Marine Omega-3 Phospholipids: Metabolism and Biological Activities
Abstract
:1. Introduction
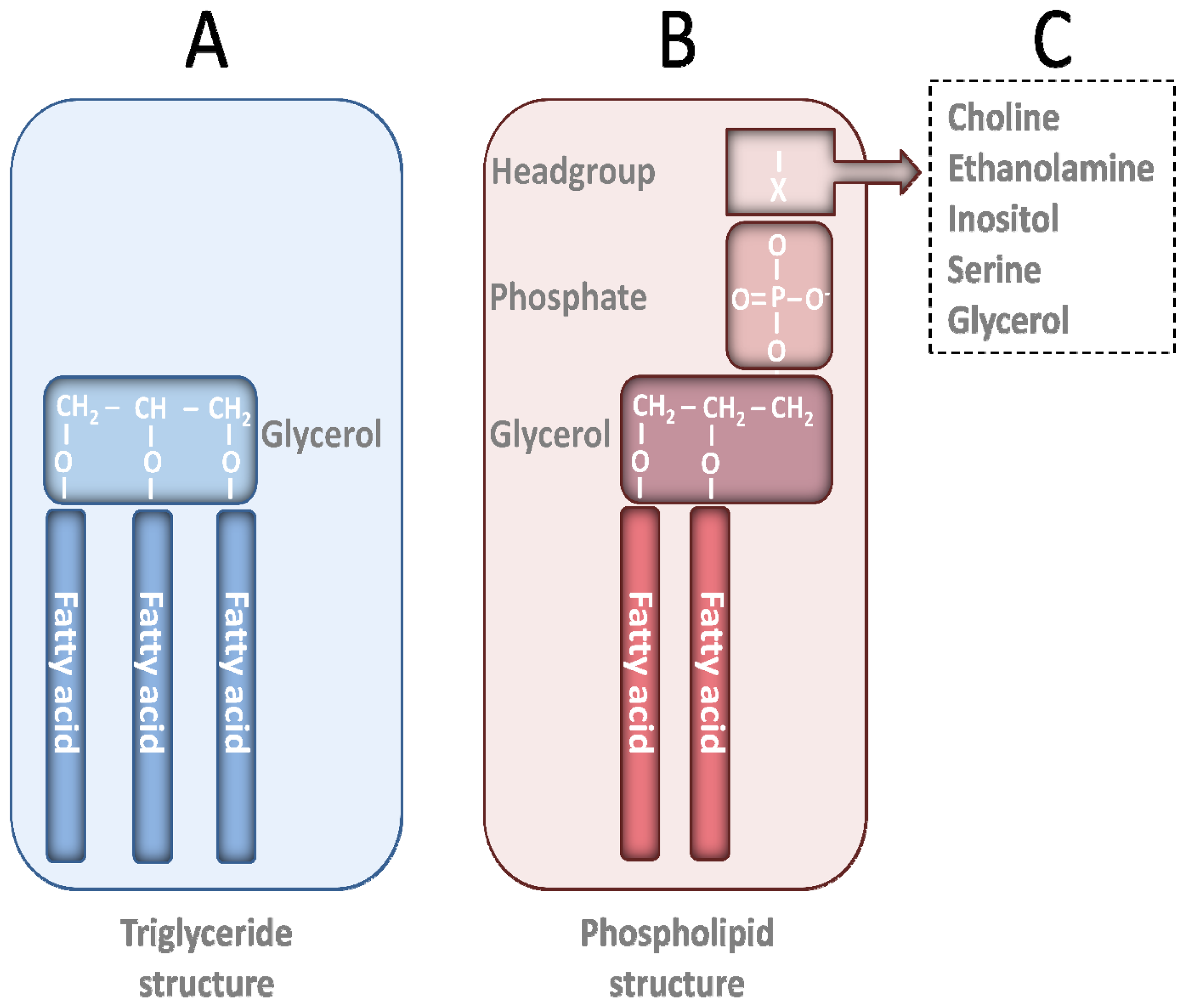
2. PL Classes
3. Sources of Marine PLs
3.1. Fish Roe
3.2. Krill Oil
3.3. Fish
4. Different Tissue Distribution of FAs from PL and TG Ester Forms
5. Health Effects of Non-Marine and Marine PLs
5.1. PLs
5.2. Choline
5.3. n-3 PUFAs
5.4. n-3 PLs
5.4.1. KO
5.4.2. Fish and Fish Roe
6. Future Perspectives
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Wassall, S.R.; Stillwell, W. Polyunsaturated fatty acid-cholesterol interactions: Domain formation in membranes. Biochimi. Biophys. Acta 2009, 1788, 24–32. [Google Scholar]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Dietet. Assoc 2009, 109, 668–679. [Google Scholar]
- Bang, H.O.; Dyerberg, J.; Nielsen, A.B. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1971, 1, 1143–1145. [Google Scholar]
- Dyerberg, J.; Bang, H.O.; Hjorne, N. Fatty acid composition of the plasma lipids in Greenland Eskimos. Am. J. Clin. Nutr 1975, 28, 958–966. [Google Scholar]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc 2011, 70, 215–231. [Google Scholar]
- Banni, S.; Di Marzo, V. Effect of dietary fat on endocannabinoids and related mediators: Consequences on energy homeostasis, inflammation and mood. Mol. Nutr. Food Res 2010, 54, 82–92. [Google Scholar]
- Lu, F.S.; Nielsen, N.S.; Timm-Heinrich, M.; Jacobsen, C. Oxidative stability of marine phospholipids in the liposomal form and their applications. Lipids 2011, 46, 3–23. [Google Scholar]
- Higuchi, T.; Shirai, N.; Suzuki, H. Effects of dietary herring roe lipids on plasma lipid, glucose, insulin, and adiponectin concentrations in mice. J. Agric. Food Chem 2006, 54, 3750–3755. [Google Scholar]
- Shirai, N.; Higuchi, T.; Suzuki, H. Effect of lipids extracted from a salted herring roe food product on maze-behavior in mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 451–456. [Google Scholar]
- Hayashi, H.; Tanaka, Y.; Hibino, H.; Umeda, Y.; Kawamitsu, H.; Fujimoto, H.; Amakawa, T. Beneficial effect of salmon roe phosphatidylcholine in chronic liver disease. Curr. Med. Res. Opin 1999, 15, 177–184. [Google Scholar]
- Shirai, N.; Higuchi, T.; Suzuki, H. Analysis of lipid classes and the fatty acid composition of the salted fish roe food products, Ikura, Tarako, Tobiko and Kazunoko. Food Chem 2006, 94, 61–67. [Google Scholar]
- Lieber, C.S.; Robins, S.J.; Li, J.; DeCarli, L.M.; Mak, K.M.; Fasulo, J.M.; Leo, M.A. Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterology 1994, 106, 152–159. [Google Scholar]
- Loy, R.; Heyer, D.; Williams, C.L.; Meck, W.H. Choline-induced spatial memory facilitation correlates with altered distribution and morphology of septal neurons. Adv. Exp. Med. Biol 1991, 295, 373–382. [Google Scholar]
- Phleger, C.F.; Nelson, M.M.; Mooney, B.D.; Nichols, P.D. Interannual and between species comparison of the lipids, fatty acids and sterols of Antarctic krill from the US AMLR Elephant Island survey area. Comp. Biochem. Physiol. B Biochem. Mol. Biol 2002, 131, 733–747. [Google Scholar]
- Tou, J.C.; Jaczynski, J.; Chen, Y.C. Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev 2007, 65, 63–77. [Google Scholar]
- Le Grandois, J.; Marchioni, E.; Zhao, M.; Giuffrida, F.; Ennahar, S.; Bindler, F. Investigation of natural phosphatidylcholine sources: Separation and identification by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS2) of molecular species. J. Agric. Food Chem 2009, 57, 6014–6020. [Google Scholar]
- Winther, B.; Hoem, N.; Berge, K.; Reubsaet, L. Elucidation of phosphatidylcholine composition in krill oil extracted from Euphausia superba. Lipids 2011, 46, 25–36. [Google Scholar]
