Anticoagulant, Antioxidant and Antitumor Activities of Heterofucans from the Seaweed Dictyopteris delicatula
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
2.2. Anticoagulant Activity by APTT and PT Assays
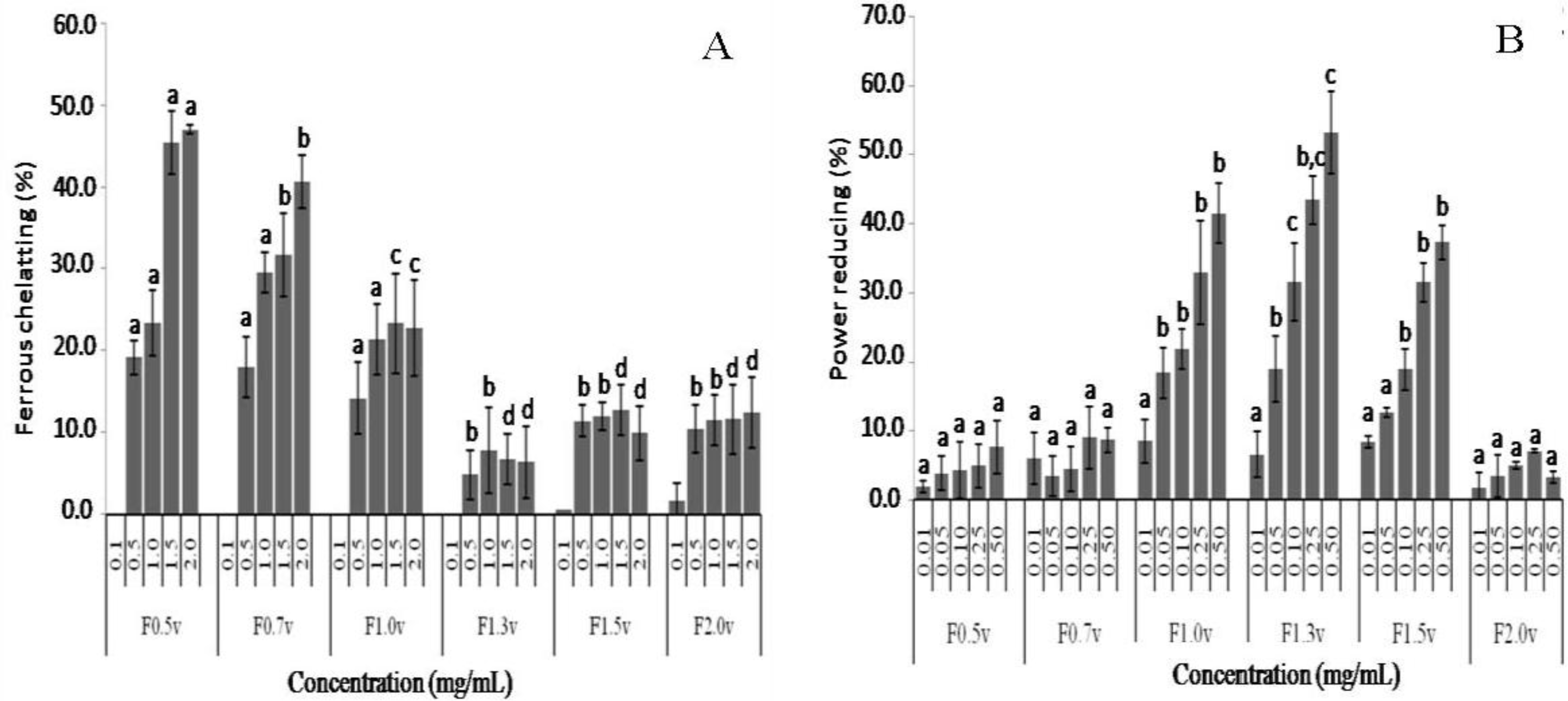
2.3. Antioxidant Activity
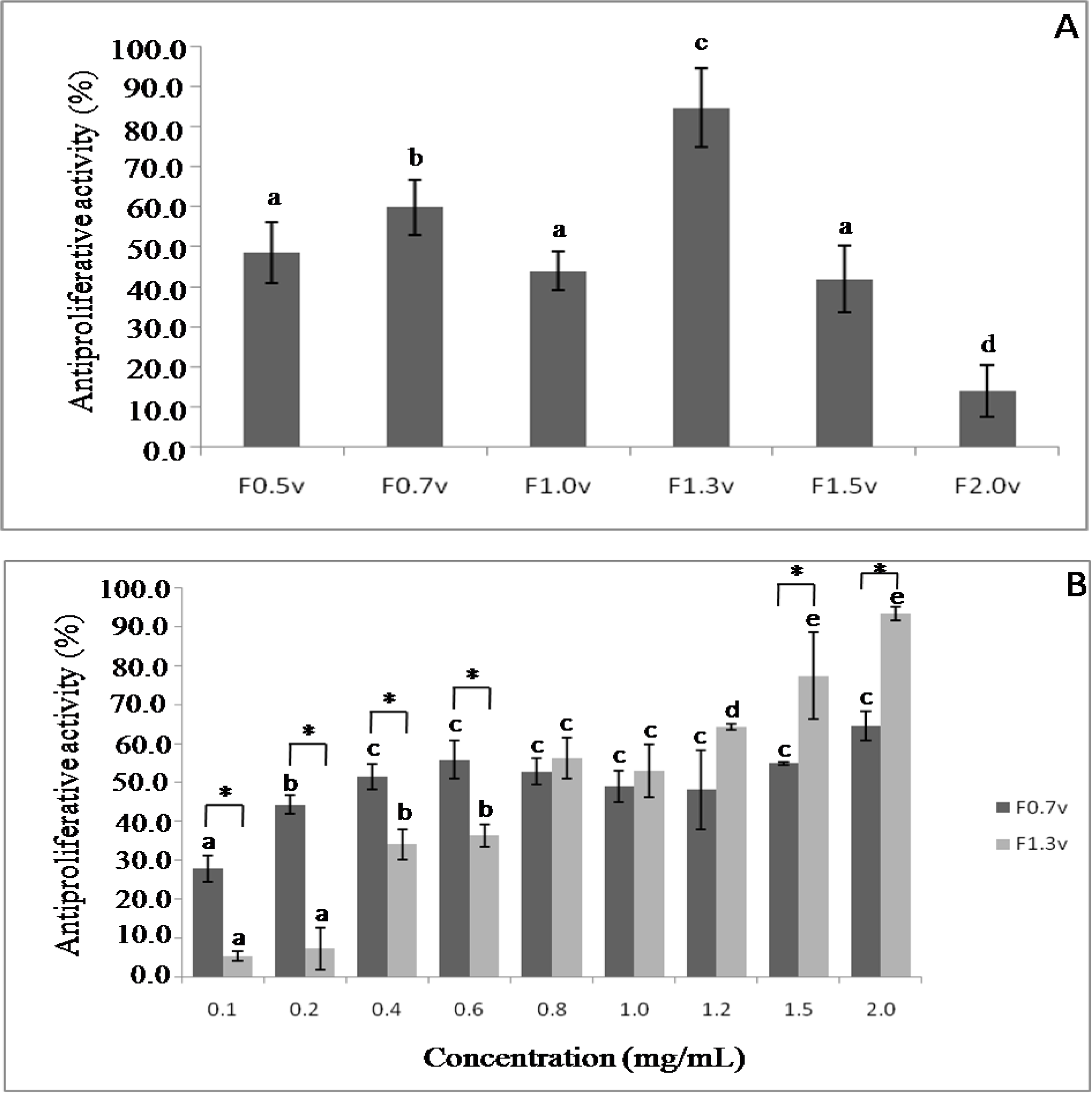
2.4. Antiproliferative Activity
3. Experimental Section
3.1. Materials
3.2. Extraction of Polysaccharides
3.3. Chemical Analysis and Monosaccharide Composition
3.4. Anticoagulant Activity
3.5. Antioxidant Activity
3.5.1. Hydroxyl Radical Scavenging Activity Assay
3.5.2. Superoxide Radical Scavenging Activity Assay
3.5.3. Ferric Chelating
3.6. Cell Proliferation Studies
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Barroso, EMA; Costa, LS; Medeiros, VP; Cordeiro, SL; Costa, MSSP; Franco, CRC; Nader, HB; Leite, EL. A non-anticoagulant heterofucan has antithrombotic activity in vivo. Planta Med 2008, 74, 712–718. [Google Scholar]
- Rocha, HAO; Franco, CRC; Trindade, ES; Carvalho, LCM; Veiga, SS; Leite, EL; Dietrich, CP; Nader, HB. A fucan from the brown seaweed Spatoglossum schröederi inhibits Chinese hamster ovary cell adhesion to several extracellular matrix proteins. Braz. J. Med. Biol. Res 2001, 34, 621–626. [Google Scholar]
- Hyun, JH; Kim, SC; Kang, JI; Kim, MK; Boo, HJ; Kwon, JM; Koh, YS; Hyun, JW; Park, DB; Yoo, ES; Kang, HK. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull 2009, 32, 1760–1764. [Google Scholar]
- Ohta, Y; Lee, JB; Hayashi, H; Hayashi, T. Isolation of galactan from Codium fragile and its antiviral effects. Biol. Pharm. Bull 2009, 32, 892–898. [Google Scholar]
- Bilan, MI; Usov, AI. Structural analysis of fucoidans. Nat. Prod. Commun 2008, 3, 1639–1648. [Google Scholar]
- Jiao, G; Yu, G; Zhang, J; Ewart, HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar]
- Michel, G; Nyval-Collen, P; Barbeyron, T; Czjzek, M; Helbert, W. Bioconversion of red seaweed galactans: a focus on bacterial agarases and carrageenases. Appl. Microbiol. Biotechnol 2006, 71, 23–33. [Google Scholar]
