Novel Microsatellite Markers of Meretrix petechialis and Cross-species Amplification in Related Taxa (Bivalvia: Veneroida)
Abstract
:1. Introduction
2. Results
2.1. Microsatellite Marker Isolation and Polymorphism Analyses
2.2. Genetic Variability of Meretrix petechialis Populations
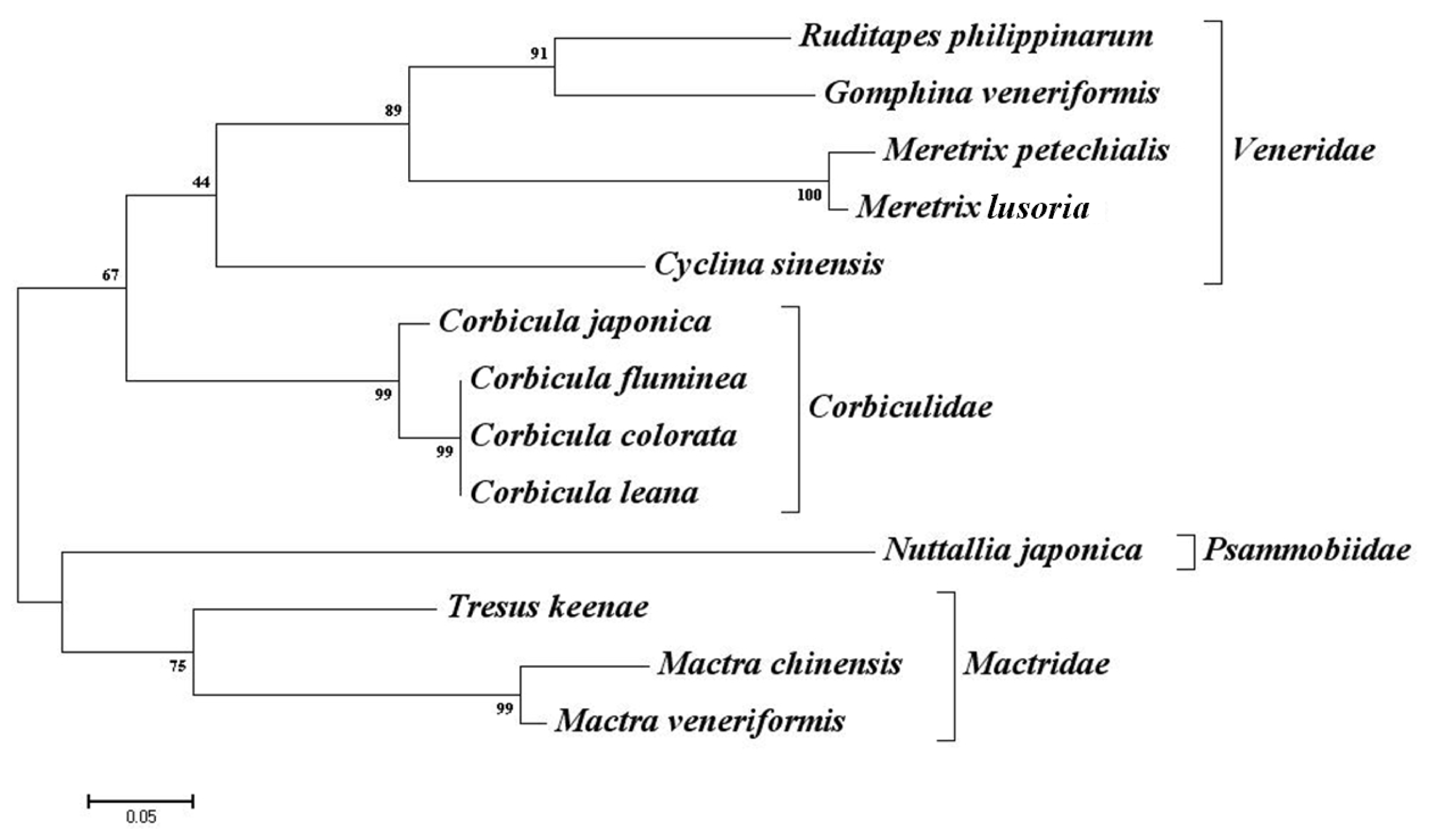
2.3. Cross-Species Amplification
3. Discussion
4. Experimental Section
4.1. Sample Collection
4.2. Library Construction and Sequencing
4.3. Primer Design and Genotyping
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Yamakawa, Y.A.; Yamaguchi, M.; Imai, H. Genetic relationships among species of Meretrix (Mollusca: Veneridae) in the Western Pacific Ocean. Pac. Sci 2008, 62, 385–394. [Google Scholar]
- Yoosukh, W.; Matsukuma, A. Taxonomic study on Meretrix (Mollusca: Bivalvia) from Thailand. Phuket. Mar. Biol. Cent. Spec. Publ 2001, 25, 451–460. [Google Scholar]
- Henmi, Y. Hamaguri no Seibutsugaku. Uchino, A., Ed.; Seibundo: Tokyo, Japan, 2009; pp. 81–121. [Google Scholar]
- Yamashita, Y.; Satoh, S.; Kim, K.; Henmi, Y.; Nagata, H.; Yamamoto, S.; Ikeguchi, A.; Mizuma, Y.; Nawa, J.; Takashima, U. Silent tidal flat: Current situations of Meretrix spp. in the coastal area of Japan and Korea. Rep. Takagi Fund Citiz. Sci 2004, 1, 85–91. [Google Scholar]
- Hong, J.S.; Yamashita, H.; Sato, S. The Saemangeum reclamation project in South Korea threatens to collapse a unique mollusk, ectosymbiotic bivalve species attached to the shell of Lingula anatina. Plankton Benthos Res 2007, 2, 70–75. [Google Scholar]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol 1992, 141, 173–216. [Google Scholar]
- Passamonti, M.; Scali, V. Gender associated mitochondrial DNA heteroplasmy in the venerid clam Tapes philippinarum (Mollusca: Bivalvia). Curr. Genet 2001, 39, 117–124. [Google Scholar]
- Breton, S.; Doucet-Beaupré, H.D.; Stewart, D.T.; Hoeh, W.R.; Blier, P.U. The unusual system of doubly uniparental inheritance of mtDNA: Isn’t one enough? Trends Genet 2007, 23, 465–474. [Google Scholar]
- Bierne, N.; Launey, S.; Naciri-Graven, Y.; Bonhomme, F. Early effect of inbreeding as revealed by microsatellite analyses on Ostrea edulis larvae. Genetics 1998, 148, 1893–1906. [Google Scholar]
- Holland, B.S. Invasion without a bottleneck: Microsatellite variation in natural and invasive populations of the brown mussel Perna perna (L). Mar. Biotechnol 2001, 3, 407–415. [Google Scholar]
- Selvamani, M.J.P.; Degnan, S.M.; Paetkau, D.; Degnan, B.M. Highly polymorphic microsatellite loci in the Heron Reef population of the tropical abalone Haliotis asinina. Mol. Ecol 2000, 9, 1184–1186. [Google Scholar]
- Li, Q.; Park, C.; Kobayashi, T.; Kijima, A. Inheritance of microsatellite DNA markers in the Pacific abalone Haliotis discus hannai. Mar. Biotechnol. 2003, 5, 331–338. [Google Scholar]