- Hjaltason, B.; Haraldsson, G.G. Fish Oils and Lipids from Marine Sources. In Modifying Lipids for Use in Food; Gunstone, F.D., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 57–79. [Google Scholar]
- Xu, R.; Hung, J.B.; German, J.B. Effects of dietary lipids on the fatty acid composition of triglycerides and phospholipids in tissues of white sturgeon. Aquacult. Nutr 1996, 2, 101–109. [Google Scholar]
- Polvi, S.M.; Ackman, R.G. Atlantic salmon (Salmo salar) muscle lipids and their response to alternative dietary fatty acid sources. J. Agric. Food Chem 1992, 40, 1001–1007. [Google Scholar]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis 2012, 11, 144. [Google Scholar]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 2011, 46, 37–46. [Google Scholar]
- Carlier, H.; Bernard, A.; Caselli, C. Digestion and absorption of polyunsaturated fatty acids. Reprod. Nutr. Dev 1991, 31, 475–500. [Google Scholar]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab 2009, 296, E1183–E1194. [Google Scholar]
- Friedman, H.I.; Nylund, B. Intestinal fat digestion, absorption, and transport. A review. Am. J. Clin. Nutr 1980, 33, 1108–1139. [Google Scholar]
- Krimbou, L.; Hassan, H.H.; Blain, S.; Rashid, S.; Denis, M.; Marcil, M.; Genest, J. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J. Lipid Res 2005, 46, 1668–1677. [Google Scholar]
- Magun, A.M.; Mish, B.; Glickman, R.M. Intracellular apoA-I and apoB distribution in rat intestine is altered by lipid feeding. J. Lipid Res 1988, 29, 1107–1116. [Google Scholar]
- Wang, H.; Du, J.; Lu, S.; Yao, Y.; Hunter, F.; Black, D.D. Regulation of intestinal apolipoprotein A-I synthesis by dietary phosphatidylcholine in newborn swine. Lipids 2001, 36, 683–687. [Google Scholar]
- Brunham, L.R.; Kruit, J.K.; Iqbal, J.; Fievet, C.; Timmins, J.M.; Pape, T.D.; Coburn, B.A.; Bissada, N.; Staels, B.; Groen, A.K.; et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest 2006, 116, 1052–1062. [Google Scholar]
- Forester, G.P.; Tall, A.R.; Bisgaier, C.L.; Glickman, R.M. Rat intestine secretes spherical high density lipoproteins. J. Biol. Chem 1983, 258, 5938–5943. [Google Scholar]
- Green, P.H.; Tall, A.R.; Glickman, R.M. Rat intestine secretes discoid high density lipoprotein. J. Clin. Invest 1978, 61, 528–534. [Google Scholar]
- Preiss-Landl, K.; Zimmermann, R.; Hammerle, G.; Zechner, R. Lipoprotein lipase: The regulation of tissue specific expression and its role in lipid and energy metabolism. Curr. Opin. Lipidol 2002, 13, 471–481. [Google Scholar]
- Yamashita, S.; Hirano, K.; Sakai, N.; Matsuzawa, Y. Molecular biology and pathophysiological aspects of plasma cholesteryl ester transfer protein. Biochim. Biophys. Acta 2000, 1529, 257–275. [Google Scholar]
- Amate, L.; Gil, A.; Ramirez, M. Dietary long-chain polyunsaturated fatty acids from different sources affect fat and fatty acid excretions in rats. J. Nutr 2001, 131, 3216–3221. [Google Scholar]
- Sala-Vila, A.; Campoy, C.; Castellote, A.I.; Garrido, F.J.; Rivero, M.; Rodriguez-Palmero, M.; Lopez-Sabater, M.C. Influence of dietary source of docosahexaenoic and arachidonic acids on their incorporation into membrane phospholipids of red blood cells in term infants. Prostaglandins Leukotrienes Essent. Fatty Acids 2006, 74, 143–148. [Google Scholar]
- Sala-Vila, A.; Castellote, A.I.; Campoy, C.; Rivero, M.; Rodriguez-Palmero, M.; Lopez-Sabater, M.C. The source of long-chain PUFA in formula supplements does not affect the fatty acid composition of plasma lipids in full-term infants. J. Nutr 2004, 134, 868–873. [Google Scholar]
- Valenzuela, A.; Nieto, S.; Sanhueza, J.; Nunez, M.J.; Ferrer, C. Tissue accretion and milk content of docosahexaenoic acid in female rats after supplementation with different docosahexaenoic acid sources. Ann. Nutr. Metab 2005, 49, 325–332. [Google Scholar]
