Effects of White Rice, Brown Rice and Germinated Brown Rice on Antioxidant Status of Type 2 Diabetic Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gamma-Aminobutyric Acid (GABA) and Total Phenolic Contents, and Antioxidant Potentials
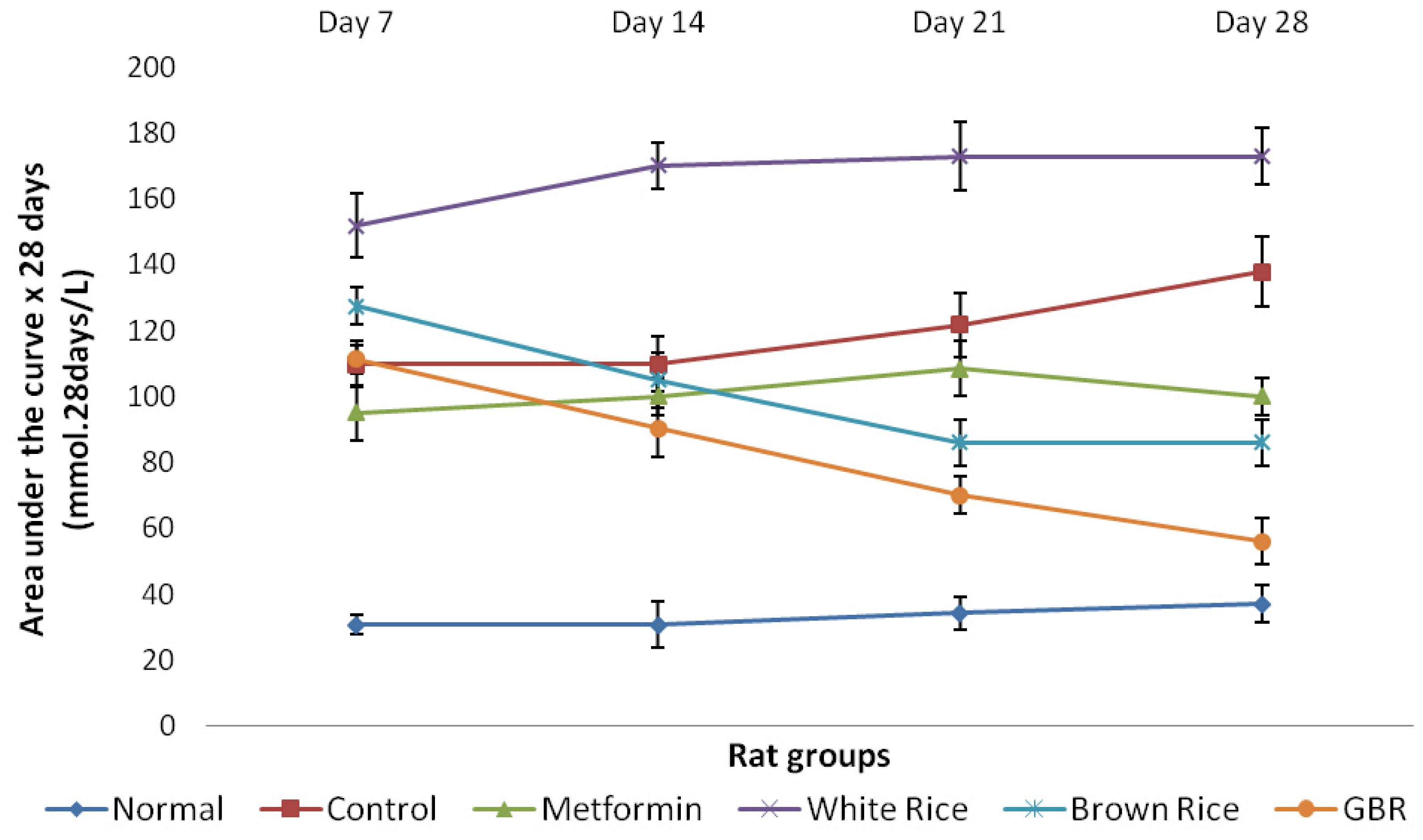
2.2. Food Consumption and Glucose Analysis
2.3. Liver Enzymes, Urea, and Creatinine
2.4. Plasma Total Antioxidant Status
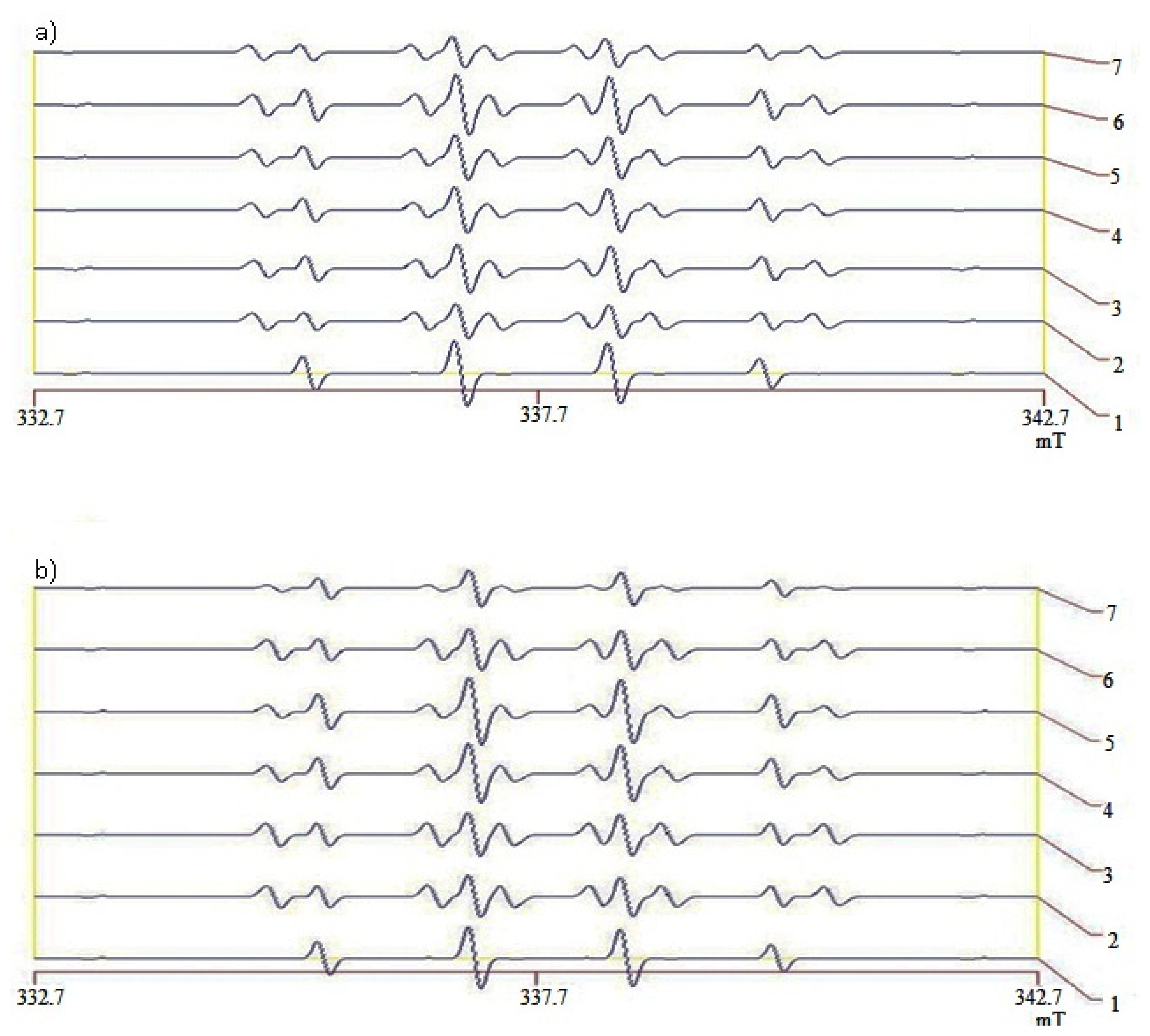
2.5. Hydroxyl Radical Scavenging Capacity of Liver and Kidneys
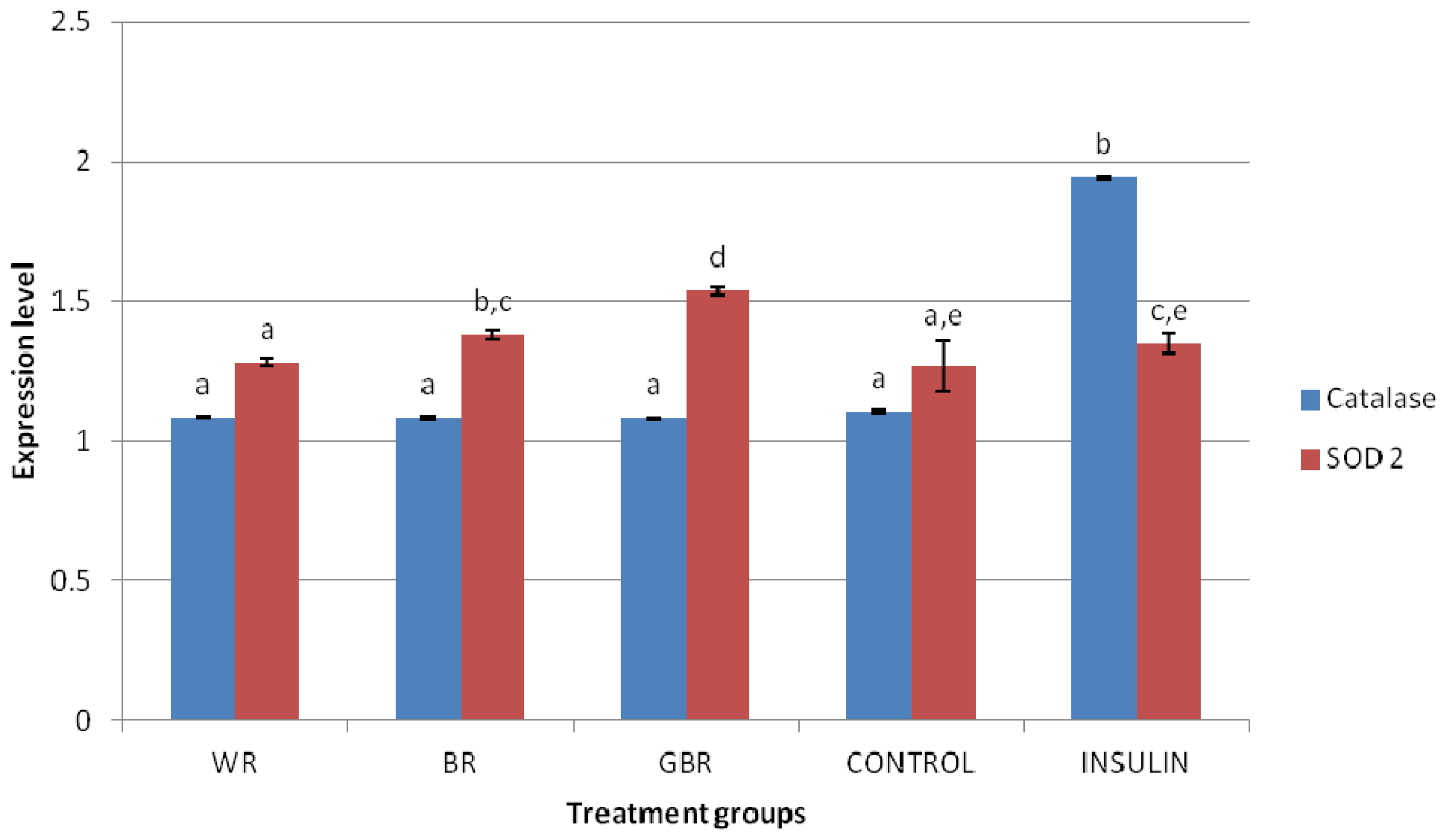
2.6. HEPG2 Antioxidant mRNA Expression
3. Experimental Section
3.1. Chemicals
3.2. Germination of Brown Rice
3.3. Gamma-Aminobutyric Acid and Total Phenolic Contents, and Antioxidant Properties
3.4. Animal Handling, Feeding and Induction of Diabetes
3.5. Plasma Total Antioxidant Status, Glucose, and Liver- and Kidney-Function Tests
3.6. Electron Spin Resonance (ESR) Spectroscopy
3.7. Cell Culture
3.8. Hepatic Antioxidant mRNA Expression Analysis
3.8.1. RNA Isolation
3.8.2. Primer Design
3.8.3. Reverse Transcription and Polymerase Chain Reaction (PCR)
3.8.4. GeXP Multiplex Data Analysis
4. Conclusions
Acknowledgments
References
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar]
- Chang, Y.C.; Chuang, L.M. The role of oxidative stress in the pathogenesis of type 2 diabetes: From molecular mechanism to clinical implication. Am. J. Transl. Res 2010, 2, 316–331. [Google Scholar]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996, 19, 257–267. [Google Scholar]