- Abdelkrim, J.; Robertson, B.C.; Stanton, J.-A.L.; Gemmell, N.J. Fast, cost effective development of species-specific microsatellite markers by genomic sequencing. BioTechniques 2009, 46, 185–191. [Google Scholar]
- Kang, J.H.; Park, J.Y.; Jo, H.S. Rapid Development of microsatellite markers with 454 pyrosequencing in a vulnerable fish, the mottled skate, Raja pulchra. Int. J. Mol. Sci 2012, 13, 7199–7211. [Google Scholar]
- Hamilton, M.; Pincus, E.L.; di Fiore, A.; Fleischer, R.C. Universal linker and ligation procedures for construction of genomic DNA libraries enriched for microsatellites. Biotechniques 1999, 27, 500–507. [Google Scholar]
- Liu, Z.; Tan, G.; Kucuktas, H.; Li, P.; Karsi, A.; Yant, D.R.; Dunham, R.A. High levels of conservation at microsatellite loci among Ictalurid catfishes. J. Hered 1999, 90, 307–312. [Google Scholar]
- Wu, L.; Kaufman, L.; Fuerst, P.A. Isolation of microsatellite markers in Astatoreochromis alluaudi and their cross-species amplifications in other African cichlids. Mol. Ecol 1999, 8, 895–897. [Google Scholar]
- Kuang, Y.Y.; Tong, G.X.; Xu, W.; Yin, J.S.; Sun, X.W. Analysis of genetic diversity in the endangered Hucho taimen from China. Acta Ecol. Sin 2009, 29, 92–97. [Google Scholar]
- Sekino, M.; Hara, M. Microsatellite DNA loci in Pacic abalone Haliotis discus discus (Mollusca, Gastropoda, Haliotidae). Mol. Ecol. Notes 2001, 1, 8–10. [Google Scholar]
- Hedgecock, D.; Li, G.; Hubert, S.; Bucklin, K.; Ribes, V. Widespread null alleles and poor cross-species amplification of microsatellite DNA loci cloned from the Pacific oyster, Crassostrea gigas. J. Shellfish Res 2004, 23, 379–385. [Google Scholar]
- Li, Q.; Yu, H.; Yu, R.H. Genetic variability assessed by microsatellites in cultured populations of the Pacific oyster (Crassostrea gigas) in China. Aquaculture 2006, 259, 95–102. [Google Scholar]
- Panova, M.; Mäkinen, T.; Fokin, M.; André, C.; Johannesson, K. Microsatellite cross species amplification in the genus Littorina and detection of null alleles in Littorina saxatilis. J. Molluscan Stud 2008, 74, 111–117. [Google Scholar]
- Zouros, E.; Foltz, D.W. Possible explanations of heterozygote deficiency in bivalve mollusks. Malacologia 1984, 25, 583–591. [Google Scholar]
- Launey, S.; Ledu, C.; Boudry, P.; Bonhomme, F.; Naciri-Graven, Y. Geographic structure in the European flat oyster (Ostrea edulis L.) as revealed by microsatellite polymorphism. J. Hered 2002, 93, 331–338. [Google Scholar]
- Eackles, M.S.; King, T.L. Isolation and characterization of microsatellite loci in Lampsilis abrupta (Bivalvia: Unionidae) and cross-species amplification within the genus. Mol. Ecol. Notes 2002, 2, 559–562. [Google Scholar]
