Possible Time-Dependent Effect of Ions and Hydrophilic Surfaces on the Electrical Conductivity of Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
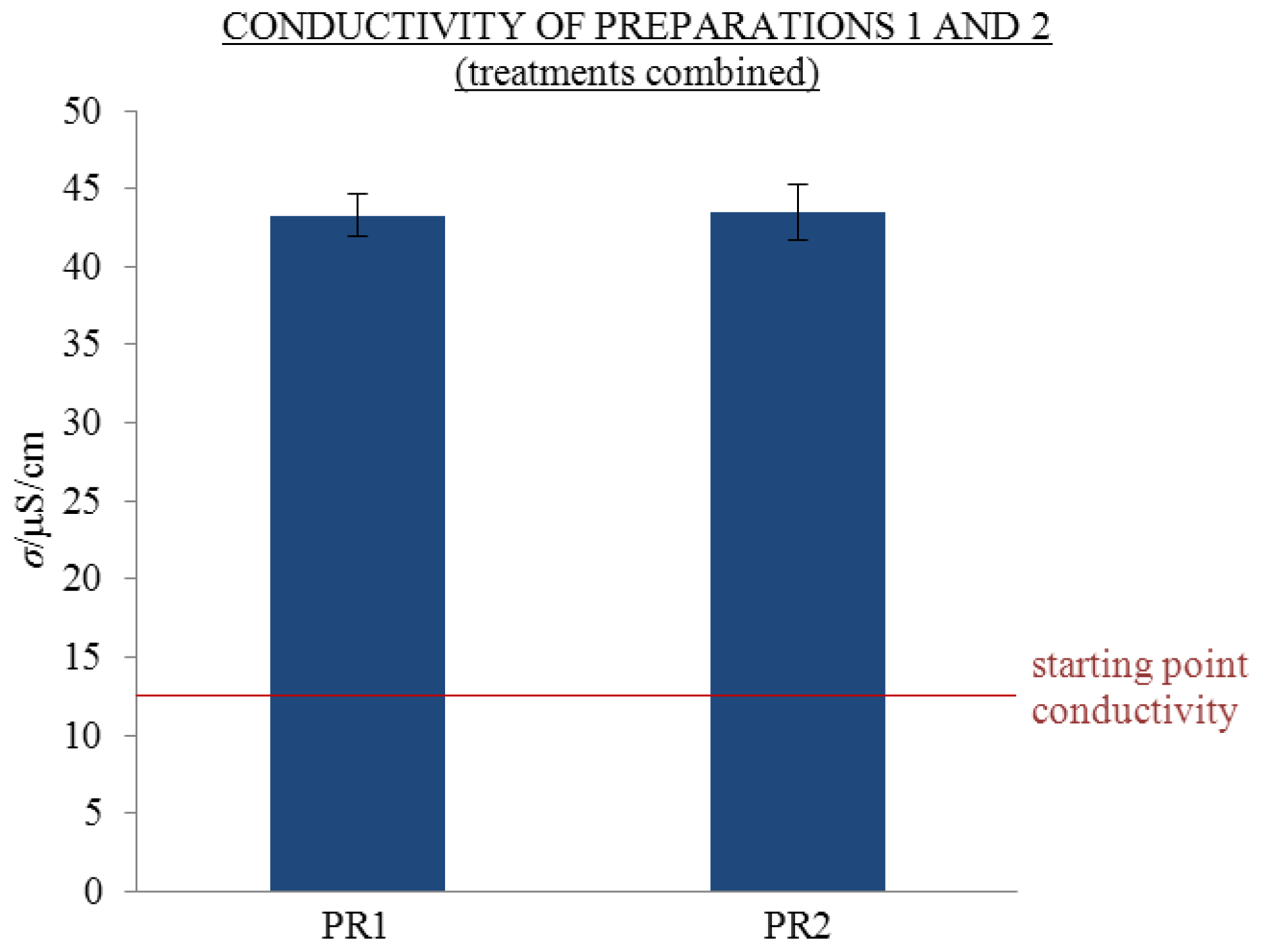
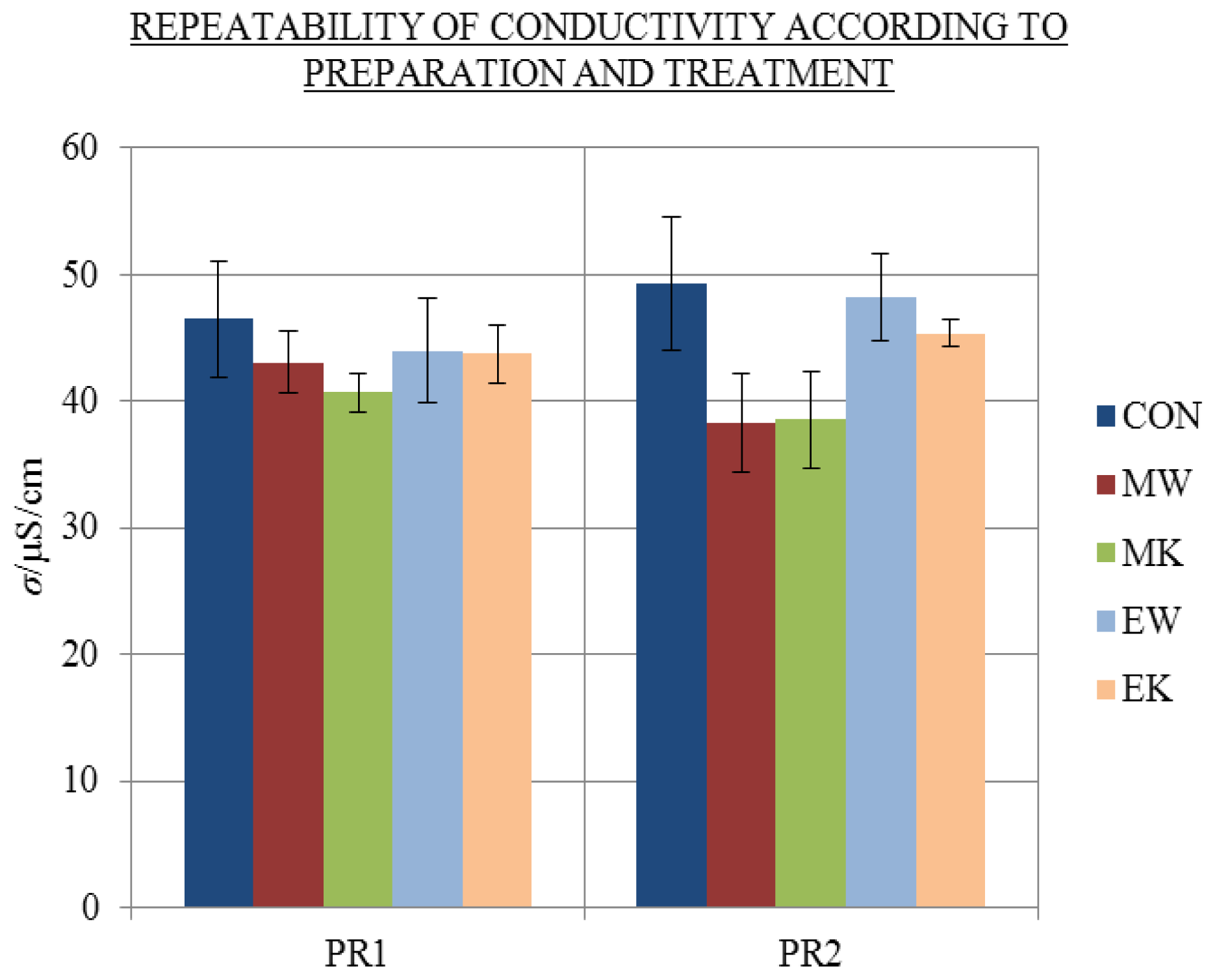
2.1. Repeatability of Conductivity Measurements and Influence of Treatment
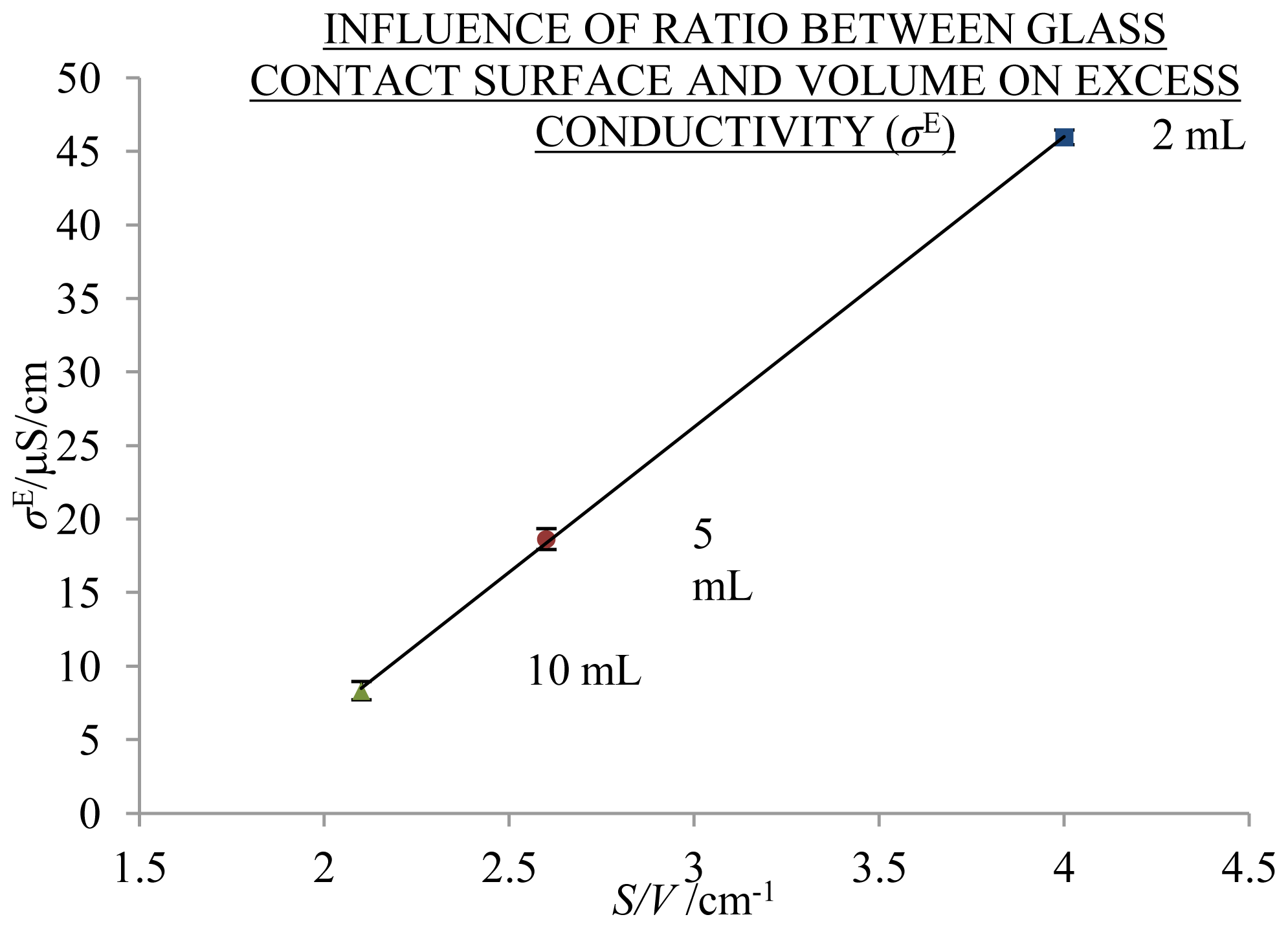
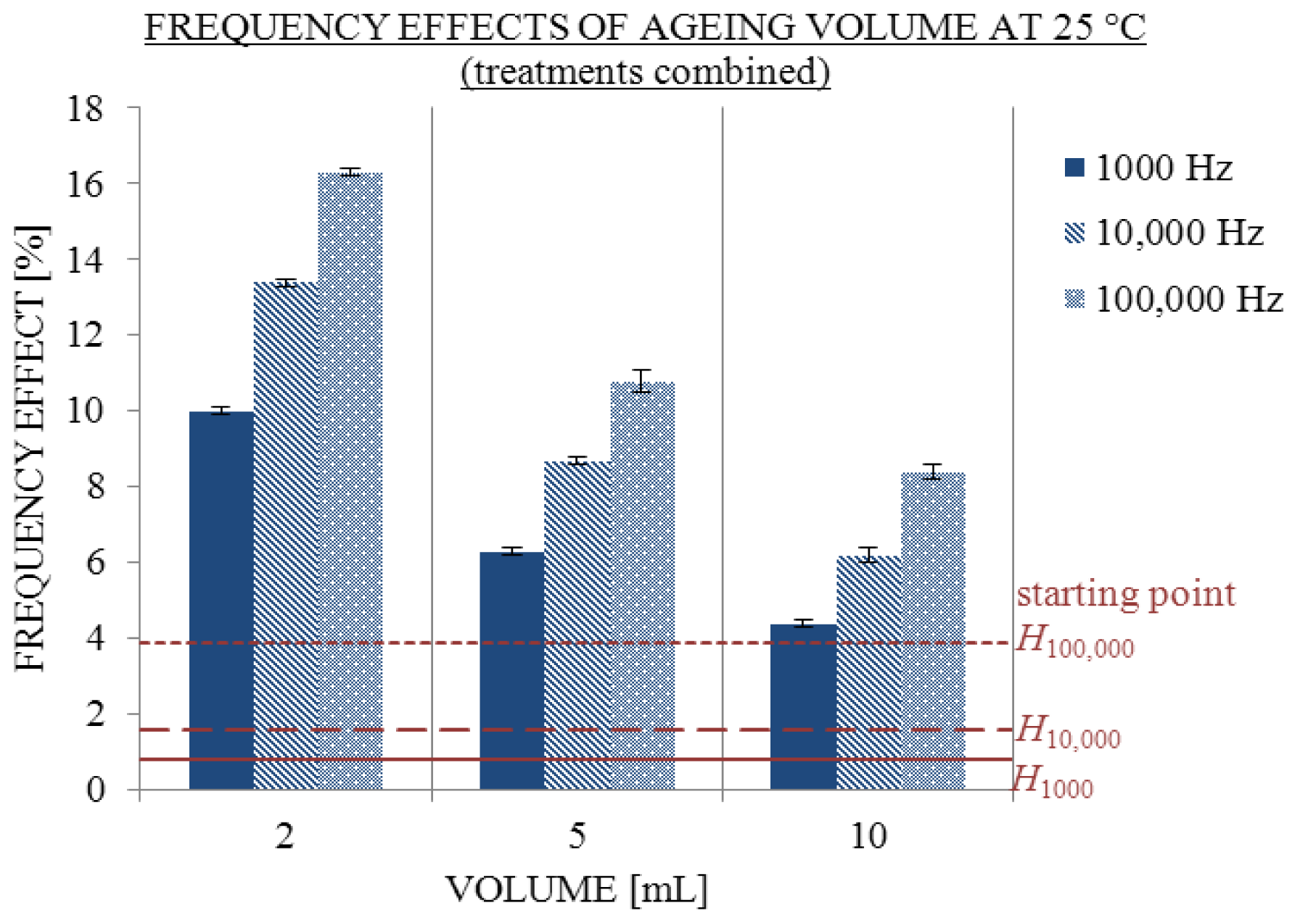
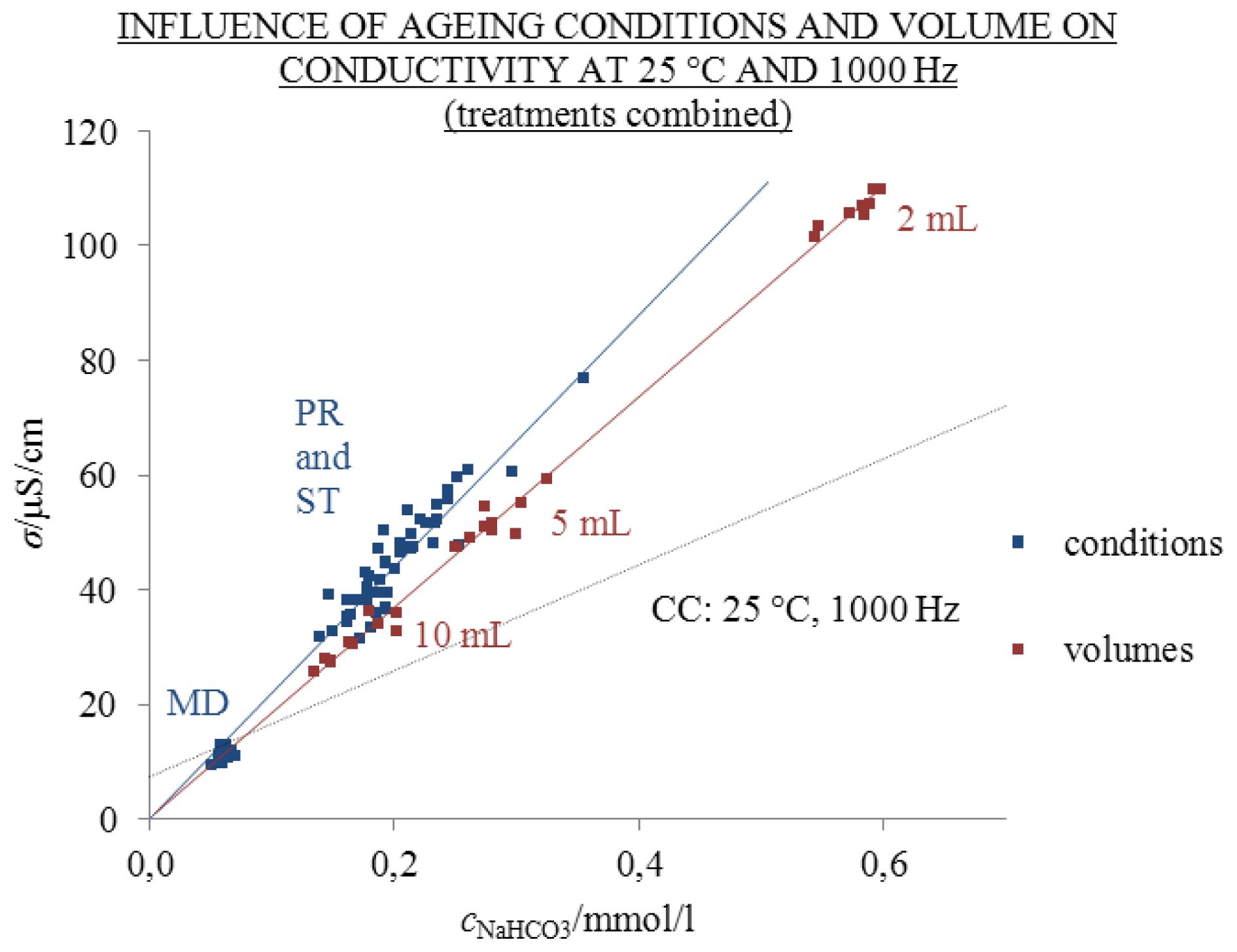