- World Health Organization. Diabetes Fact sheet. World Health Organization. 2011. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/index.html accessed on 21 May 2012.
- Rebolledo, O.R.; Actis Dato, S.M. Postprandial hyperglycemia and hyperlipidemia-generated glycoxidative stress: Its contribution to the pathogenesis of diabetes complications. Eur. Rev. Med. Pharmacol. Sci 2005, 9, 191–208. [Google Scholar]
- Ceriello, A.; Bortolotti, N.; Motz, E.; Crescentini, A.; Lizzio, S.; Russo, A.; Tonutti, L.; Taboga, C. Meal-generated oxidative stress in type 2 diabetic patients. Diabetes Care 1998, 21, 1529–1533. [Google Scholar]
- Miller, J.B.; Pang, E.; Bramall, L. Rice: A high or low glycemic index food? Am. J. Clin. Nutr 1992, 56, 1034–1036. [Google Scholar]
- Sun, Q.; Spiegelman, D.; van Dam, R.M.; Holmes, M.D.; Malik, V.S.; Willett, W.C.; Hu, F.B. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch. Intern. Med 2010, 170, 961–969. [Google Scholar]
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol 2005, 59, 1–6. [Google Scholar]
- Panlasigui, L.N.; Thompson, L.U. Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int. J. Food Sci. Nutr 2006, 57, 151–158. [Google Scholar]
- Patil, S.B.; Khan, M.K. Germinated brown rice as a value added rice product: A review. J. Food Sci. Technol 2011, 48, 661–667. [Google Scholar]