- Vadopalas, B.; Leclair, L.L.; Bentzen, P. Microsatellite and allozyme analyses reveal few genetic differences among spatially distinct aggregations of geoduck clam (Panopea abrupta, Conrad 1849). J. Shellfish Res 2004, 23, 693–706. [Google Scholar]
- Barbará, T.; Palma-Silva, C.; Paggi, G.M.; Bered, F.; Fay, M.F.; Lexer, C. Cross-species transfer of nuclear microsatellite markers: potential and limitations. Mol. Ecol 2007, 16, 3759–3767. [Google Scholar]
- Waterson, G.A. Allele frequencies after a bottleneck. Theor. Popul. Biol 1984, 26, 387–407. [Google Scholar]
- Luikart, G.; Cornuet, J.M. Empirical evaluation of a test for identifying recent bottlenecked populations from allele frequency data. Conservat. Biol 1998, 12, 228–237. [Google Scholar]
- Torii, H.; Sato, S.; Hamaguchi, M.; Henmi, Y.; Yamashita, H. The comparison of shell morphology and genetic relationship between Meretrix lusoria and M. petechialis in Japan and Korea. Plankton Benthos Res 2010, 5, 231–241. [Google Scholar]
- Yamakawa, Y.A.; Imai, H. Hybridization between Meretrix lusoria and the alien congeneric species M. petechialis in Japan as demonstrated using DNA markers. Aquat. Invasions 2012, 7, 327–336. [Google Scholar]
- Morgan, T.S.; Rogers, A.S. Specificity and sensitivity of microsatellite makers for the identification of larvae. Mar. Biol 2001, 139, 967–973. [Google Scholar]
- Lu, X.; Wang, H.; Liu, B.; Lin, J. Microsatellite-based genetic and growth analysis for a diallel mating design of two stocks of the clam, Meretrix meretrix. Aquat. Res 2012, 43, 260–270. [Google Scholar]
- Lu, X.; Wang, H.; Liu, B.; Xiang, J. An effective method for parentage determination of the clam (Meretrix meretrix) based on SSR and COI markers. Aquaculture 2011, 318, 223–228. [Google Scholar]
- Li, Q.; Liu, S.K.; Kong, L.F. Microsatellites within genes and ESTs of the Pacific oyster Crassostrea gigas and their transferability in five other Crassostrea species. Electron. J. Biotechnol 2009, 12, 15–16. [Google Scholar]
- Asahida, T.; Kobayashi, T.; Saitoh, K.; Nakayama, I. Tissue preservation and total DNA extraction from fish at ambient temperature using buffers containing high concentration of urea. Fish. Sci 1996, 62, 727–730. [Google Scholar]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural population. Mol. Ecol 1998, 7, 639–655. [Google Scholar]
- Rousset, F. Genepop 007: A complete reimplementation of the Genepop software for Windows and Linux. Mol. Ecol. Resour 2008, 8, 103–106. [Google Scholar]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar]
- Goudet, J. FSTAT, A program to estimate and test gene diversities and fixation indices (Version 2.9.3). 2001. Available online: http://www.unil.ch/izea/software/fstat.html accessed on 16 July 2012.