- Cunha, PLR; Paula, RCM; Feitosa, JPA. Polysaccharides from brazilian biodiversity: An opportunity to charge knowledge into economic value. Quim. Nova 2009, 32, 649–660. [Google Scholar]
- Costa, LS; Fidelis, GP; Cordeiro, SL; Oliveira, RM; Sabry, DA; Câmara, RBG; Nobre, LTDB; Costa, MSSP; Almeida-Lima, J; Farias, EHC; Leite, EL; Rocha, HAO. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother 2010, 64, 21–28. [Google Scholar]
- Leite, EL; Medeiros, MGL; Rocha, HAO; Farias, GGM; Silva, LF; Chavante, SF; Abreu, LD; Dietrich, CP; Nader, HB. Structure and pharmacological activities of a sulfated xylofucoglucuronan from the alga Spatoglossum schröederi. Plant Sci 1998, 132, 215–228. [Google Scholar]
- Dietrich, CP; Farias, GGM; Abreu, LRD; Leite, EL; Silva, LF; Nader, HB. A new approach for characterization of polysaccharides from algae: Presence of four main acidic polysaccharides in three specie of the class Phaeophycea. Plant Sci 1995, 108, 143–153. [Google Scholar]
- Albuquerque, IRL; Queiroz, KCS; Alves, LG; Santos, EA; Leite, EL; Rocha, HAO. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res 2004, 37, 167–171. [Google Scholar]
- Silva, TMA; Alves, LG; Queiroz, KCS; Santos, MGL; Marques, CT; Chavante, SF; Rocha, HAO; Leite, EL. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz. J. Med. Biol. Res 2005, 38, 523–533. [Google Scholar]
- Ponce, NMA; Pujol, CA; Damonte, EB. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res 2003, 338, 153–165. [Google Scholar]
- Bilan, MI; Zakharova, AN; Grachev, AA; Shashkov, AS; Nifant’ev, NE; Usov, AI. Polysaccharides of algae: 60. Fucoidan from the Pacific brown alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Russ. J. Bioorg. Chem 2007, 33, 44–53. [Google Scholar]
- Chevolot, L; Mulloy, B; Ratiskol, J; Foucault, A; Colliec-Jouault, S. A disaccharide repeat unit is the structure in fucoidans from two species of brown algae. Carbohydr. Res 2001, 330, 529–535. [Google Scholar]
- Marais, MF; Joseleau, JP. A fucoidan fraction from Ascophyllum nodosum. Carbohydr. Res 2001, 336, 155–159. [Google Scholar]