- Valenzuela, A.; Valenzuela, V.; Sanhueza, J.; Nieto, S. Effect of supplementation with docosahexaenoic acid ethyl ester and sn-2 docosahexaenyl monoacylglyceride on plasma and erythrocyte fatty acids in rats. Ann. Nutr. Metab 2005, 49, 49–53. [Google Scholar]
- Amate, L.; Gil, A.; Ramirez, M. Feeding infant piglets formula with long-chain polyunsaturated fatty acids as triacylglycerols or phospholipids influences the distribution of these fatty acids in plasma lipoprotein fractions. J. Nutr 2001, 131, 1250–1255. [Google Scholar]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thies, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204, discussion 215–221. [Google Scholar]
- Picq, M.; Chen, P.; Perez, M.; Michaud, M.; Vericel, E.; Guichardant, M.; Lagarde, M. DHA metabolism: Targeting the brain and lipoxygenation. Mol. Neurobiol 2010, 42, 48–51. [Google Scholar]
- Graf, B.A.; Duchateau, G.S.; Patterson, A.B.; Mitchell, E.S.; van Bruggen, P.; Koek, J.H.; Melville, S.; Verkade, H.J. Age dependent incorporation of 14C-DHA into rat brain and body tissues after dosing various 14C-DHA-esters. Prostaglandins Leukotrienes Essent. Fatty Acids 2010, 83, 89–96. [Google Scholar]
- Wijendran, V.; Huang, M.C.; Diau, G.Y.; Boehm, G.; Nathanielsz, P.W.; Brenna, J.T. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr. Res 2002, 51, 265–272. [Google Scholar]
- Batetta, B.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Sanna, F.; Bisogno, T.; Uda, S.; et al. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J. Nutr 2009, 139, 1495–501. [Google Scholar]
- Di Marzo, V.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Bisogno, T.; Collu, M.; Batetta, B.; et al. Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoylglycerol but not N-acylethanolamine levels in the brain of obese Zucker rats. Int. Dairy J 2010, 20, 231–235. [Google Scholar]
- Gamoh, S. Krill-derived phospholipids rich in n-3 fatty acid improve spatial memory in adult rats. J. Agric. Sci 2011, 3, 3–12. [Google Scholar]
- Thies, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem 1992, 59, 1110–1116. [Google Scholar]
- Thies, F.; Pillon, C.; Moliere, P.; Lagarde, M.; Lecerf, J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol 1994, 267, R1273–R1279. [Google Scholar]
- Cansell, M. Marine phospholipids as dietary carriers of long-chain polyunsaturated fatty acids. Lipid Technol 2010, 22, 223–226. [Google Scholar]
- Cansell, M.; Moussaoui, N.; Petit, A.P.; Denizot, A.; Combe, N. Feeding rats with liposomes or fish oil differently affects their lipid metabolism. Eur. J. Lipid Sci. Technol 2006, 108, 459–467. [Google Scholar]
- Cansell, M.; Nacka, F.; Combe, N. Marine lipid-based liposomes increase in vivo FA bioavailability. Lipids 2003, 38, 551–559. [Google Scholar]
- Cansell, M.S.; Battin, A.; Degrace, P.; Gresti, J.; Clouet, P.; Combe, N. Early dissimilar fates of liver eicosapentaenoic acid in rats fed liposomes or fish oil and gene expression related to lipid metabolism. Lipids 2009, 44, 237–247. [Google Scholar]
- Cole, G.M.; Ma, Q.L.; Frautschy, S.A. Dietary fatty acids and the aging brain. Nutr. Rev 2010, 68, S102–S111. [Google Scholar]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol 2006, 63, 1545–1550. [Google Scholar]
- Lemaitre-Delaunay, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Armstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [(13)C]DHA in phosphatidylcholine. J. Lipid Res 1999, 40, 1867–1874. [Google Scholar]
- Bunea, R.; El Farrah, K.; Deutsch, L. Evaluation of the effects of neptune krill oil on the clinical course of hyperlipidemia. Altern. Med. Rev 2004, 9, 420–428. [Google Scholar]