- Ito, Y.; Mizukuchi, A.; Kise, M.; Aoto, H.; Yamamoto, S.; Yoshihara, R.; Yokoyama, J. Postprandial blood glucose and insulin responses to pre-germinated brown rice in healthy subjects. J. Med. Invest 2005, 52, 159–164. [Google Scholar]
- Hsu, T.F.; Kise, M.; Wang, M.F.; Ito, Y.; Yang, M.D.; Aoto, H.; Yoshihara, R.; Yokoyama, J.; Kunii, D.; Yamamoto, S. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J. Nutr. Sci. Vitaminol 2008, 54, 163–168. [Google Scholar]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Yusof, R.M.; Hussin, A.S.M.; Hamid, A.; Abd Manap, M.Y. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J. Sci. Food Agric 2010, 90, 245–251. [Google Scholar]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem 2004, 52, 4808–4813. [Google Scholar]
- Kim, D.J.; Oh, S.K.; Yoon, M.R.; Chun, A.; Choi, I.S.; Lee, D.H.; Lee, J.S.; Yu, K.W.; Kim, Y.K. The change in biological activities of brown rice and germinated brown rice. J. Korean Soc. Food Sci. Nutr 2011, 40, 781–789. [Google Scholar]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Hussin, A.S.M.; Hamid, A.; Abd Manap, M.Y. Effect of pre-germination time on amino acid profile and gamma amino butyric acid (GABA) contents in different varieties of Malaysian brown rice. Int. J. Food Prop 2011, 14, 1386–1399. [Google Scholar]
- Charoenthaikij, P.; Jangchud, K.; Jangchud, A.; Prinyawiwatkul, W.; Tungtrakul, P. Germination conditions affect selected quality of composite wheat-germinated brown rice flour and bread formulations. J. Food Sci 2010, 75, S312–S318. [Google Scholar]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci 2002, 3, 715–727. [Google Scholar]
