yields and labeling of the materials.
Two kinds of stationary phases were obtained: (i) xerogels X0 were synthesized by co-condensation of X and template with zero concentration (0) of cross-linker, and (ii) xerogels X1–X3 were synthesized by co-condensation of X, template with monofunctional Q0 and T0 cross-linkers such as Si(OEt)4, Si(OMe)4 and MeSi(OMe)3, respectively.
2.2. Solid-State NMR Spectroscopic Investigations
Due to cross-linking effects, the solubility of the polymeric materials X0–X3 is rather limited. Therefore, solid-state NMR spectroscopy was used as a powerful technique for their characterization.
29Si CP/MAS NMR spectroscopy
Silane functionality and bonding chemistry can easily be determined by 29Si CP/MAS NMR spectroscopy. The universal chemical shift and symbols for these 29Si function groups are collected in Scheme 2.
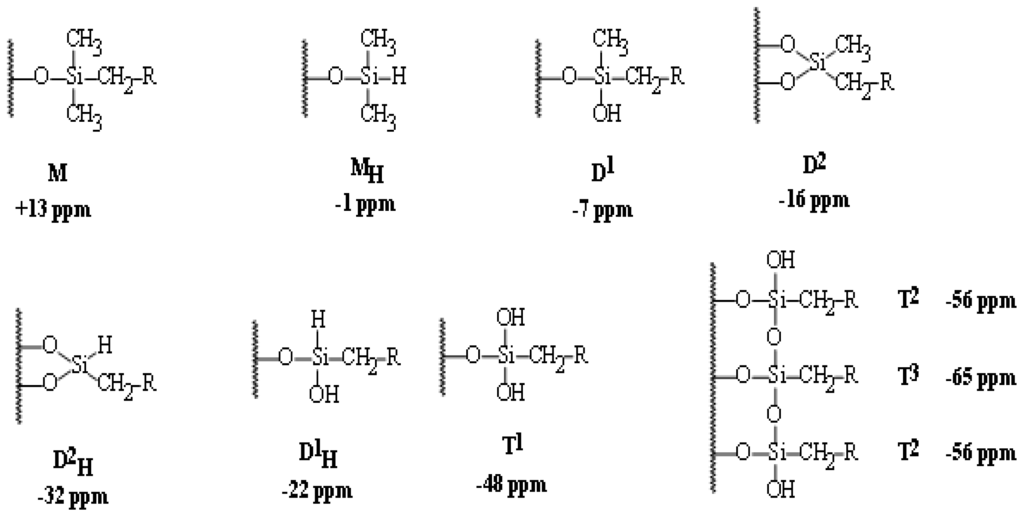
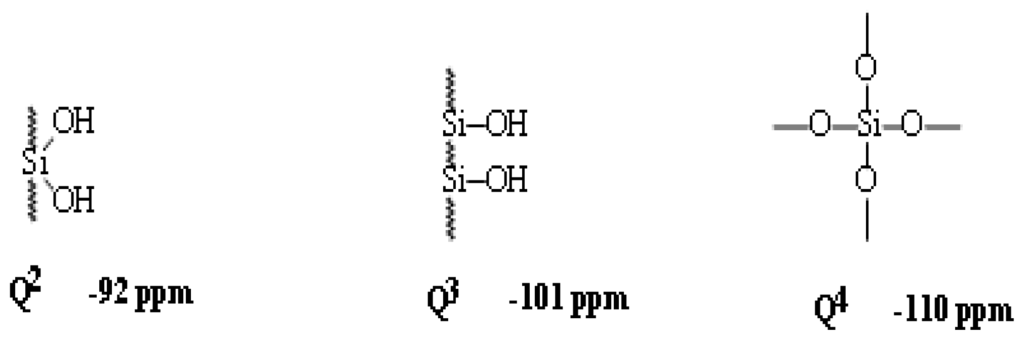
Scheme 2.
The universal 29Si chemical shifts, symbols and orders of silyl species.
The average chemical shifts for T2 (δ = −58.8), and T3 (δ = −65.1), Q3 (δ = −101.9), Q4 (δ = −109.5) species are significantly changed by the incorporation of the different types of co-condensation agents and are in agreement with values reported in the literature for comparable systems [37]. Since all silicon atoms are in direct proximity of protons the Hartmann–Hahn [29–33] match could efficiently be achieved.
The signal assignment of silyl fragments can be summarized as follows: a higher degree of cross-linking of silicon species and/or an increase of oxygen neighbors leads to an upfield shift in NMR spectra. Difunctional species (Dn) appear in the region of −7 to −20 ppm, tri-functional species (Tn) from −49 to −66 ppm, and signals from the native silica (Qn) from −91 to −110 ppm. The high condensation degrees which were obtained through the sol-gel processes and monitored by 29Si NMR is mainly resonated to the employment of T2, T3, Q3 and Q4 as cross-linkers [28–35].
All the spectra of xerogels (X0–X3) show signals for substructures corresponding to T and Q functions only, the D or M silyl fragment has not been observed, as seen in Figure 1. X0–X3 showed T silyl fragment only in general 3 order predominated over 2, which supports the full cross-linked organosiloxane species, demonstrating the incorporation of the function groups within the framework walls of the mesostructures. T2 silyl fragment indicted that one Si-OH function group of the sector or cross-linkers MeSi(OMe)3 was not incorporated in the sol-gel process. X0 and X3 revealed only T silyl fragment, and this was expected since no Q co-condensation agents were used. X1 and X2 showed both T and Q with 2 and 3 order, because both the sector T silyl fragment and Q silyl fragment of the co-condensation agents were investigated in the sol-gel process; Q predominated over T due to the concentrations used [9:1] respectively. The respective NMR spectra of the different materials’ phases and the native silica are shown in Figure 1.