- Banni, S.; Carta, G.; Murru, E.; Cordeddu, L.; Giordano, E.; Sirigu, A.R.; Berge, K.; Vik, H.; Maki, K.C.; Di Marzo, V.; et al. Krill oil significantly decreases 2-arachidonoylglycerol plasma levels in obese subjects. Nutr. Metab.(Lond) 2011, 8, 7. [Google Scholar] [Green Version]
- Deutsch, L. Evaluation of the effect of neptune krill oil on chronic inflammation and arthritic symptoms. J. Am. Coll. Nutr 2007, 26, 39–48. [Google Scholar]
- Skarpańska-Stejnborn, A.; Pilaczyńska-Szcześniak, L.; Basta, P.; Foriasz, J.; Arlet, J. Effects of supplementation with neptune krill oil (Euphasia Superba) on selected redox parameters and pro-Inflammatory markers in athletes during exhaustive exercise. J. Hum. Kinet 2010, 25, 49–57. [Google Scholar]
- Sampalis, F.; Bunea, R.; Pelland, M.F.; Kowalski, O.; Duguet, N.; Dupuis, S. Evaluation of the effects of neptune krill oil on the management of premenstrual syndrome and dysmenorrhea. Altern. Med. Rev 2003, 8, 171–179. [Google Scholar]
- Richter, Y.; Herzog, Y.; Cohen, T.; Steinhart, Y. The effect of phosphatidylserine-containing omega-3 fatty acids on memory abilities in subjects with subjective memory complaints: A pilot study. Clin. Interv. Aging 2010, 5, 313–316. [Google Scholar]
- Vaisman, N.; Kaysar, N.; Zaruk-Adasha, Y.; Pelled, D.; Brichon, G.; Zwingelstein, G.; Bodennec, J. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: Effect of dietary n-3 fatty acids containing phospholipids. Am. J. Clin. Nutr 2008, 87, 1170–1180. [Google Scholar]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Griinari, M.; Berge, K.; Vik, H.; Hubacher, R.; Rains, T.M. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr. Res 2009, 29, 609–615. [Google Scholar]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis 2011, 10, 145. [Google Scholar]
- Fosshaug, L.E.; Berge, R.K.; Beitnes, J.O.; Berge, K.; Vik, H.; Aukrust, P.; Gullestad, L.; Vinge, L.E.; Oie, E. Krill oil attenuates left ventricular dilatation after myocardial infarction in rats. Lipids Health Dis 2011, 10, 245. [Google Scholar]
- Higuchi, T.; Shirai, N.; Suzuki, H. Effects of herring roe on plasma lipid, glucose, insulin and adiponectin levels, and hepatic lipid contents in mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 230–236. [Google Scholar]
- Rossmeisl, M.; Jilkova, Z.M.; Kuda, O.; Jelenik, T.; Medrikova, D.; Stankova, B.; Kristinsson, B.; Haraldsson, G.G.; Svensen, H.; Stoknes, I.; et al. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS One 2012, 7, e38834. [Google Scholar]
- Ferramosca, A.; Conte, A.; Burri, L.; Berge, K.; De Nuccio, F.; Giudetti, A.M.; Zara, V. A krill oil supplemented diet suppresses hepatic steatosis in high-fat fed rats. PLoS One 2012, 7, e38797. [Google Scholar]
- Piscitelli, F.; Carta, G.; Bisogno, T.; Murru, E.; Cordeddu, L.; Berge, K.; Tandy, S.; Cohn, J.S.; Griinari, M.; Banni, S.; et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high fat-fed mice. Nutr.Metab 2011, 8, 1–16. [Google Scholar]
- Tandy, S.; Chung, R.W.S.; Wat, E.; Kamili, A.; Berge, K.; Griinari, M.; Cohn, J.S. Dietary krill oil supplementation reduces hepatic steatosis, glycemia and hypercholesterolemia in high-fat fed mice. J. Agric. Food Chem 2009, 57, 9339–9345. [Google Scholar]
- Moriya, H.; Hosokawa, M.; Miyashita, K. Combination effect of herring roe lipids and proteins on plasma lipids and abdominal fat weight of mouse. J. Food Sci 2007, 72, C231–C234. [Google Scholar]
- Bjorndal, B.; Burri, L.; Wergedahl, H.; Svardal, A.; Bohov, P.; Berge, R.K. Dietary supplementation of herring roe and milt enhances hepatic fatty acid catabolism in female mice transgenic for hTNFalpha. Eur. J. Nutr 2012, 51, 741–753. [Google Scholar]