- Nakagawa, T.; Yokozawa, T.; Kim, H.J.; Shibahara, N. Protective effects of gamma-aminobutyric acid in rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol 2005, 51, 278–282. [Google Scholar]
- Burton-Freeman, B. Postprandial metabolic events and fruit-derived phenolics: A review of the science. Br. J. Nutr 2010, 104, S1–S14. [Google Scholar]
- Stumvoll, M.; Goldstein, B.J.; Haeften, T.W.V. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar]
- DeFronzo, R.A.; Goodman, A.M. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The multicenter metformin study group. N. Engl. J. Med 1995, 333, 541–549. [Google Scholar]
- Michalopoulos, G.K.; DeFrances, M.C. Liver regeneration. Science 1997, 276, 60–66. [Google Scholar]
- Thomas, D.M.; Coles, G.A.; Williams, J.D. What does the renal reserve mean? Kidney Int 1994, 45, 411–416. [Google Scholar]
- Usuki, S.; Ito, Y.; Morikawa, K.; Kise, M.; Ariga, T.; Rivner, M.; Yu, R.K. Effect of pre-germinated brown rice intake on diabetic neuropathy in streptozotocin-induced diabetic rats. Nutr. Metab 2007, 4, 25. [Google Scholar]
- Mattson, M.P. Dietary factors, hormesis and health. Ageing Res. Rev 2008, 7, 43–48. [Google Scholar]
- Ismail, M.; Al-Naqeep, G.; Chan, K.W. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic. Biol. Med 2010, 48, 664–672. [Google Scholar]