2.2. Influence of Ageing Volume
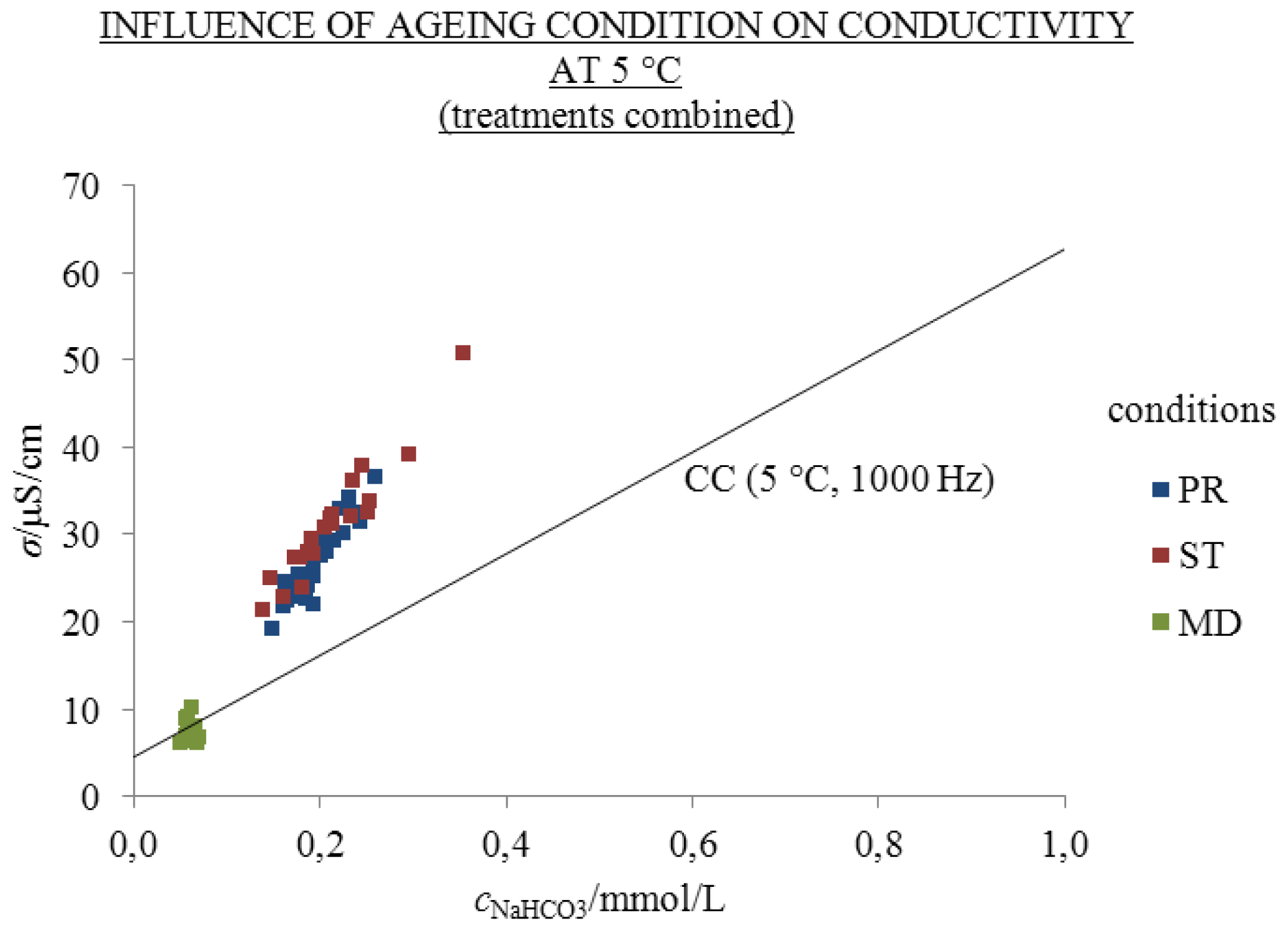
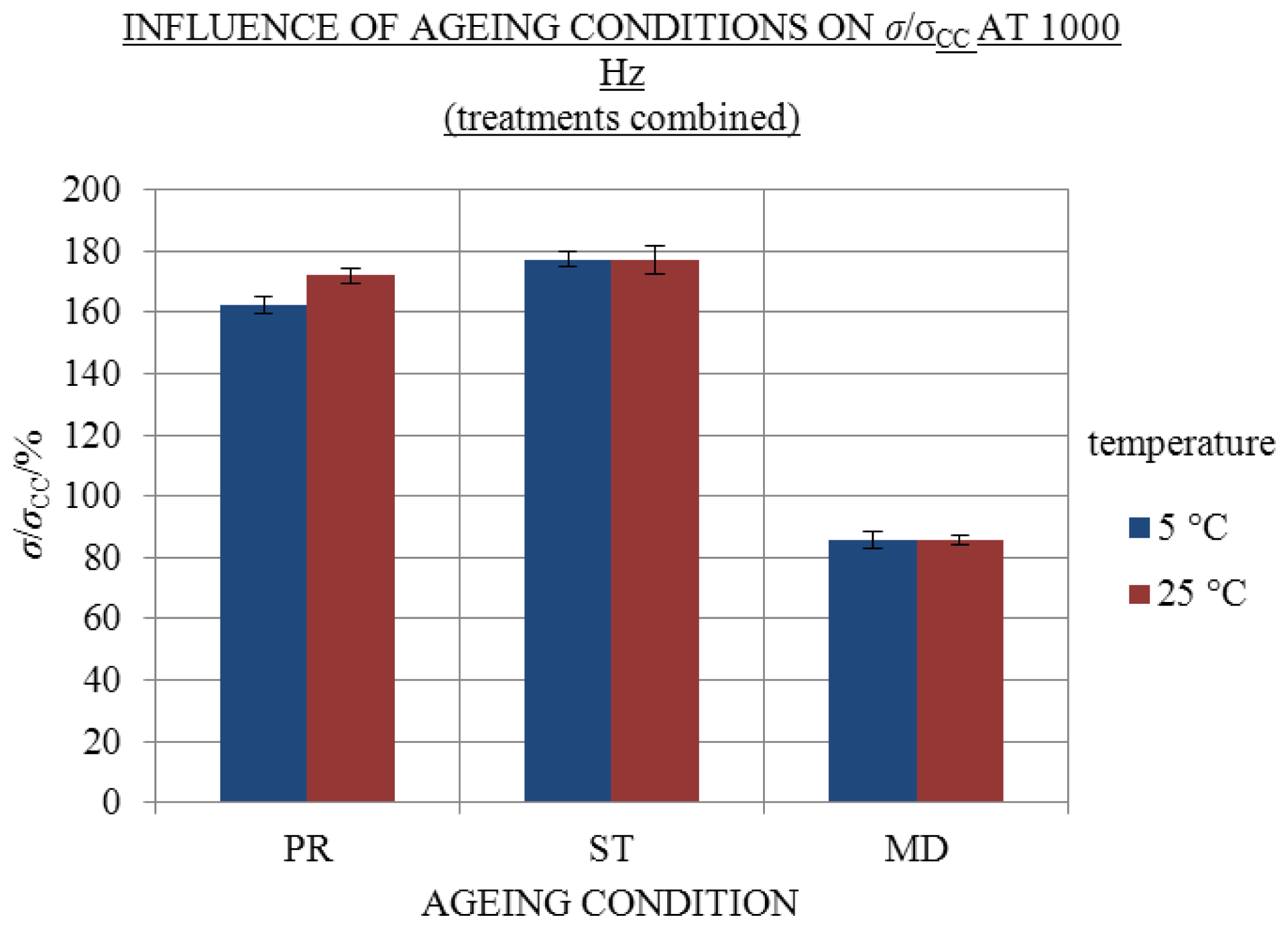
2.3. Influence of Ageing Condition and Temperature
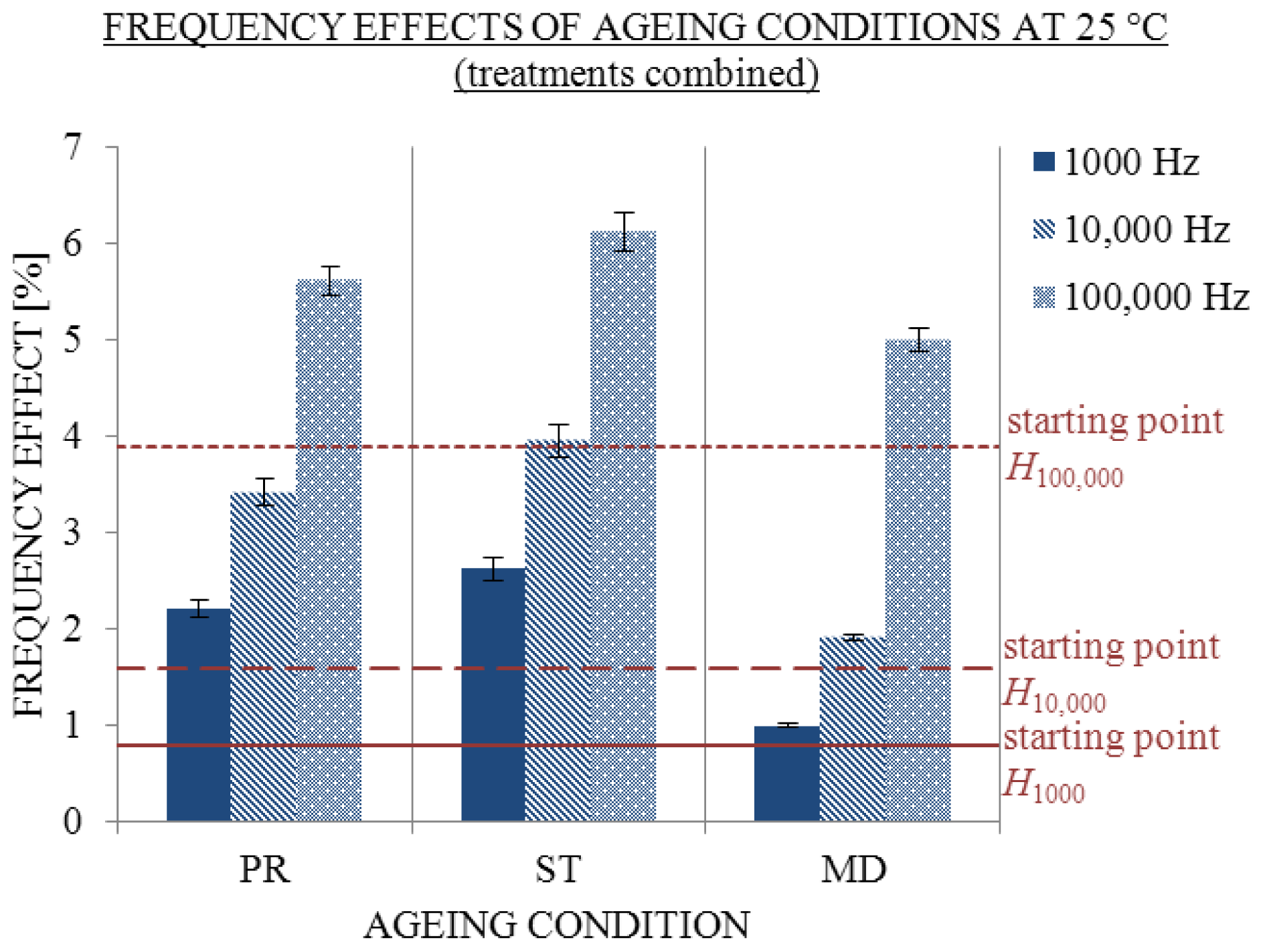
2.4. Influence of CO2 Absorption
2.5. Influence of Ions on Time-Related Changes in Water Structure
3. Experimental Section
3.1. Materials
3.2. Procedures
3.2.1. Mechanical Treatment
3.2.2. Electrical Treatment