- Vigerust, N.F.; Bjorndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-alpha. Eur. J. Nutr. 2012. [Google Scholar] [CrossRef]
- Grimstad, T.; Bjorndal, B.; Cacabelos, D.; Aasprong, O.G.; Janssen, E.A.; Omdal, R.; Svardal, A.; Hausken, T.; Bohov, P.; Portero-Otin, M.; et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scand. J. Gastroenterol 2012, 47, 49–58. [Google Scholar]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskeletal Disord 2010, 11, 136. [Google Scholar]
- Lee, B.; Sur, B.J.; Han, J.J.; Shim, I.; Her, S.; Lee, H.J.; Hahm, D.H. Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1085–1093. [Google Scholar]
- Lukas, R.; Gigliotti, J.C.; Smith, B.J.; Altman, S.; Tou, J.C. Consumtion of different sources of omega-3 polyunsaturated fatty acids by growing female rats affects long bone mass and microarchitecture. Bone 2011, 49, 455–462. [Google Scholar]
- Ferramosca, A.; Conte, L.; Zara, V. A krill oil supplemented diet reduces the activities of the mitochondrial tricarboxylate carrier and of the cytosolic lipogenic enzymes in rats. J. Anim. Physiol. Anim. Nutr. 2011, 1–12. [Google Scholar]
- Burri, L.; Berge, K.; Wibrand, K.; Berge, R.K.; Barger, J.L. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front. Genet 2011, 2, 1–8. [Google Scholar]
- Hung, M.C.; Shibasaki, K.; Yoshida, R.; Sato, M.; Imaizumi, K. Learning behaviour and cerebral protein kinase C, antioxidant status, lipid composition in senescence-accelerated mouse: Influence of a phosphatidylcholine-vitamin B12 diet. Br. J. Nutr 2001, 86, 163–171. [Google Scholar]
- Chung, S.Y.; Moriyama, T.; Uezu, E.; Uezu, K.; Hirata, R.; Yohena, N.; Masuda, Y.; Kokubu, T.; Yamamoto, S. Administration of phosphatidylcholine increases brain acetylcholine concentration and improves memory in mice with dementia. J. Nutr 1995, 125, 1484–1489. [Google Scholar]
- Schneider, H.; Braun, A.; Fullekrug, J.; Stremmel, W.; Ehehalt, R. Lipid based therapy for ulcerative colitis-modulation of intestinal mucus membrane phospholipids as a tool to influence inflammation. Int. J. Mol. Sci 2010, 11, 4149–4164. [Google Scholar]
- Buang, Y.; Wang, Y.M.; Cha, J.Y.; Nagao, K.; Yanagita, T. Dietary phosphatidylcholine alleviates fatty liver induced by orotic acid. Nutrition 2005, 21, 867–873. [Google Scholar]
- O’Brien, B.C.; Andrews, V.G. Influence of dietary egg and soybean phospholipids and triacylglycerols on human serum lipoproteins. Lipids 1993, 28, 7–12. [Google Scholar]
- Jager, R.; Purpura, M.; Geiss, K.R.; Weiss, M.; Baumeister, J.; Amatulli, F.; Schroder, L.; Herwegen, H. The effect of phosphatidylserine on golf performance. J. Int. Soc. Sports Nutr 2007, 4, 23. [Google Scholar]
- Jager, R.; Purpura, M.; Kingsley, M. Phospholipids and sports performance. J. Int. Soc. Sports Nutr 2007, 4, 5. [Google Scholar]
- An, B.K.; Nishiyama, H.; Tanaka, K.; Ohtani, S.; Iwata, T.; Tsutsumi, K.; Kasai, M. Dietary safflower phospholipid reduces liver lipids in laying hens. Poult. Sci 1997, 76, 689–695. [Google Scholar]
- Imaizumi, K.; Mawatari, K.; Murata, M.; Ikeda, I.; Sugano, M. The contrasting effect of dietary phosphatidylethanolamine and phosphatidylcholine on serum lipoproteins and liver lipids in rats. J. Nutr 1983, 113, 2403–2411. [Google Scholar]
- Iwata, T.; Hoshi, S.; Takehisa, F.; Tsutsumi, K.; Furukawa, Y.; Kimura, S. The effect of dietary safflower phospholipid and soybean phospholipid on plasma and liver lipids in rats fed a hypercholesterolemic diet. J. Nutr. Sci. Vitaminol. (Tokyo) 1992, 38, 471–479. [Google Scholar]
- Kabir, Y.; Ide, T. Effect of dietary soybean phospholipid and fats differing in the degree of unsaturation on fatty acid synthesis and oxidation in rat liver. J. Nutr. Sci. Vitaminol. (Tokyo) 1995, 41, 635–645. [Google Scholar]