- Halliwell, B. Free radicals, antioxidants, and human disease curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar]
- Kaput, J.; Rodriguez, R.L. Nutritional genomics: The next frontier in the postgenomic era. Physiol. Genomics 2004, 16, 166–177. [Google Scholar]
- Rozan, P.; Kuo, Y.H.; Lambein, F. Free amino acids present in commercially available seedlings sold for human consumption: A potential hazard for consumers. J. Agric. Food Chem 2000, 48, 716–723. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 2005, 91, 571–577. [Google Scholar]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.S.; Cho, M.; Choi, H.K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem 2010, 121, 429–436. [Google Scholar]
- Imam, M.U.; Ismail, M. Effects of brown rice and white rice on expression of xenobiotic metabolism genes in type 2 diabetic rats. Int. J. Mol. Sci 2012, 13, 8597–8608. [Google Scholar]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res 2005, 52, 313–320. [Google Scholar]
- Katakam, P.V.G.; Ujhelyi, M.R.; Hoenig, M.; Miller, A.W. Metformin improves vascular function in insulin-resistant rats. Hypertension 2000, 35, 108–112. [Google Scholar]
- Tai, M.M. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care 1994, 17, 152–154. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- National Center for Biotechnology. Information GenBank Database. Available online: http://www.ncbi.nlm.nih.gov/nucleotide/ accessed on 2 March 2010.