| Primer name | Core sequence | Primer (5′→3′) | Annealing temp. | Amplicon size | No. Allele | Ho | He | GenBank accession No. | |
|---|---|---|---|---|---|---|---|---|---|
| Mp01-nfrdi | (AC)34 | F R | GACCGCGATCGTATACAAGTCCC TCCGTGCATGTGTGCCTATATCC | 48 | 179–199 | 4 | 0.372 | 0.306 | JX017338 |
| Mp02-nfrdi | (AC)14 | F R | CATGGGAAGCAGGCGGTTTGTTG ACATACGTGTGCGCATGCGTGTG | 52 | 102–164 | 9 | 0.327 | 0.287 | JX017339 |
| Mp03-nfrdi | (AC)29 | F R | TCGTATACAAGTCCCGGTCCTGG TCCGTGCATGTGTGCCTATATCC | 50 | 149–155 | 2 | 0.118 | 0.111 | JX017340 |
| Mp04-nfrdi | (AC)24 | F R | GGATTCCAGTTTAGCCCTCTC TATACACAGCGCAAGGTGAAC | 52 | 246–286 | 13 | 0.244 | 0.218 | JX017341 |
| Mp05-nfrdi | (AC)28 | F R | CCATATTTGACAGCAGTTTCGTC CAAAGTATCGCCTGAACCTGAC | 50 | 78–92 | 6 | 0.369 | 0.34 | JX017342 |
| Mp06-nfrdi | (AC)11 | F R | ACAGGACCTGATCGTGAACAC CAGGCCGAGTGCAGAAGTGGA | 50 | 168–196 | 2 | 0.14 | 0.13 | JX017343 |
| Mp07-nfrdi | (AC)31 | F R | GTATACAAGTCCCGGTCCTGT CATCCGTGCATGTGTGCCTAT | 48 | 103–173 | 5 | 0.675 | 0.455 | JX017344 |
| Mp08-nfrdi | (AC)37 | F R | TATAGTTCGGACGGACATGGACA GCCCAAGAGTTGAACATCAGGTT | 48 | 129–227 | 43 | 0.603 | 0.594 | JX017345 |
| Mp09-nfrdi | (GT)5CT(GT)7 CT(GT)3 | F R | ACATACGTGTGCGCATGCATGTG GCAGGCGGTTTGGCTGGCAGGTC | 58 | 209–267 | 6 | 0.394 | 0.381 | JX017346 |
| Mp10-nfrdi | (AC)10 | F R | AGGACCTGATCCTGAACACAC TCTGCATGACTGTCTGTCTCC | 48 | 188 | 1 | - | - | JX017347 |
| Mp11-nfrdi | (GT)11 | F R | CCGAGTGCAGAAGAGGAACACAC CACACAGGACCTGATCGTGAACA | 48 | 118 | 1 | - | - | JX017348 |
| Mp12-nfrdi | (AC)35 | F R | ACAGACCAAACCATCCTTATCCC CTATTTTCAAGCCAGGCAGAATG | 50 | 93 | 1 | - | - | JX017349 |
| Mp13-nfrdi | (AC)8 | F R | TTAACCCGGCCACCCACCTATAC CGTATTTGTGCGCATGCGTGTGC | 50 | 83 | 1 | - | - | JX017350 |
| Mp14-nfrdi | (GT)10 | F R | GCAGCAGGCCGAGTGCAGAAG CGTTGGGGCACCGCGACCACA | 48 | 165 | 1 | - | - | JX017351 |
| Mp15-nfrdi | (GT)11TT(GT)16 (AT)2(GT)2GA(GT)9 | F R | GCCTATATCTGCGTATGTGCATC AGGGGACCGCGATCGTATACAAG | 48 | 112 | 1 | - | - | JX017352 |
| Microsatellite loci | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pop. | Mp01-nfrdi | Mp02-nfrdi | Mp03-nfrdi | Mp04-nfrdi | Mp05-nfrdi | Mp06-nfrdi | Mp07-nfrdi | Mp08-nfrdi | Mp09-nfrdi | Means | |
| GC | N | 35 | 35 | 35 | 34 | 33 | 35 | 35 | 35 | 35 | 34.7 |
| Na | 3 | 5 | 2 | 7 | 2 | 2 | 2 | 18 | 3 | 5.3 | |
| AR | 2.8 | 4.6 | 2.0 | 6.5 | 2.0 | 2.0 | 2.0 | 15.7 | 2.8 | 4.9 | |
| R | 179–199 | 102–136 | 149–155 | 246–286 | 78–92 | 168–196 | 143–173 | 129–205 | 209–247 | ||