- LeBlanc, M.J.; Brunet, S.; Bouchard, G.; Lamireau, T.; Yousef, I.M.; Gavino, V.; Levy, E.; Tuchweber, B. Effects of dietary soybean lecithin on plasma lipid transport and hepatic cholesterol metabolism in rats. J. Nutr. Biochem 2003, 14, 40–48. [Google Scholar]
- Polichetti, E.; Diaconescu, N.; De La Porte, P.L.; Malli, L.; Portugal, H.; Pauli, A.M.; Lafont, H.; Tuchweber, B.; Yousef, I.; Chanussot, F. Cholesterol-lowering effect of soyabean lecithin in normolipidaemic rats by stimulation of biliary lipid secretion. Br. J. Nutr 1996, 75, 471–478. [Google Scholar]
- Polichetti, E.; Janisson, A.; de la Porte, P.L.; Portugal, H.; Leonardi, J.; Luna, A.; La Droitte, P.; Chanussot, F. Dietary polyenylphosphatidylcholine decreases cholesterolemia in hypercholesterolemic rabbits: Role of the hepato-biliary axis. Life Sci 2000, 67, 2563–2576. [Google Scholar]
- Shirouchi, B.; Nagao, K.; Inoue, N.; Ohkubo, T.; Hibino, H.; Yanagita, T. Effect of dietary omega-3 phosphatidylcholine on obesity-related disorders in obese Otsuka Long-Evans Tokushima fatty rats. J. Agric. Food Chem 2007, 55, 7170–7176. [Google Scholar]
- Lieber, C.S. New concepts of the pathogenesis of alcoholic liver disease lead to novel treatments. Curr. Gastroenterol. Rep 2004, 6, 60–65. [Google Scholar]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar]
- Turecky, L.; Kupcova, V.; Szantova, M.; Uhlikova, E. Plasma lipid parameters in patients with alcoholic fatty liver after treatment with essential phospholipids. Bratisl. Lek. Listy 2003, 104, 227–231. [Google Scholar]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar]
- Cohn, J.S.; Wat, E.; Kamili, A.; Tandy, S. Dietary phospholipids, hepatic lipid metabolism and cardiovascular disease. Curr. Opin. Lipidol 2008, 19, 257–262. [Google Scholar]
- Dasgupta, S.; Bhattacharyya, D.K. Dietary effect of eicosapentaenoic acid (EPA) containing soyphospholipid. J. Oleo Sci 2007, 56, 563–568. [Google Scholar]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr 2003, 133, 1302–1307. [Google Scholar]
- Deiana, S.; Platt, B.; Riedel, G. The cholinergic system and spatial learning. Behav. Brain Res 2011, 221, 389–411. [Google Scholar]
- McDaniel, M.A.; Maier, S.F.; Einstein, G.O. “Brain-specific” nutrients: A memory cure?”. Nutrition 2003, 19, 957–975. [Google Scholar]
- Da Costa, K.A.; Cochary, E.F.; Blusztajn, J.K.; Garner, S.C.; Zeisel, S.H. Accumulation of 1,2-sn-diradylglycerol with increased membrane-associated protein kinase C may be the mechanism for spontaneous hepatocarcinogenesis in choline-deficient rats. J. Biol. Chem 1993, 268, 2100–2105. [Google Scholar]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J 1991, 5, 2093–2098. [Google Scholar]
- Zeisel, S.H. Choline: Needed for normal development of memory. J. Am. Coll. Nutr 2000, 19, 528S–531S. [Google Scholar]
- Meck, W.H.; Williams, C.L. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport 1997, 8, 3053–3059. [Google Scholar]
- Meck, W.H.; Williams, C.L. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport 1997, 8, 3045–3051. [Google Scholar]
- Meck, W.H.; Williams, C.L. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 1997, 8, 2831–2835. [Google Scholar]
- Tees, R.C. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav. Brain Res 1999, 105, 173–188. [Google Scholar]
- Williams, C.L.; Meck, W.H.; Heyer, D.D.; Loy, R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res 1998, 794, 225–238. [Google Scholar]
- Albright, C.D.; Tsai, A.Y.; Friedrich, C.B.; Mar, M.H.; Zeisel, S.H. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. Dev. Brain Res 1999, 113, 13–20. [Google Scholar]