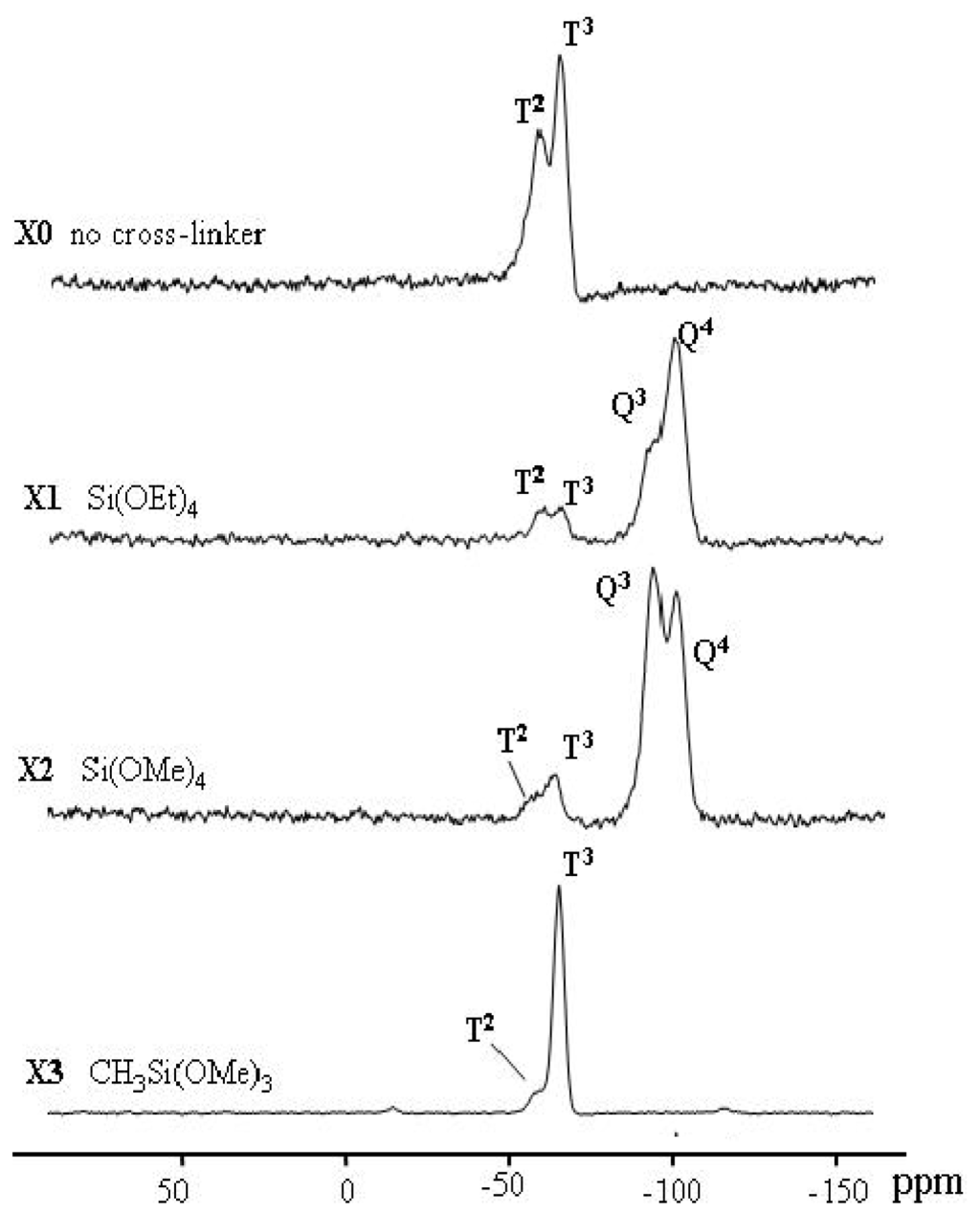
Figure 1.
29Si CP/MAS NMR spectra of X0–X3 materials which were prepared by using Si(OEt)4, Si(OMe)4 and MeSi(OMe)3 as cross-linkers.
2.3. 13C CP/MAS NMR Spectroscopy
More detailed information on bonded phase architecture was obtained from the 13C CP/MAS NMR spectra of the supported matrices X0–X3, and the corresponding signal assignments are summarized in Figure 2. Characteristic peaks at approximately δ = 14.0 ppm are assigned to the carbon atom of CH3 and the carbon atom of the silicon adjacent methyl groups (SiCH2) in the Si–O–Si substructure δ = 22.8 ppm is assigned to CH2CH3 and SiCH2CH2 are significant for all the stationary phases. Weak signals of 13C were assigned to residual Si–OR (R = Me or Et) functionalities at around δ = 17.0 and 57.9 ppm, attributed to non-hydrolyzed EtO (X1) or MeO (X2 and X3) silica functional groups, which point to a high degree of hydrolysis compared to the literature [7,9]. δ = −3.6 ppm is assigned to SiMe in case of using MeSi(OMe)3. The NMR investigation of such stationary bonded phases exhibits two signals for the main chain methylene carbons at 30.0 and 32.2 ppm (gauche/trans conformations, respectively) as shown in Figure 2 [5].