3.3. Methods
3.3.1. Conductivity Measurements
3.3.2. Analytical Determination of Impurities
3.4. Course of the Work
3.4.1. Influence of Ageing Volume
3.4.2. Influence of CO2 Absorption
3.4.3. Influence of Ageing Condition and Treatment
4. Conclusions
- In accordance with Holandino et al., but in contrast to Elia’s findings, previous treatment by mechanical shaking and repetitive dilution to extremely dilute solutions (as well as electric treatment with strong electrical impulses) performed in our laboratory had no significant influence on the conductivity of aged solutions.
- Significant excess conductivity values (σE) compared to the conductivity of chemically analogous one-day-old solutions were found at 25 and 5 °C in all aged solutions except for those aged frozen at −20 °C.
- The excess conductivity values cannot be simply attributed to the absorption of CO2 due to the independency of σ/σCC from the ratio of the volume of air above solution and the volume of solution.
- The highest σE values at 25 °C were measured in 2 mL solutions aged under condition ST—protected from daylight for 310 days (20.3 μS/cm)—and 370 days under condition PR—exposed to daylight (46.0 μS/cm). The excess conductivity values cannot be attributed to low amounts of other ions disregarded in the calculations of the theoretical conductivity. Hence, we could probably ascribe the σE values to the ability of liquid water to spontaneously develop autothixotropic or gel-like properties, where ions and hydrophilic surfaces seem to play an important role. The autothixotropic properties enhance proton hopping mechanism in aqueous solutions and, therefore, increase the conductivity values.