- Albright, C.D.; Zeisel, S.H. Choline deficiency causes increased localization of transforming growth factor-beta1 signaling proteins and apoptosis in the rat liver. Pathobiology 1997, 65, 264–270. [Google Scholar]
- Holmes-McNary, M.Q.; Loy, R.; Mar, M.H.; Albright, C.D.; Zeisel, S.H. Apoptosis is induced by choline deficiency in fetal brain and in PC12 cells. Brain Res. Dev. Brain Res 1997, 101, 9–16. [Google Scholar]
- Xu, X.; Gammon, M.D.; Zeisel, S.H.; Bradshaw, P.T.; Wetmur, J.G.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M.; Chen, J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J 2009, 23, 4022–4028. [Google Scholar]
- Xu, X.; Gammon, M.D.; Zeisel, S.H.; Lee, Y.L.; Wetmur, J.G.; Teitelbaum, S.L.; Bradshaw, P.T.; Neugut, A.I.; Santella, R.M.; Chen, J. Choline metabolism and risk of breast cancer in a population-based study. FASEB J 2008, 22, 2045–2052. [Google Scholar]
- Lee, J.E.; Giovannucci, E.; Fuchs, C.S.; Willett, W.C.; Zeisel, S.H.; Cho, E. Choline and betaine intake and the risk of colorectal cancer in men. Cancer Epidemiol. Biomarkers Prev 2010, 19, 884–887. [Google Scholar]
- Jump, D.B.; Botolin, D.; Wang, Y.; Xu, J.; Christian, B.; Demeure, O. Fatty acid regulation of hepatic gene transcription. J. Nutr 2005, 135, 2503–2506. [Google Scholar]
- Arita, M.; Yoshida, M.; Hong, S.; Tjonahen, E.; Glickman, J.N.; Petasis, N.A.; Blumberg, R.S.; Serhan, C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2005, 102, 7671–7676. [Google Scholar]
- Davidson, M.H. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am. J. Cardiol 2006, 98, 27i–33i. [Google Scholar]
- Hoffman, D.R.; Boettcher, J.A.; Diersen-Schade, D.A. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: A review of randomized controlled trials. Prostaglandins Leukotrienes Essent. Fatty Acids 2009, 81, 151–158. [Google Scholar]
- Iso, H.; Kobayashi, M.; Ishihara, J.; Sasaki, S.; Okada, K.; Kita, Y.; Kokubo, Y.; Tsugane, S. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) study cohort I. Circulation 2006, 113, 195–202. [Google Scholar]
- Serini, S.; Piccioni, E.; Calviello, G. Dietary n-3 PUFA vascular targeting and the prevention of tumor growth and age-related macular degeneration. Curr. Med. Chem 2009, 16, 4511–4526. [Google Scholar]
- Von Schacky, C.; Angerer, P.; Kothny, W.; Theisen, K.; Mudra, H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med 1999, 130, 554–562. [Google Scholar]
- Weitz, D.; Weintraub, H.; Fisher, E.; Schwartzbard, A.Z. Fish oil for the treatment of cardiovascular disease. Cardiol. Rev 2010, 18, 258–263. [Google Scholar]
- Wu, M.; Harvey, K.A.; Ruzmetov, N.; Welch, Z.R.; Sech, L.; Jackson, K.; Stillwell, W.; Zaloga, G.P.; Siddiqui, R.A. Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int. J. Cancer 2005, 117, 340–348. [Google Scholar]
- Yurko-Mauro, K. Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr. Alzheimer Res 2010, 7, 190–196. [Google Scholar]
- Horrobin, D.F. Interactions between n-3 and n-6 essential fatty acids (EFAs) in the regulation of cardiovascular disorders and inflammation. Prostaglandins Leukotrienes Essent. Fatty Acids 1991, 44, 127–131. [Google Scholar]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol 2003, 60, 940–946. [Google Scholar]
- Richardson, A.J.; Puri, B.K. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 233–239. [Google Scholar]
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F.B.; et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 2012, 345, e6698. [Google Scholar]
- Hooper, L.; Thompson, R.L.; Harrison, R.A.; Summerbell, C.D.; Ness, A.R.; Moore, H.J.; Worthington, H.V.; Durrington, P.N.; Higgins, J.P.; Capps, N.E.; et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ 2006, 332, 752–760. [Google Scholar]