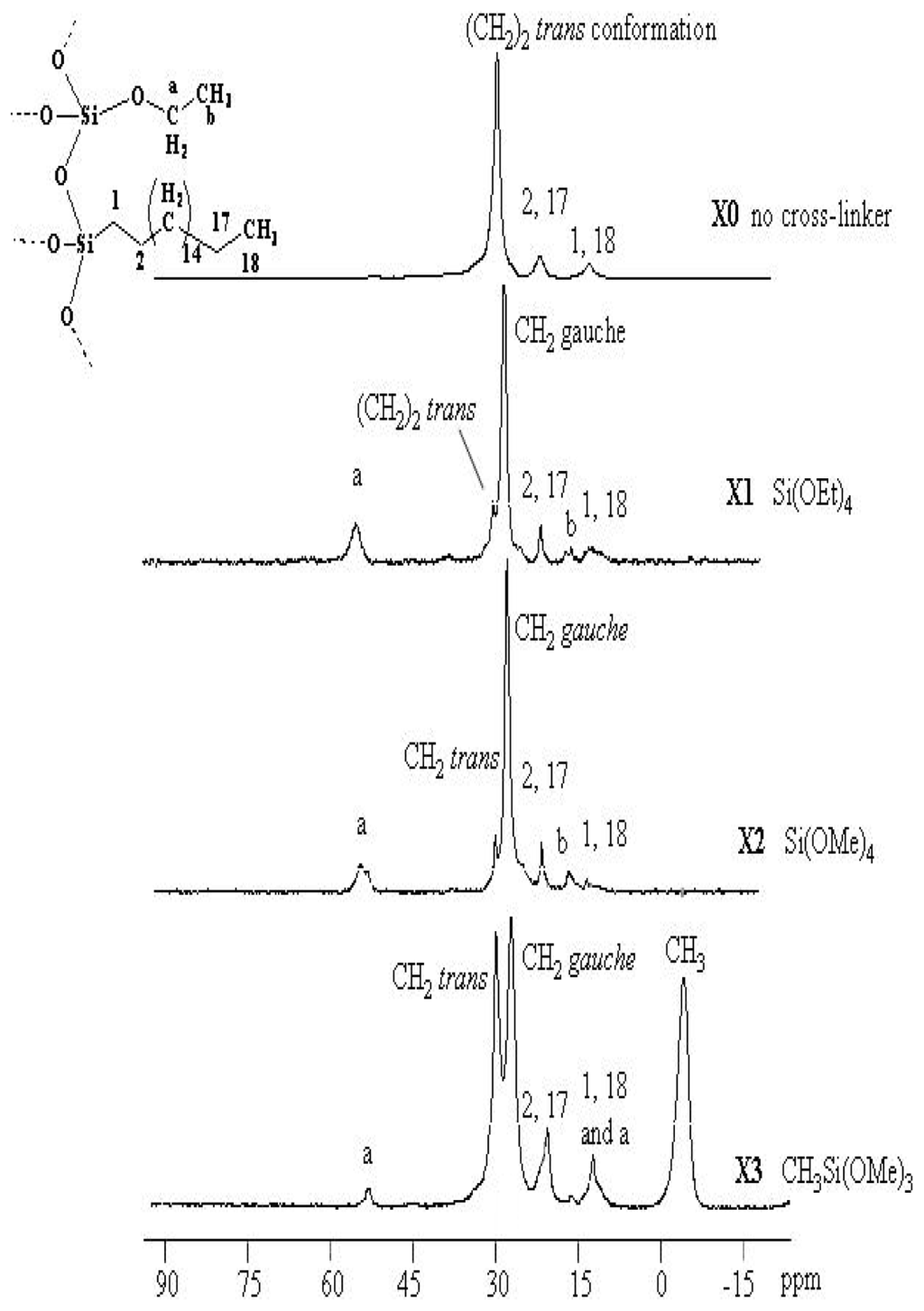
Figure 2.
13C CP/MAS NMR spectra of X0-X3 materials.
The samples X1, X2 and X3 show two peaks related to the main chain (CH2)14 at 30.0 and 32.0 ppm which represent two different conformations of the alkyl chain. The signal at 32.8 ppm characterizes trans conformations, and the signal at 30.0 ppm reveals the existence of gauche conformations. The effect of different motilities is only visible in the 13C CP/MAS NMR spectra of the xerogels containing alkyl chains with 18 carbon atoms at least; the alkyl chain order is found to increase with increasing chain length from C18 to C34 [36].
The NMR investigation of C18 bonded phases here exhibits only one signal for the main chain: methylene carbons at 32.8 belong to the trans conformation when no co-condensation agent was investigated (X0). When TESO or TMSO (Q0) was introduced to the sol-gel, the presence of the Me-Si function group in the cross-linker plays a key role in the conformation; the trans conformation was encouraged. The main reason of such a phenomenon is not yet clear, it could be related to the un-hydrolysis Me-Si steric factor of remaining Me on the X3 polysiloxane surface affecting the C18 chain not to bend away to form a gauche conformation, thus enhancing the C–H Wander Val forces to form sort of stabilizing straight line chains as in Scheme 3. This proposal is compatible with the decrease in the trans conformation at the expense of the alternative gauche conformation increasing upon increasing the temperature [9,28–30].