Supplementary Information
ijms-13-04048-s001.pdfAcknowledgments
References
- Zheng, J.M.; Pollack, G.H. Long range forces extending from polymer-gel surfaces. Phys. Rev. E 2003, 68. [Google Scholar] [CrossRef]
- Zheng, J.-M.; Chin, W.-C.; Khijniak, E.; Khijniak, E., Jr; Pollack, G.H. Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Adv. Colloid Interface Sci. 2006, 127, 19–27. [Google Scholar]
- Pollack, G.H.; Figueroa, X.; Zhao, Q. Molecules, water, and radiant energy: New clues for the origin of life. Int. J. Mol. Sci 2009, 10, 1419–1429. [Google Scholar]
- Guckenberger, R.; Heim, M.; Cevc, G.; Knapp, H.F.; Wiegräbe, W.; Hillebrand, A. Scanning tunneling microscopy of insulators and biological specimens based on lateral conductivity of ultrathin water films. Science 1994, 266, 1538–1540. [Google Scholar]
- Sasaki, N. Dielectric properties of slightly hydrated collagen: Time—water content superposition analysis. Biopolymers 1984, 23, 1725–1734. [Google Scholar]
- Elia, V.; Napoli, E.; Niccoli, M.; Marchettini, N.; Tiezzi, E. New physico—Chemical properties of extremely diluted solutions. A conductivity study at 25 °C in relation to ageing. J. Solut. Chem 2008, 37, 85–96. [Google Scholar]
- Elia, V.; Niccoli, M. New physico—Chemical properties of extremely diluted aqueous solutions. J. Therm. Anal. Calorim 2004, 75, 815–836. [Google Scholar]
- Elia, V.; Napoli, E.; Niccoli, M.; Nonatelli, L.; Ramaglia, A.; Ventimiglia, E. New physico—Chemical properties of extremely diluted aqueous solutions. A calorimetric and conductivity study at 25 °C. J. Therm. Anal. Calorim 2004, 78, 331–342. [Google Scholar]
- Elia, V.; Marchese, M.; Montanino, M.; Napoli, E.; Niccoli, M.; Nonatelli, L.; Ramaglia, A. Hydrohysteretic phenomena of “extremely diluted solutions” induced by mechanical treatments. A calorimetric and conductometric study at 25 °C. J. Solut. Chem 2005, 34, 947–960. [Google Scholar]
- Elia, V.; Elia, L.; Montanino, M.; Napoli, E.; Niccoli, M.; Nonatelli, L. Conductometric studies of the serially diluted and agitated solutions on an anomalous effect that depends on the dilution process. J. Mol. Liq 2007, 135, 158–165. [Google Scholar]
- Elia, V.; Napoli, E.; Niccoli, M. A molecular model of interaction between extremely diluted solutions and NaOH solutions used as titrant. Conductometric and pHmetric titrations. J. Mol. Liq 2009, 148, 45–50. [Google Scholar]
- Elia, V.; Napoli, E.; Niccoli, M. Thermodynamic parameters for the binding process of the OH−ion with the dissipative structures. Calorimetric and conductometric titrations. J. Therm. Anal. Calorim 2010, 102, 1111–1118. [Google Scholar]
- Han, J.; Zhou, X.; Liu, H. Ab initio simulation on the mechanism of proton transport in water. J. Power Sources 2006, 161, 1420–1427. [Google Scholar]
- Lapid, H.; Agmon, N.; Petersen, M.K.; Voth, G.A. A bond-order analysis of the mechanism for hydrated proton mobility in liquid water. J. Chem. Phys 2005, 122, 014506:1–014506:16. [Google Scholar]
- Mohammed, O.F.; Pines, D.; Pines, E.; Nibbering, E.T.J. Aqueous bimolecular proton transfer in acid-base neutralization. Chem. Phys 2007, 341, 240–257. [Google Scholar]
- Siwick, B.J.; Bakker, H.J. On the role of water in intermolecular proton-transfer reactions. J. Am. Chem. Soc 2007, 129, 13412–13420. [Google Scholar]
- Xantheas, S.S. Computational chemistry. Dances with hydrogen cations. Nature 2009, 457, 673–674. [Google Scholar]
- Vybíral, B. The Comprehensive Experimental Research on the Autothixotropy of Water. In Water and the Cell; Pollack, G.H., Cameron, I., Wheatley, D., Eds.; Springer: New York, NY, USA, 2006; pp. 299–314. [Google Scholar]
- Vybíral, B.; Voráček, P. Long term structural effects in water: Autothixotropy of water and its hysteresis. Homeopathy 2007, 96, 183–188. [Google Scholar]
- Jerman, I.; Ružič, R.; Krašovec, R.; Škarja, M.; Mogilnicki, L. Electrical transfer of molecule information into water, its storage, and bioeffects on plants and bacteria. Electromagn. Biol. Med 2005, 24, 341–353. [Google Scholar]
- Verdel, N.; Jerman, I.; Bukovec, P. The “autothixotropic” phenomenon of water and its role in proton transfer. Int. J. Mol. Sci 2011, 12, 7481–7494. [Google Scholar]
- Holandino, C.; Harduim, R.; da Veiga, V.F.; Garcia, S.; Zacharias, C.R. Modeling physical-chemical properties of high dilutions: An electrical conductivity study. Int. J. High Dilution Res 2008, 7, 165–173. [Google Scholar]