- Hussein, MM; Abdel, A; Salem, HM. Sulfated heteropolysaccharides from Padina pavoia. Phytochemistry 1980, 19, 2131–2132. [Google Scholar]
- Duarte, MER; Cardoso, MA; Noseda, MD; Cerezo, AS. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Polym 2001, 333, 281–293. [Google Scholar]
- Kloareg, B; Quatrano, RS. Structure of cell wall of marine algae and ecophysiological function of matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev 1988, 26, 259–315. [Google Scholar]
- Nishino, T; Nishioka, C; Ura, H; Nagumo, T. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus sulfated fucan. Carbohydr. Res 1994, 255, 213–224. [Google Scholar]
- Li, B; Lu, F; Wei, X; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar]
- Cuzzocrea, S; Riley, DP; Caputi, AP; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation and ischemia/reperfusion injury. Pharmacol. Rev 2001, 53, 135–159. [Google Scholar]
- Kaeffer, B; Benard, C; Lahaye, M; Blottiere, HM; Cherbut, C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial cells. Planta Med 1999, 65, 527–531. [Google Scholar]
- Zhang, Z; Zhang, Q; Wang, J; Song, H; Zhang, H; Niu, X. Chemical modification and influence of function groups on the in vitro-antioxidant activities of porphyran from Porphyra haitanensi. Carbohydr. Polym 2010, 79, 290–295. [Google Scholar]
- Souza, MCR; Marques, CT; Dore, CMG; Silva, FRF; Rocha, HAO; Leite, EL. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Psychol 2007, 19, 153–160. [Google Scholar]
- Wang, J; Zhang, Q; Zhang, Z; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol 2008, 42, 127–132. [Google Scholar]
- Holtkamp, AD; Kelly, S; Ulber, R; Lang, S. Fucoidans and fucoidanases—Focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol 2009, 82, 1–11. [Google Scholar]
- Ye, J; Li, Y; Teruya, K; Katakura, Y; Ichikawa, A; Eto, H; Hosoi, M; Hosoi, M; Nishimoto, S; Shirahata, S. Enzyme-digested fucoidan extracts derived from seaweed Mozuku of Cladosiphon novae-caledoniae Kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology 2005, 47, 117–126. [Google Scholar]