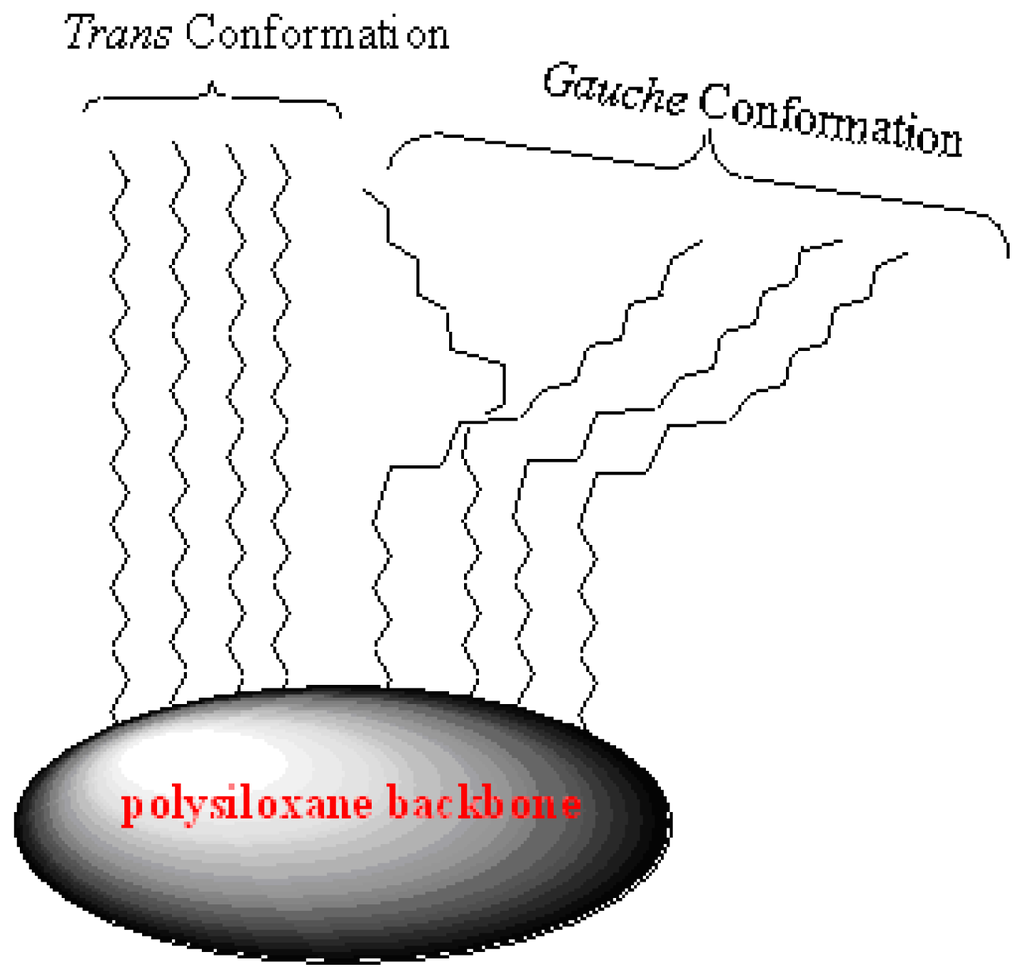
Scheme 3.
Schematic illustration of trans and gauche alkyl chain arrangements.
Here we report a new method at room temperature to control the gauche/trans conformation ratio of the alkyl chains in the stationary phase by using fixed concentrations of several type of T0 and Q0 cross-linkers.
2.4. IR Investigations
The IR spectra of the desired materials, X0, X1, X2 and X3 in particular, show several peaks that are attributed to stretching and bending. The broad intensive stretching vibrations at 2980–2840 cm−1 and bending vibrations at 1150–950 cm−1 belonging to (vCH) of the SiCH2(CH2)16CH3 functional groups were the main IR active functional groups. A typical example of the IR behavior of X3 s illustrated in Figure 3b.
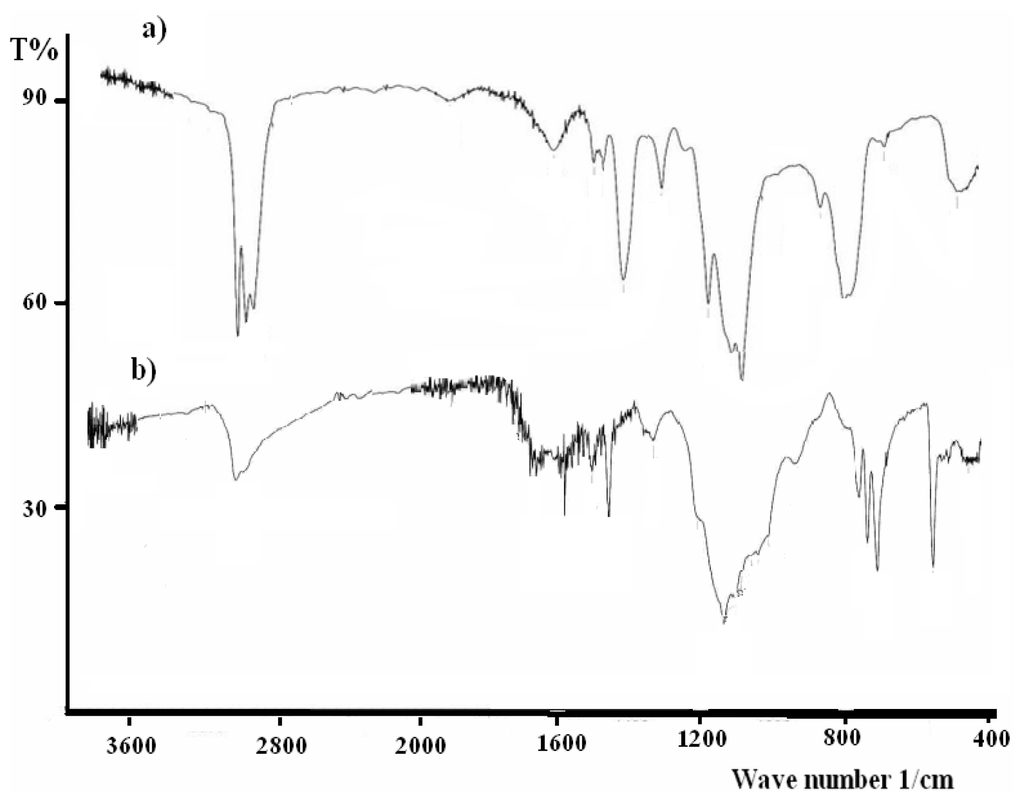
Figure 3.