- Holysz, L.; Szczes, A.; Chibowski, E. Effects of a static magnetic field on water and electrolyte solutions. J. Colloid Interface Sci 2007, 316, 996–1002. [Google Scholar]
- Turton, D.A.; Hunger, J.; Hefter, G.; Buchner, R.; Wynne, K. Glasslike behavior in aqueous electrolyte solutions. J. Chem. Phys 2008, 128, 161102:1–161102:4. [Google Scholar]
- Lobyshev, V.I.; Shikhlinskaya, R.E.; Ryzhikov, B.D. Experimental evidence for intrinsic luminescence of water. J. Mol. Liq 1999, 82, 73–81. [Google Scholar]
- Bešter-Rogač, M.; Habe, D. Modern advances in electrical conductivity measurements of solutions. Acta Chim. Slov 2006, 53, 391–395. [Google Scholar]
- McGrail, B.P.; Icenhower, J.P.; Shuh, D.K.; Liu, P.; Darab, J.G.; Baer, D.R.; Thevuthasen, S.; Shutthanandan, V.; Engelhard, M.H.; Booth, C.H.; et al. The structure of Na2O-Al2O3-SiO2 glass: Impact on sodium ion exchange in H2O and D2O. J. Non Cryst. Solids 2001, 296, 10–26. [Google Scholar]
- Shirai, M.; Tamamushi, B. Dielectric studies on colloidal solutions. I. High-frequency conductivity of aqueous solutions of paraffin-chain salts. Bull. Chem. Soc. Jpn 1955, 28, 545–548. [Google Scholar]
- Wachter, R.; Barthel, J. Untersuchungen zur temperaturabhängigkeit der eigenschaften von elektrolytlösungen ii. Bestimmung der leitfähigkeit über einen groβen temperaturbereich. Ber. Bunsenges. Phys. Chem 1979, 83, 634–642. [Google Scholar]






| Temperature (°C) | σ/σCC (%) ± RSE (%) | ||
|---|---|---|---|
| PR | ST | MD | |
| 25 | 171.9% ± 1.4% | 176.8% ± 2.6% | 85.6% ± 1.8% |
| 5 | 162.3% ± 1.5% | 177.2% ± 1.5% | 85.6% ± 3.2% |
| Ageing | VFLASK | VSOLUTION | VAIR | VCO2/VSOLUTION | S/V | NaHCO3 | σ/σCC1000 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AVG | SE | AVG | SE | N | ||||||
| d | mL | mL | mL | % | cm−1 | mmol/L | mmol/L | % | % | / |
| 310 | 2.5 | 2 | 0.5 | 0.01 | 3.9 | 0.19 | 0.01 | 171.9 | 2.4 | 28 |
| 370 | 20 | 2 | 18 | 0.35 | 4.0 | 0.58 | 0.01 | 176.3 | 0.8 | 8 |
| 370 | 20 | 5 | 15 | 0.12 | 2.6 | 0.28 | 0.01 | 156.7 | 1.9 | 10 |
| 370 | 20 | 10 | 10 | 0.04 | 2.1 | 0.17 | 0.01 | 136.8 | 2.2 | 10 |
| Treatment | Donor | Abbreviation |
|---|---|---|
| no treatment—control | / | CON |
| mechanical treatment to 10C | Milli-q water | MW |
| KCl | MK | |
| electrical treatment | Milli-q water | EW |
| KCl | EK |
| cNaHCO3 | σ (25 °C) | Δ (25 °C) | σ (5 °C) | Δ (5 °C) |
|---|---|---|---|---|
| mmol/L | μS/cm | μS/cm | μS/cm | μS/cm |
| 0.05 | 12.5 | 0.1 | 7.5 | 0.0 |
| 0.10 | 16.8 | 0.2 | 10.5 | 0.1 |
| 0.20 | 25.7 | 0.5 | 16.3 | 0.2 |
| 0.30 | 34.6 | 0.1 | 22.1 | 0.0 |
| 0.40 | 44.4 | 0.4 | 28.0 | 0.4 |
| 0.50 | 53.9 | 0.2 | 33.6 | 0.2 |
| 0.60 | 63.7 | 1.3 | 39.5 | 0.0 |
| 0.80 | 83.1 | 1.8 | 50.7 | 0.3 |
| 1.00 | 98.0 | 0.0 | / | / |
| T (°C) | 120 Hz | 1000 Hz | 10,000 Hz | 100,000 Hz |
|---|---|---|---|---|
| 25 | σCC = 90.1cNaHCO3 + 8 | σCC = 92.4cNaHCO3 + 7 | σCC = 93.4cNaHCO3 + 7 | σCC = 95.2cNaHCO3 + 8 |
| R2 = 0.9983 | R2 = 0.9983 | R2 = 0.9983 | R2 = 0.9983 | |
| 5 | σCC = 56.7cNaHCO3 + 5 | σCC = 58.1cNaHCO3 + 5 | σCC = 59.0cNaHCO3 + 4 | σCC = 56.0cNaHCO3 + 2 |
| R2 = 0.9996 | R2 = 0.9997 | R2 = 0.9996 | R2 = 0.9973 |
| Condition | Influences |
|---|---|
| PR | exposed to daylight |
| ST | protected from daylight |
| MD | at low temperatures: −20 °C |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Verdel, N.; Jerman, I.; Krasovec, R.; Bukovec, P.; Zupancic, M. Possible Time-Dependent Effect of Ions and Hydrophilic Surfaces on the Electrical Conductivity of Aqueous Solutions. Int. J. Mol. Sci. 2012, 13, 4048-4068. https://doi.org/10.3390/ijms13044048
Verdel N, Jerman I, Krasovec R, Bukovec P, Zupancic M. Possible Time-Dependent Effect of Ions and Hydrophilic Surfaces on the Electrical Conductivity of Aqueous Solutions. International Journal of Molecular Sciences. 2012; 13(4):4048-4068. https://doi.org/10.3390/ijms13044048
Chicago/Turabian StyleVerdel, Nada, Igor Jerman, Rok Krasovec, Peter Bukovec, and Marija Zupancic. 2012. "Possible Time-Dependent Effect of Ions and Hydrophilic Surfaces on the Electrical Conductivity of Aqueous Solutions" International Journal of Molecular Sciences 13, no. 4: 4048-4068. https://doi.org/10.3390/ijms13044048
APA StyleVerdel, N., Jerman, I., Krasovec, R., Bukovec, P., & Zupancic, M. (2012). Possible Time-Dependent Effect of Ions and Hydrophilic Surfaces on the Electrical Conductivity of Aqueous Solutions. International Journal of Molecular Sciences, 13(4), 4048-4068. https://doi.org/10.3390/ijms13044048