| Ho | 0.400 | 0.286 | 0.114 | 0.353 | 0.303 | 0.057 | 0.714 | 0.543 | 0.571 | 0.372 | |
| He | 0.330 | 0.260 | 0.109 | 0.319 | 0.261 | 0.056 | 0.466 | 0.549 | 0.467 | 0.365 | |
| FIS | −0.212 | −0.097 | −0.045 | −0.107 | −0.161 | −0.015 | −0.533 * | 0.011 | −0.224 | −0.084 | |
| MA | N | 58 | 57 | 58 | 58 | 58 | 58 | 58 | 58 | 57 | 57.8 |
| Na | 2 | 6 | 2 | 8 | 2 | 2 | 3 | 26 | 4 | 6.7 | |
| AR | 2.0 | 4.9 | 2.0 | 5.5 | 2.0 | 2.0 | 2.5 | 16.9 | 3.0 | 5.2 | |
| R | 179–199 | 102–164 | 149–155 | 246–280 | 78–92 | 168–196 | 103–173 | 129–227 | 209–267 | ||
| Ho | 0.431 | 0.263 | 0.138 | 0.345 | 0.379 | 0.155 | 0.603 | 0.586 | 0.333 | 0.355 | |
| He | 0.341 | 0.243 | 0.130 | 0.308 | 0.331 | 0.144 | 0.430 | 0.651 | 0.309 | 0.374 | |
| FIS | −0.264 | −0.084 | −0.065 | −0.119 | −0.146 | −0.075 | −0.403 * | 0.099 | −0.078 | −0.051 | |
| Mean all pops. | N | 46.5 | 46 | 46.5 | 46 | 45.5 | 46.5 | 46.5 | 46.5 | 46 | |
| Na | 2.5 | 5.5 | 2 | 7.5 | 2 | 2 | 2.5 | 22 | 3.5 | ||
| AR | 2.4 | 4.8 | 2 | 6 | 2 | 1.9914 | 2.3 | 16.3 | 2.9 | ||
| R | 179–199 | 102–164 | 149–155 | 246–286 | 78–92 | 168–196 | 103–173 | 129–227 | 209–267 | ||
| Ho | 0.386 | 0.275 | 0.126 | 0.349 | 0.341 | 0.106 | 0.659 | 0.565 | 0.452 | ||
| He | 0.336 | 0.252 | 0.119 | 0.314 | 0.296 | 0.1 | 0.448 | 0.6 | 0.388 | ||
| FIS | −0.238 | −0.091 | −0.055 | −0.113 | −0.154 | −0.045 | −0.468 * | −0.055 | −0.151 | ||
| Taxonomy | Veneridae | Psammobiidae | Mactridae | Corbiculidae | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Meretrix petechialis | Meretrix lousoria | Gomphina veneriformis | Cyclina sinensis | Ruditapes philippinarum | Nuttallia japonica | Mactra chinensis | Mactra veneriformis | Tresus keenae | Corbicula japonica | Corbicula leana | Corbicula fluminea | Corbicula colorata | Transferability * |
| Number of Individuals | 95 | 7 | 2 | 2 | 9 | 3 | 1 | 2 | 1 | 2 | 2 | 4 | 2 | |
| Mp01-nfrdi | 179–199 | 179 | - | 91–139 | - | 179–199 | - | - | - | - | 77 | - | 83 | 41.7 (16.7) |
| Mp02-nfrdi | 102–164 | - | 132 | 64–124 | 112–306 | 222 | - | 146 | 146–150 | 276–334 | 242–300 | 82–178 | 82 | 83.3 (50.0) |
| Mp03-nfrdi | 149–155 | 149 | - | 143 | 117 | - | - | 65 | - | - | - | - | - | 33.3 (0) |
| Mp04-nfrdi | 246–286 | - | - | - | - | - | - | - | - | - | - | - | - | 0/0 |
| Mp05-nfrdi | 78–92 | 78–92 | - | 82–332 | - | 112 | - | 214 | - | 108 | - | 96 | - | 50.0 (16.7) |
| Mp06-nfrdi | 168–196 | 168 | 126–296 | 126–298 | 72–122 | 126–140 | 196 | 82–228 | 164 | 98 | 166 | - | 132 | 91.7 (33.3) |