| Extract (70% ethanolic) | TPC (mg GAE/g extract) # | ABTS scavenging activity (mg TEAC/g extract) # | DPPH free radical scavenging assay *,# |
|---|---|---|---|
| WR | 0.60 ± 0.45 a | 1.78 ± 0.44 a | 18.41 ± 0.46 a |
| BR | 3.17 ± 1.68 b | 2.67 ± 0.24 b | 6.82 ± 0.23 b |
| GBR | 12.01 ± 0.2 c | 6.31 ± 0.57 c | 5.27 ± 0.17 c |
| Parameter | Rat group | ||||||
|---|---|---|---|---|---|---|---|
| Normal | Control (untreated diabetic) | Metformin | WR | BR | GBR | ||
| Glucose (mmol/L) | Baseline | 4.6 ± 0.5 a | 14.9 ± 2.2 b | 14.7 ± 4.1 b | 19.1 ± 2 b | 18.4 ± 2.8 b | 17.3 ± 2.5 b |
| Food consumption (kcal/100 g body weight/day) * | Baseline | 30.5 ± 3.7 a | 34.0 ± 6.0 a | 30.7 ± 6.0 a | 33.2 ± 8.3 a | 30.5 ± 6.7 a | 33.2 ± 8.3 a |
| ALT (U/l) * | Baseline | 58.32 ± 1.03 a | 61.52 ± 2.17 a | 61.36 ± 2.47 a | 61.52 ± 6.05 a | 60.36 ± 4.09 a | 58.91 ± 1.34 a |
| Final | 54.82 ± 1.44 a | 62.44 ± 1.75 b | 68.11 ± 1.55 c | 67.76 ± 2.54 c | 71.83 ± 2.58 c | 54.20 ± 1.43 a | |
| AST (U/l) * | Baseline | 77.22 ± 3.29 a | 77.7 ± 2.51 a | 75.07 ± 2.35 a | 76.64 ± 1.75 a | 72.97 ± 3.49 a | 74.00 ± 3.38 a |
| Final | 77.37 ± 1.16 a | 84. 69 ± 2.54 b | 75.30 ± 2.26 a | 81.94 ± 1.23 b | 74.21± 2.23 a,c | 70.82 ± 2.13 c | |
| GGT (U/l) * | Baseline | 2.18 ± 0.43 a | 2.70 ± 0.03 b | 2.68 ± 0.08 b | 3.00 ± 0.38 b | 2.83 ± 0.20 b | 2.78 ± 0.06 b |
| Final | 1.92 ± 0.12 a | 2.94 ± 0.18 b | 2.64 ± 0.16 b | 3.24 ± 0.20 c | 2.69 ± 0.16 b | 2.61 ± 0.16 b | |
| Urea (mmol/L) | Baseline | 5.14 ± 0.04 a | 5.54 ± 0.45 a | 5.32 ± 0.17 a | 5.37 ± 0.27 a | 5.21 ± 0.43 a | 5.23 ± 0.21 a |
| Final | 5.31 ± 0.06 a | 8.42 ± 0.34 b | 7.82 ± 0.16 c | 9.77 ± 0.39 d | 8.18 ± 0.65 b,c | 9.20 ± 0.74 b,d | |
| Creatinine (μmol/L) | Baseline | 53.86 ± 0.84 a | 57. 78 ± 1.26 b | 58.55 ± 1.32 b | 60.83 ± 5.06 b | 58.25 ± 1.67 b | 58.32 ± 1.89 b |
| Final | 53.80 ± 1.20 a | 59.92 ± 0.36 b | 60.42 ± 0.41 b | 62.96 ± 0.78 c | 60.76 ± 0.86 b | 57.56 ± 0.45 d | |
| TAS (mmol/L) | Baseline | 2.12 ± 0.12 a | 2.22 ± 0.04 a | 2.18 ± 0.04 a | 2.14 ± 0.03 a | 2.23 ± 0.09 a | 2.09 ± 0.09 a |
| Final | 2.13 ± 0.04 a | 1.84 ± 0.02 b | 2.03 ± 0.02 c | 2.05 ± 0.02 c | 2.22 ± 0.01 d | 2.10 ± 0.02 a | |
| Rat Groups | Liver | Kidney | ||
|---|---|---|---|---|