Infra-red spectra (a and b) of X and X3, before and after sol-gel, respectively.
In order to confirm the full sol-gel reaction, the structural vibration behaviors of these compounds against the infrared spectra of X as the starting material and X3 as xerogel before and after the sol-gel processes were investigated and compared, such as the typical examples in Figure 3.
The broad intensive stretching vibrations at 2980–2840 cm−1 of (vCH) belong to the SiOCH2CH3 functional groups of X starting material as in Figure 3a totally disappeared after the sol-gel process to prepare the complex X3 as seen in Figure 3b, which strongly confirms the completeness of the sol-gel process formation.
2.5. Surface Structure of the Materials
The surface area data (BET) of such materials determined by the sol-gel procedure was found to equal 1000–1500 m2/g, 0.5–1.3 cm3/g pore volume, and 13–20 Ǻ pore size [37].
SEM micrographs of the X2 and X3 powder are given in Figure 4. Morphological features of the samples show the difference between spherical shapes, colloidal and porous structure and irregular particles mainly with the size 0.5–2 μm. All powders consisted of hard sub micrometer agglomerates, which are composed of fine crystallites. Generally, these agglomerate particles are hard to break down even with a long ultra-sonication time.
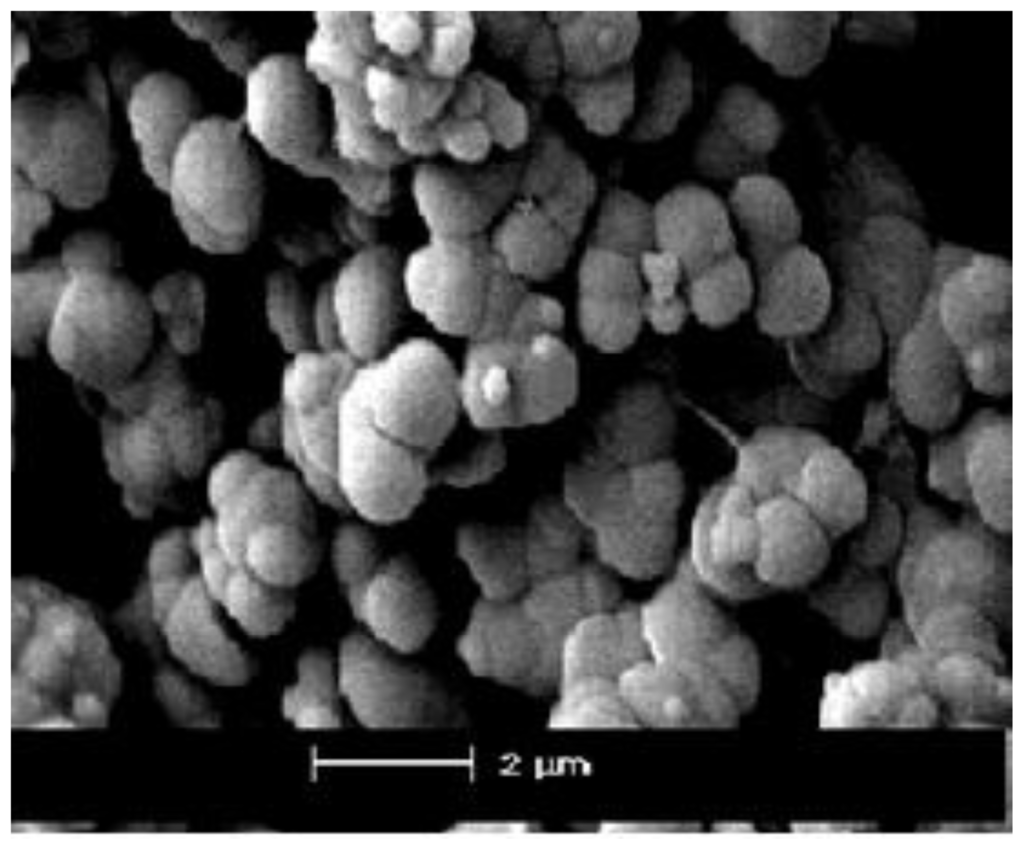
Figure 4.
Scanning electron micrograph (SEM) of X2.