| Mp07-nfrdi | 103–173 | 143–173 | - | 91–111 | - | 67 | - | - | - | - | 155–175 | - | 93 | 41.7 (25.0) |
| Mp08-nfrdi | 129–227 | - | 87–123 | 89–99 | 137–245 | 159–161 | 169–253 | - | 215 | 101 | 243–245 | 111–135 | 245 | 83.3 (58.3) |
| Mp09-nfrdi | 209–267 | - | - | - | - | - | - | - | - | - | - | - | - | 0/0 |
| Mp10-nfrdi | 188 | 188 | 196 | 192–198 | 94–108 | - | 110–178 | 182 | 112 | 266–270 | - | 100–166 | - | 75.0 (41.7) |
| Mp11-nfrdi | 118 | - | 132–138 | - | - | 132–146 | 156 | - | 94–178 | - | - | - | 138 | 41.7 (25.0) |
| Mp12-nfrdi | 93 | - | 65–111 | 93 | 95–123 | - | - | 121–161 | - | - | - | - | - | 33.3 (25.0) |
| Mp13-nfrdi | 83 | - | 71 | - | 65–87 | 115–215 | 91–219 | 91–139 | 199 | - | 105 | 83 | - | 66.7 (33.3) |
| Mp14-nfrdi | 165 | - | 157 | 157 | 129–131 | 75 | - | 101 | - | - | - | - | 239–243 | 50.0 (16.7) |
| Mp15-nfrdi | 112 | 76–112 | - | - | 102 | - | - | 222 | - | - | - | 126–350 | - | 33.3 (16.7) |
| Transferability * | - | 46.7 (20.0) | 53.0 (26.7) | 66.7 (40.0) | 60 (46.7) | 60.0 (33.3) | 33.3 (20.0) | 60.0 (20.0) | 40.0 (13.3) | 33.3 (13.3) | 40.0 (20.0) | 40.0 (26.7) | 46.7 (6.7) | |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kang, J.-H.; Kim, B.-H.; Park, J.-Y.; Lee, J.-M.; Jeong, J.-E.; Lee, J.-S.; Ko, H.-S.; Lee, Y.-S. Novel Microsatellite Markers of Meretrix petechialis and Cross-species Amplification in Related Taxa (Bivalvia: Veneroida). Int. J. Mol. Sci. 2012, 13, 15942-15954. https://doi.org/10.3390/ijms131215942
Kang J-H, Kim B-H, Park J-Y, Lee J-M, Jeong J-E, Lee J-S, Ko H-S, Lee Y-S. Novel Microsatellite Markers of Meretrix petechialis and Cross-species Amplification in Related Taxa (Bivalvia: Veneroida). International Journal of Molecular Sciences. 2012; 13(12):15942-15954. https://doi.org/10.3390/ijms131215942
Chicago/Turabian StyleKang, Jung-Ha, Byeng-Hak Kim, Jung-Youn Park, Jung-Mi Lee, Ji-Eun Jeong, Jun-Sang Lee, Hyun-Sook Ko, and Yong-Seok Lee. 2012. "Novel Microsatellite Markers of Meretrix petechialis and Cross-species Amplification in Related Taxa (Bivalvia: Veneroida)" International Journal of Molecular Sciences 13, no. 12: 15942-15954. https://doi.org/10.3390/ijms131215942
APA StyleKang, J.-H., Kim, B.-H., Park, J.-Y., Lee, J.-M., Jeong, J.-E., Lee, J.-S., Ko, H.-S., & Lee, Y.-S. (2012). Novel Microsatellite Markers of Meretrix petechialis and Cross-species Amplification in Related Taxa (Bivalvia: Veneroida). International Journal of Molecular Sciences, 13(12), 15942-15954. https://doi.org/10.3390/ijms131215942