- Marik, P.E.; Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clin. Cardiol 2009, 32, 365–372. [Google Scholar]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar]
- Wang, C.; Harris, W.S.; Chung, M.; Lichtenstein, A.H.; Balk, E.M.; Kupelnick, B.; Jordan, H.S.; Lau, J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am. J. Clin. Nutr 2006, 84, 5–17. [Google Scholar]
- Ligresti, A.; Petrosino, S.; Di Marzo, V. From endocannabinoid profiling to “endocannabinoid therapeutics”. Curr. Opin. Chem. Biol 2009, 13, 321–331. [Google Scholar]
- Taylor, L.A.; Pletschen, L.; Arends, J.; Unger, C.; Massing, U. Marine phospholipids—A promising new dietary approach to tumor-associated weight loss. Support. Care Cancer 2010, 18, 159–170. [Google Scholar]
| Area of study | Population characteristics | Treatment | Main findings | References |
|---|---|---|---|---|
| Cardiovascular | Dyslipidemia | KO | Improved blood lipids | [56] |
| Obesity | Normal to obese | KO | Changed endocannabinoid levels | [57] |
| Inflammation | Arthritis | KO | Reduced arthritic symptoms | [58] |
| Athletes | KO | Reduced oxidative damage | [59] | |
| PMS | Women | KO | Reduced dysmenorrhea | [60] |
| Brain | Memory complains | n-3 PS 1 | Improved word recall | [61] |
| Eye | ADHD children | n-3 PLs 2 | Improved attention | [62] |
| Liver | Chronic liver disease | Roe 3 | Improved lipid parameters | [10] |
| Bioavailability | Healthy | KO | Increased n-3 FA blood levels | [22,63,64] |
| Area of study | Animal model | Treatment | Main findings | References |
|---|---|---|---|---|
| Cardiovascular | Heart failure (r) | KO | Attenuated heart remodeling | [65] |
| Healthy (m) | Roe 1 | Improved blood lipids and adiponectin | [8,66] | |
| Obesity | High fat diet (m) | n-3 PLs 2 | Improved metabolic profile | [67] |
| High-fat diet (r) | KO | Decreased body weight | [68] | |
| High-fat diet (m) | KO | Reduced endocannabinoid biosynthesis | [69] | |
| High-fat diet (m) | KO | Decreased hepatic steatosis | [70] | |
| Genetic obesity (r) | KO | Decreased hepatic and heart lipids | [44] | |
| High-fat diet (m) | Roe 3 | Reduced abdominal fat | [71] | |
| Inflammation | TNFα overexpression (m) | Roe 3, KO | Increased hepatic β-oxidation | [72–74] |
| Ulcerative colitis (r) | KO | Reduced oxidative stress | [75] | |
| Arthritis (m) | KO | Reduced arthritis scores | ||
| Brain | Healthy (r) | n-3 PLs 4 | Improved memory function | [46] |
| Genetic obesity (r) | KO | Increased DHA level in brain | [45] | |
| Healthy (r) | n-3 PS 5 | Improved learning and memory | [76] | |
| Healthy (m) | Roe 1 | Improved learning capacity | [9] | |
| Bone | Growing females (r) | KO | Did not improve bone mass/architecture | [77] |
| Other | Healthy (r) | KO | Decreased hepatic lipogenesis | [78] |
| Healthy (m) | KO | Beneficial hepatic gene regulation | [79] | |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. Int. J. Mol. Sci. 2012, 13, 15401-15419. https://doi.org/10.3390/ijms131115401
Burri L, Hoem N, Banni S, Berge K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. International Journal of Molecular Sciences. 2012; 13(11):15401-15419. https://doi.org/10.3390/ijms131115401
Chicago/Turabian StyleBurri, Lena, Nils Hoem, Sebastiano Banni, and Kjetil Berge. 2012. "Marine Omega-3 Phospholipids: Metabolism and Biological Activities" International Journal of Molecular Sciences 13, no. 11: 15401-15419. https://doi.org/10.3390/ijms131115401
APA StyleBurri, L., Hoem, N., Banni, S., & Berge, K. (2012). Marine Omega-3 Phospholipids: Metabolism and Biological Activities. International Journal of Molecular Sciences, 13(11), 15401-15419. https://doi.org/10.3390/ijms131115401