3. Experimental
3.1. General Methods
All reactions were carried out in an inert atmosphere (argon) by using standard high vacuum and Schlenk-line techniques unless otherwise noted. n-octadecyltriethoxysilane was purchased from ABCR GmbH, Germany. Prior to use, n-hexane and Et2O were distilled from both LiAlH4 and sodium/benzophenone, respectively. All other chemicals were obtained from Merck KGaA Darmstadt, Germany, and were used without further purification.
3.2. Method of Characterization
13C CP/MAS NMR spectra were recorded on a Bruker ASX 300 spectrometer (Bruker GmbH, Rheinstetten, Germany) at a spinning rate of 4000 Hz with 7 mm double bearing rotors of ZrO2 and proton of 90° pulse length and 7 μs was used. The contact time and delay time were 3 ms and 3 s, respectively with the line broadening of 30 Hz.
29Si CP/MAS NMR spectra were also collected on a Bruker ASX 300 NMR spectrometer. Representative samples of 200–250 mg were spun at 3500 Hz using 7 mm double bearing ZrO2 rotors. The spectra were obtained with a cross-polarization contact time of 5 ms. The pulse interval time was 1 s. Typically, 1.5 k FIDs with an acquisition time of 30 ms were accumulated in 1 kb data points and zero-filling to 8 kb prior to Fourier transformation. Line broadening of 30 Hz and respective spectral width were found for all spectra at about 20 kHz.
Scanning electron microscopy was performed on a JEOL JSM-6380 LA scanning electron microscope (SEM).
3.3. General Procedure for the Sol-Gel Processes
2.2 × 10−2 mmol of n-hexadecylamine (template) in 50 mL of absolute ethanol was completely dissolved. The addition of 2.4 × 10−3 mmol of n-octadecyltriethoxysilane (selector) to the template solution and stirring for 3 h is an important step for self-assembly. 2.2 × 10−2 mmol of the corresponding cross-linker was added and stirred for 10 h, at 35 °C with a molar substrate [1:9:9] [Selector:Template:Cross-linker] respectively. An excess (5 g) of distilled water was added drop wise to a stirred solution. This mixture was stirred for 24 h at room temperature until a gel was formed, then the solvent was removed by reducing the pressure. For the removal of n-hexadecylamine the crude xerogels were placed in a Soxhlet extractor containing 300 mL ethanol and the mixture was refluxed for 3 days. Subsequently the gels were washed three times with n-hexane and ether (100 mL) respectively, and dried under vacuum for 12 h.
Polysiloxanyloctane (X0)
A mixture of 1 g (2.4 × 10−3 mmol) of n-octyltrimethoxysilane and 5.2 g (2.2 × 10−2 mmol) n-hexadecylamine were sol-gel processed in ethanol and water to yield a colorless swollen gel. After purification and drying 0.6 g of white powder was formed. 13C CP/MAS NMR δ = 14.0 (SiCH2 and CH3), 22.8 (SiCH2CH2 and CH2CH3), 32.8 [(CH2)14 trans conformation]. 29Si CP/MAS NMR δ = −57.8 (T2), −67.1 (T3).
Polysiloxanyloctane (X1)
A mixture of 1 g (2.4 × 10−3 mmol) of n-octyltrimethoxysilane, 5.2 g (2.2 × 10−2 mmol) n-hexadecylamine and 5.5 g (2.2 × 10−2 mmol) of TEOS were sol-gel processed in ethanol and water to yield a colorless swollen gel. After purification and drying 5.2 g of white powder was formed. 13C CP/MAS NMR: δ = 13.2 (SiCH2 and CH3), 16.6 (OCH2CH3), 22.8 (SiCH2CH2 and CH2CH3), 29.9 [(CH2)14 gauche conformation], 32.0 [(CH2)14 trans conformation], 59.3 (OCH2). 29Si CP/MAS NMR: δ= −56.2 (T2), −66.8 (T3), −101.9 (Q3), −109.5 (Q4).
Polysiloxanyloctane (X2)
A mixture of 1 g (2.4 × 10−3 mmol) of n-octyltrimethoxysilane, 5.2 g (2.2 × 10−2 mmol) n-hexadecylamine and 5.5 g (2.2 × 10−2 mmol) of TMOS were sol-gel processed in ethanol and water to yield a colorless swollen gel. After purification and drying 4.2 g of white powder was formed. 13C CP/MAS NMR: δ = 13.2 (SiCH2 and CH3), 16.6 (OCH2CH3), 22.8 (SiCH2CH2 and CH2CH3), 29.9 [(CH2)14 gauche conformation], 32.0 [(CH2)14 trans conformation], 57.8 (OCH2).29Si CP/MAS NMR: δ = −57.2 (T2), −67.8 (T3), −101.5 (Q3), −109.9 (Q4).
Polysiloxanyldodecane (X3)
A mixture of 1g (2.4 × 10−3 mmol) of n-octyltrimethoxysilane, 5.2 g (2.2 × 10−2 mmol) n-hexadecylamine and 5.2 g (2.2 × 10−2 mmol) of MeSi(OMe)3 were sol-gel processed in ethanol and water to yield a colorless swollen gel. After purification and drying 4.5 g of white powder was formed. 13C CP/MAS NMR: δ = −3.6 (CH3Si), 14.0 (SiCH2 and CH3), 17.2 (OCH2CH3), 22.8 (SiCH2CH2 and CH2CH3), 29.9 [(CH2)14 gauche conformation], 32.8 9 [(CH2)14 trans conformation], 57.4 (OCH2). 29Si CP/MAS NMR: δ = −57.2 (T2), −67.8 (T3).