| Scavenging activity (%) | DMSO equivalent | Scavenging activity (%) | DMSO equivalent | |
| Normal | 44.5 ± 4 a | 79.8 ± 1.2 a | 39.0 ± 2 a | 68.8 ± 5.2 a |
| Control | 21.5 ± 3 b | 33.8 ± 3.2 b | 26.0 ± 3 b | 42.8 ± 3.2 b |
| Metformin | 22.0 ± 6 b | 34.8 ± 2.8 b | 13.0 ± 3 c | 16.8 ± 3.2 c |
| White rice | 20.5 ± 3 b | 31.8 ± 3.2 b | 0 d | 0 d |
| Brown rice | 13.0 ± 2 c | 16.8 ± 5.2 c | 39.0 ± 5 a | 68.8 ± 0.8 a |
| GBR | 22.0 ± 2 b | 34.8 ± 5.2 b | 39.0 ± 3 a | 68.8 ± 3.2 a |
| Gene name (Accession number) * | Primer sequences * (with universal tag) | |
|---|---|---|
| Forward | Reverse | |
| Catalase [NM_001752] | AGGTGACACTATAGAATAGCTCAGCTGACACAGTTCGT | GTACGACTCACTATAGGGACCATTCGCATTAACCAGCTT |
| Superoxide dismutase 2 [NM_000636] | AGGTGACACTATAGAATACAAGCGTGACTTTGGGTCTT | GTACGACTCACTATAGGGAGGGCTTCACTTCTTGCAAAC |
| Actb [NM_001101] a | AGGTGACACTATAGAATAGATCATTGCTCCTCCTGAGC | GTACGACTCACTATAGGGAAAAGCCATGCCAATCTCATC |
| GAPDH [NM_002046] a,# | AGGTGACACTATAGAATAAAGGTGAAGGTCGGAGTCAA | GTACGACTCACTATAGGGAGATCTCGCTCCTGGAAGATG |
| EEF1A1 [NM_001402] a | AGGTGACACTATAGAATACACACGGCTCACATTGCAT | GTACGACTCACTATAGGGACACGAACAGCAAAGCGA |
| Kanr b | ||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Imam, M.U.; Musa, S.N.A.; Azmi, N.H.; Ismail, M. Effects of White Rice, Brown Rice and Germinated Brown Rice on Antioxidant Status of Type 2 Diabetic Rats. Int. J. Mol. Sci. 2012, 13, 12952-12969. https://doi.org/10.3390/ijms131012952
Imam MU, Musa SNA, Azmi NH, Ismail M. Effects of White Rice, Brown Rice and Germinated Brown Rice on Antioxidant Status of Type 2 Diabetic Rats. International Journal of Molecular Sciences. 2012; 13(10):12952-12969. https://doi.org/10.3390/ijms131012952
Chicago/Turabian StyleImam, Mustapha Umar, Siti Nor Asma Musa, Nur Hanisah Azmi, and Maznah Ismail. 2012. "Effects of White Rice, Brown Rice and Germinated Brown Rice on Antioxidant Status of Type 2 Diabetic Rats" International Journal of Molecular Sciences 13, no. 10: 12952-12969. https://doi.org/10.3390/ijms131012952
APA StyleImam, M. U., Musa, S. N. A., Azmi, N. H., & Ismail, M. (2012). Effects of White Rice, Brown Rice and Germinated Brown Rice on Antioxidant Status of Type 2 Diabetic Rats. International Journal of Molecular Sciences, 13(10), 12952-12969. https://doi.org/10.3390/ijms131012952