- Rocha, HAO; Moraes, FA; Trindade, ES; Franco, CRC; Torquato, RJS; Veiga, SS; Valente, AP; Mourão, PAS; Leite, EL; Nader, HB; Dietrich, CP. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schroederi—An ideal antithrombotic agent? J. Biol. Chem 2005, 280, 41278–41288. [Google Scholar]
- Dubois, M; Gilles, KA; Hamilton, JK; Rebers, PA; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem 1956, 28, 250–256. [Google Scholar]
- Farndale, RW; Buttle, DJ; Barrett, AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar]
- Dodgson, KS; Price, RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J 1962, 84, 106–110. [Google Scholar]
- Spector, J. Refinement of the coomassie blue method of protein qualification. A simple and linear spectrofotometric assay of 0.5 to 50 μg of protein. Anal. Biochem 1978, 86, 142–146. [Google Scholar]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem 1952, 195, 19–23. [Google Scholar]

| Fucans | Total sugar (%) | Sulfate (%) | Sulfate/Total sugar (%/%) | Proteins (%) | Molar ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fuc | Gal | Glc | Man | Xyl | Gluc A | |||||
| F0.5v | 89.1 | 14.1 | 0.16 | 0.1 | 1.0 | 2.0 | 0.3 | 0.5 | 0.7 | 0.9 |
| F0.7v | 70.0 | 17.8 | 0.25 | 0.3 | 1.0 | 3.0 | 0.4 | 0.6 | 1.5 | 2.2 |
| F1.0v | 65.0 | 19.0 | 0.29 | 0.5 | 1.0 | 1.9 | 0.2 | 0.5 | 0.8 | 1.5 |
| F1.3v | 68.2 | 14.5 | 0.21 | 0.1 | 1.0 | 2.4 | 0.3 | 0.2 | 0.6 | 0.5 |
| F1.5v | 61.3 | 15.4 | 0.25 | 0.1 | 1.0 | 1.6 | 0.2 | 0.4 | 1.3 | 1.9 |
| F2.0v | 64.3 | 16.0 | 0.25 | 0.7 | 1.0 | 1.8 | 0.2 | 0.4 | 1.6 | 2.2 |
| Sulfated polysaccharides | Concentration (mg/mL) | Inhibition (%) | |
|---|---|---|---|
| OH· | O2− | ||
| F0.5v | 0.05 | 6.1 ± 0.7 | 2.8 ± 2.1 |
| 0.1 | 9.6 ± 0.4 | 4.9 ± 3.1 | |
| 0.25 | 11.0 ± 0.9 | 5.1 ± 3.4 | |
| 0.5 | 14.4 ± 2.1 | 5.7 ± 4.0 | |
| F0.7v | 0.05 | 0.5 ± 0.7 | 3.9 ± 0.3 |
| 0.1 | 12.4 ± 3.5 | 6.1 ± 0.9 | |
| 0.25 | 13.4 ± 4.7 | 6.7 ± 3.1 | |
| 0.5 | 15.6 ± 3.8 | 4.8 ± 2.4 | |
| F1.0v | 0.05 | 6.6 ± 3.1 | 0 ± 0 |
| 0.1 | 12.7 ± 2.9 | 0 ± 0 | |
| 0.25 | 16.9 ± 2.2 | 0 ± 0 | |
| 0.5 | 17.0 ± 3.4 | 0 ± 0 | |
| F1.3v | 0.05 | 0 ± 0 | 0 ± 0 |
| 0.1 | 0 ± 0 | 0 ± 0 | |
| 0.25 | 0 ± 0 | 0 ± 0 | |