4. Conclusion
The sol-gel process offers new hybrid materials X0, X1, X2 and X3. A suitable pathway is the sol-gel processing of n-alkyl like n-octadecyltriethoxysilane with an aliphatic amine like n-hexadecylamine as template molecules and different cross-linkers such as Si(OEt)4, Si(OMe)4 (Q0) and MeSi(OMe)3 (T0) carried out in ethanol at room temperature. The structure of all xerogels (X0–X3) were determined by solid state 13C and 29Si NMR spectroscopy, infrared spectroscopy and SEM. Additionally, the mobility of the alkyl chains and the dynamic behavior of the polymer matrix depended strongly on the type of cross-linkers. At room temperature, pure trans was observed without cross-linker while trace amount of trans was detected when Q0 cross-linkers were introduced. Both trans and gauche conformations in equal amounts were recorded when T0 cross-linkers were used.
Acknowledgements
The project was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
References
- Sander, L.C.; Sharpless, K.E.; Craft, N.E.; Wise, S.A. Development of engineered stationary phases for the separation of carotenoid isomers. Anal. Chem. 1994, 66, 1667–1674. [Google Scholar]
- Sander, L.C.; Wise, S.A. Influence of stationary phase chemistry on shape recognition in liquid chromatography. Anal. Chem 1995, 67, 3284–3292. [Google Scholar]
- Karger, B.L.; Gant, J.R.; Hartkopf, A.; Weiner, P.H. Hydrophobic effects in reversed-phase liquid chromatography. J. Chromatogr 1976, 128, 65–78. [Google Scholar]
- Raitza, M.; Wegmann, J.; Bachmann, S.; Albert, K. Investigating the surface morphology of triacontyl phases with spin-diffusion solid-state NMR spectroscopy. Angew. Chem 2000, 39, 3486–3489. [Google Scholar]
- Pursch, M.; Sander, L.C.; Albert, K. Chain order and mobility of high-density C18 phases by solid-state NMR spectroscopy and liquid chromatography. Anal. Chem 1996, 68, 4107–4113. [Google Scholar]
- Lindner, E.; Al-Gharabli, S.; Warad, I.; Mayer, H.A.; Steinbrecher, S.; Plies, E.; Seiler, M.; Bertagnolli, H.Z. Supported organometallic complexes. XXXVI. Diaminediphosphine-ruthenium(II) interphase catalysts for the hydrogenation of α,β-unsaturated ketones. Z. Anorg. Allg. Chem 2003, 629, 161–171. [Google Scholar]
- Lu, Z.L.; Lindner, E.; Mayer, H.A. Applications of sol-gel-processed interphase catalysts. Chem. Rev 2002, 102, 3543–3578. [Google Scholar]
- Rolison, D.R.; Dumn, B. Electrically conductive oxide aerogels: New materials in electrochemistry. J. Mater. Chem 2001, 11, 963–980. [Google Scholar]
- Salesch, T.H.; Bachmann, S.; Brugger, S.; Rabelo-Schaefer, R.; Albert, K.; Steinbrecher, S.; Plier, E.; Mehdi, A.; Reyé, C.; Corriu, R.; et al. New inorganic-organic hybrid materials for HPLC separation obtained by direct synthesis in the presence of a surfactant. Adv. Funct. Mater 2002, 12, 134–142. [Google Scholar]
- Tang, Q.; Wu, N.; Lee, M.L. Continuous-bed columns containing sol-gel bonded octadecylsilica for capillary liquid chromatography. J. Microcolumn. Sep 2000, 12, 6–12. [Google Scholar]
- Chan, M.A.; Lam, J.L.; Lo, D. Fiber optic oxygen sensor based on phosphorescence quenching of erythrosin B trapped in silica-gel glasses. Anal. Chim. Acta 2000, 408, 33–37. [Google Scholar]
- Holder, E.; Oelkrug, D.; Egelhaaf, H.J.; Mayer, H.A.; Lindner, E. Synthesis, characterization, and luminescence spectroscopic accessibility studies of tris(2,2′-bipyridine)ruthenium(II)-labeled inorganic-organic hybrid polymers. J. Fluoresc 2002, 12, 383–395. [Google Scholar]
- Lindner, E.; Auer, F.; Schneller, T.; Mayer, H.A. Chemistry in interphases—A new approach to organometallic syntheses and catalysis. Angew. Chem. Int. Ed 1999, 38, 2154–2174. [Google Scholar]
- Matos, J.R.; Kruk, M.; Mercuri, L.P.; Jaroniec, M.; Zhao, L.; Kamiyama, T.; Terasaki, O.; Pinnavaia, T.J.; Liu, Y. Ordered mesoporous silica with large cage-like pores: Structural identification and pore connectivity design by controlling the synthesis temperature and time. J. Am. Chem. Soc 2003, 125, 821–829. [Google Scholar]
- Corriu, R.J.P.; Hoarau, C.; Mehdi, A.; Reyé, C. Study of the accessibility of phosphorus centers incorporated within ordered mesoporous organic-inorganic hybrid materials. Chem. Commun 2000, 1, 71–72. [Google Scholar]
- Jiao, J.; Sun, X.; Pinnavaia, T.J. Mesostructured silica for the reinforcement and toughening of rubbery and glassy epoxy polymers. Polymer 2009, 50, 983–989. [Google Scholar]