| 0.5 | 0 ± 0 | 0 ± 0 | |
| F1.5v | 0.05 | 0 ± 0 | 3.9 ± 2.1 |
| 0.1 | 0 ± 0 | 4.9 ± 3.1 | |
| 0.25 | 0 ± 0 | 9.6 ± 3.4 | |
| 0.5 | 0 ± 0 | 13.4 ± 2.1 | |
| F2.0v | 0.05 | 0 ± 0 | 0 ± 0 |
| 0.1 | 0 ± 0 | 0 ± 0 | |
| 0.25 | 0 ± 0 | 0 ± 0 | |
| 0.5 | 0 ± 0 | 0 ± 0 | |
| 0.1 | 43.6 ± 2.4 | 41.8 ± 4.7 | |
| 0.25 | 64.3 ± 3.0 | 72.1 ± 2.9 | |
| 0.5 | 93.7 ± 3.7 | 86.3 ± 3.1 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Magalhaes, K.D.; Costa, L.S.; Fidelis, G.P.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Albuquerque, I.R.L.; Cordeiro, S.L.; Sabry, D.A.; et al. Anticoagulant, Antioxidant and Antitumor Activities of Heterofucans from the Seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 2011, 12, 3352-3365. https://doi.org/10.3390/ijms12053352
Magalhaes KD, Costa LS, Fidelis GP, Oliveira RM, Nobre LTDB, Dantas-Santos N, Camara RBG, Albuquerque IRL, Cordeiro SL, Sabry DA, et al. Anticoagulant, Antioxidant and Antitumor Activities of Heterofucans from the Seaweed Dictyopteris delicatula. International Journal of Molecular Sciences. 2011; 12(5):3352-3365. https://doi.org/10.3390/ijms12053352
Chicago/Turabian StyleMagalhaes, Kaline Dantas, Leandro Silva Costa, Gabriel Pereira Fidelis, Ruth Medeiros Oliveira, Leonardo Thiago Duarte Barreto Nobre, Nednaldo Dantas-Santos, Rafael Barros Gomes Camara, Ivan Rui Lopes Albuquerque, Sara Lima Cordeiro, Diego Araujo Sabry, and et al. 2011. "Anticoagulant, Antioxidant and Antitumor Activities of Heterofucans from the Seaweed Dictyopteris delicatula" International Journal of Molecular Sciences 12, no. 5: 3352-3365. https://doi.org/10.3390/ijms12053352
APA StyleMagalhaes, K. D., Costa, L. S., Fidelis, G. P., Oliveira, R. M., Nobre, L. T. D. B., Dantas-Santos, N., Camara, R. B. G., Albuquerque, I. R. L., Cordeiro, S. L., Sabry, D. A., Costa, M. S. S. P., Alves, L. G., & Rocha, H. A. O. (2011). Anticoagulant, Antioxidant and Antitumor Activities of Heterofucans from the Seaweed Dictyopteris delicatula. International Journal of Molecular Sciences, 12(5), 3352-3365. https://doi.org/10.3390/ijms12053352