- Thommes, M.; Kohn, R.; Froba, M. Characterization of mesoporous solids: Pore condensation and sorption hysteresis phenomena in mesoporous molecular sieves. Stud. Surf. Sci. Catal 2002, 142, 1695–1702. [Google Scholar]
- Sforca, M.L.; Yoshida, I.V.P.; Nunes, S.P. Organic-inorganic membranes prepared from polyether diamine and epoxy silane. J. Membr. Sci 1999, 159, 197–207. [Google Scholar]
- Pinho, R.O.; Radovanovic, E.; Torriani, L.I.; Yoshida, I.V.P. Hybrid materials derived from divinylbenzene and cyclic siloxane. Eur. Polym. J 2004, 40, 615–622. [Google Scholar]
- Schubert, U. Catalysts made of organic-inorganic hybrid materials. New J. Chem 1994, 18, 1049–1058. [Google Scholar]
- Arends, I.W.; Sheldon, R.A. Activities and stabilities of heterogeneous catalysts in selective liquid phase oxidations: Recent developments. Appl. Catal. A 2001, 212, 175–187. [Google Scholar]
- Warad, I.; Al-Othman, Z.; Al-Resayes, S.; Al-Deyab, S.; Kenawy, E. Synthesis and characterization of novel inorganic-organic hybrid Ru(II) complexes and their application in selective hydrogenation. Molecules 2010, 15, 1028–1040. [Google Scholar]
- Warad, I.; Siddiqui, M.; Al-Resayes, S.; Al-Warthan, A.; Mahfouz, R. Synthesis, characterization, crystal structure and chemical behavior of [1,1-bis(diphenylphosphinomethyl) ethene]ruthenium-(II) complex toward primary alkylamine addition. Transition Met. Chem 2009, 34, 347–354. [Google Scholar]
- Sayah, R.; Flochc, M.; Framery, E.; Dufaud, V. Immobilization of chiral cationic diphosphine rhodium complexes in nanopores of mesoporous silica and application in asymmetric hydrogenation. J. Mol. Catal. A Chem 2010, 315, 51–59. [Google Scholar]
- Kang, C.; Huang, J.; He, W.; Zhang, F. Periodic mesoporous silica-immobilized palladium(II) complex as an effective and reusable catalyst for water-medium carbon-carbon coupling reactions. J. Organomet. Chem 2010, 695, 120–127. [Google Scholar]
- Baiker, A.; Grunwaldt, J.D.; Muller, C.A.; Schmid, L. Catalytic materials by design. Chimia 1998, 52, 517–524. [Google Scholar]
- Huesing, N.; Schubert, U. Aerogels-airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed 1998, 37, 22–45. [Google Scholar]
- Pursch, M.; Brindle, R.; Ellwanger, A.; Sander, L.C.; Bell, C.M.; Haendel, H.; Albert, K. Stationary interphases with extended alkyl chains: A comparative study on chain order by solid-state NMR spectroscopy. Solid State Nucl. Magn. Reson 1997, 9, 191–201. [Google Scholar]
- Pursch, M.; Strohschein, S.; Haendel, H.; Albert, K. Temperature-dependent behaviour of C30 interphases. A solid-state NMR and LC-NMR study. Anal. Chem 1996, 68, 386–393. [Google Scholar]
- Pursch, M.; Sander, L.C.; Egelhaaf, H.J.; Raitza, M.S.; Wise, S.A.; Oelkrug, D.; Albert, K. Architecture and dynamics of C22 bonded interphases. J. Am. Chem. Soc 1999, 121, 3201–3213. [Google Scholar]
- Pines, A.; Gibby, M.G.; Waugh, J.S. Proton-enhanced NMR of dilute spins in solids. J. Chem. Phys 1973, 59, 569–590. [Google Scholar]
- Andrew, E.R. Narrowing of NMR spectra of solids by high-speed specimen rotation and the resolution of chemical shift and spin multiplet structures for solids. Prog. Nucl. Magn. Reson. Spectrosc 1971, 8, 1–39. [Google Scholar]
- Koenig, J.L.; Andreis, M. Solid State NMR of Polymers; Mathias, L.J., Ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Albert, K.; Bayer, E. Characterization of bonded phases by solid-state NMR spectroscopy. J. Chromatogr 1991, 544, 345–370. [Google Scholar]
- Fontes, M.M.; Oliva, G.; Cordeiro, L.C.; Batista, A. The crystal and molecular structure of bis[1,3-bis(diphenylphosphino)propane]dichlororuthenium(II). J. Coord. Chem 1993, 30, 125–129. [Google Scholar]
- Clauss, J.; Schmidt-Rohr, K.; Adam, A.; Boeffel, C.; Spiess, H.W. Stiff macromolecules with aliphatic side chains: Side-chain mobility, conformation, and organization from 2D solid-state NMR spectroscopy. Macromolecules 1992, 25, 5208–5214. [Google Scholar]
- Maciel, G.E.; Sindorf, D.W. Silicon-29 NMR study of the surface of silica gel by cross polarization and magic-angle spinning. J. Am. Chem. Soc 1980, 102, 7606–7607. [Google Scholar]
- Mercier, L.; Pinnavaia, T. Direct synthesis of hybrid organic-inorganic nanoporous silica by a neutral amine assembly route: Structure-function control by stoichiometric, incorporation of organosiloxane molecules. Chem. Mater 2000, 12, 188–196. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).