Lycium barbarum Polysaccharides Attenuate Cisplatin-Induced Hair Cell Loss in Rat Cochlear Organotypic Cultures
Abstract
:1. Introduction
2. Results and Discussion
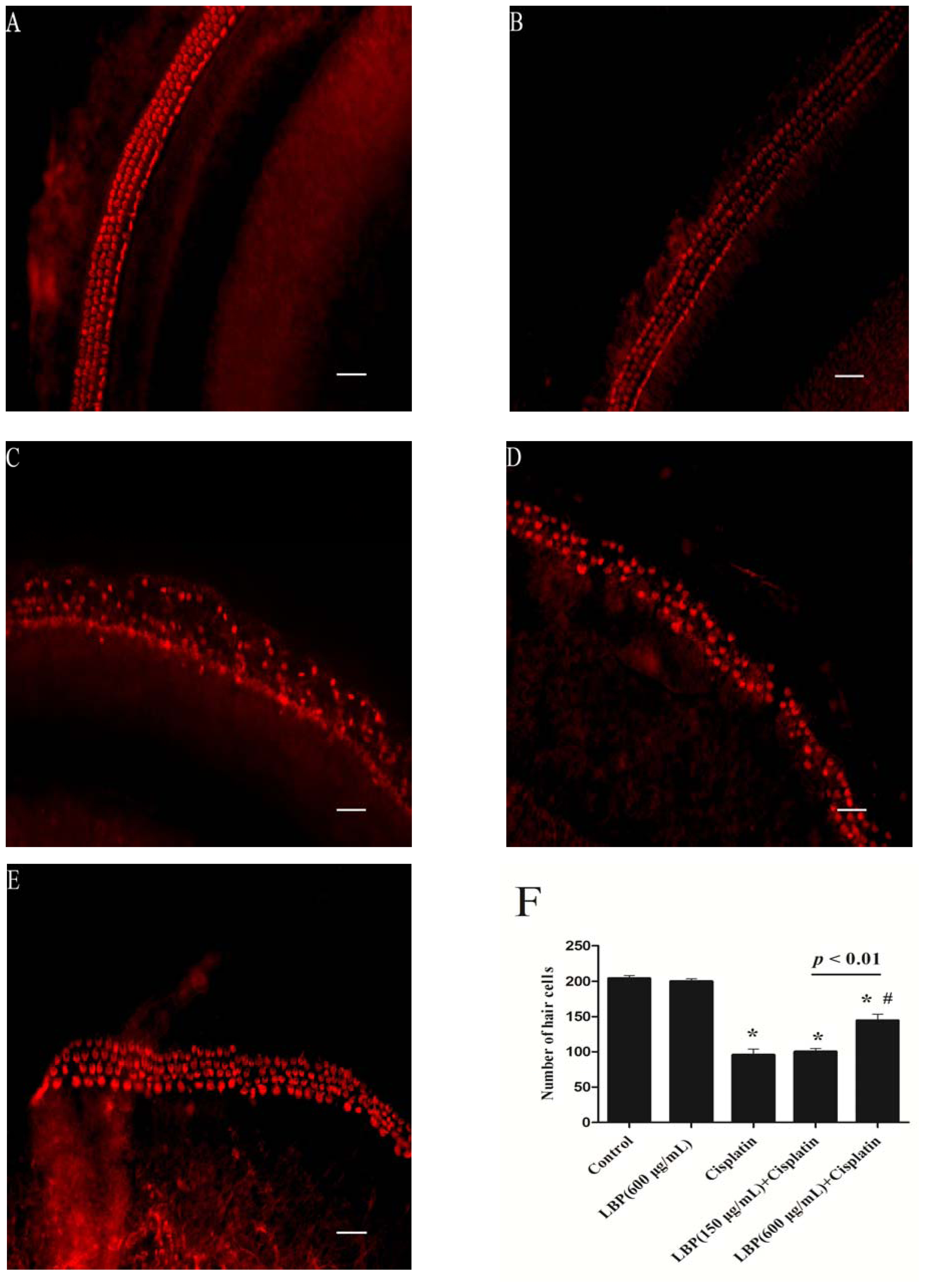
2.1. LBP Protection Against Cisplatin-Induced Hair Cell Death
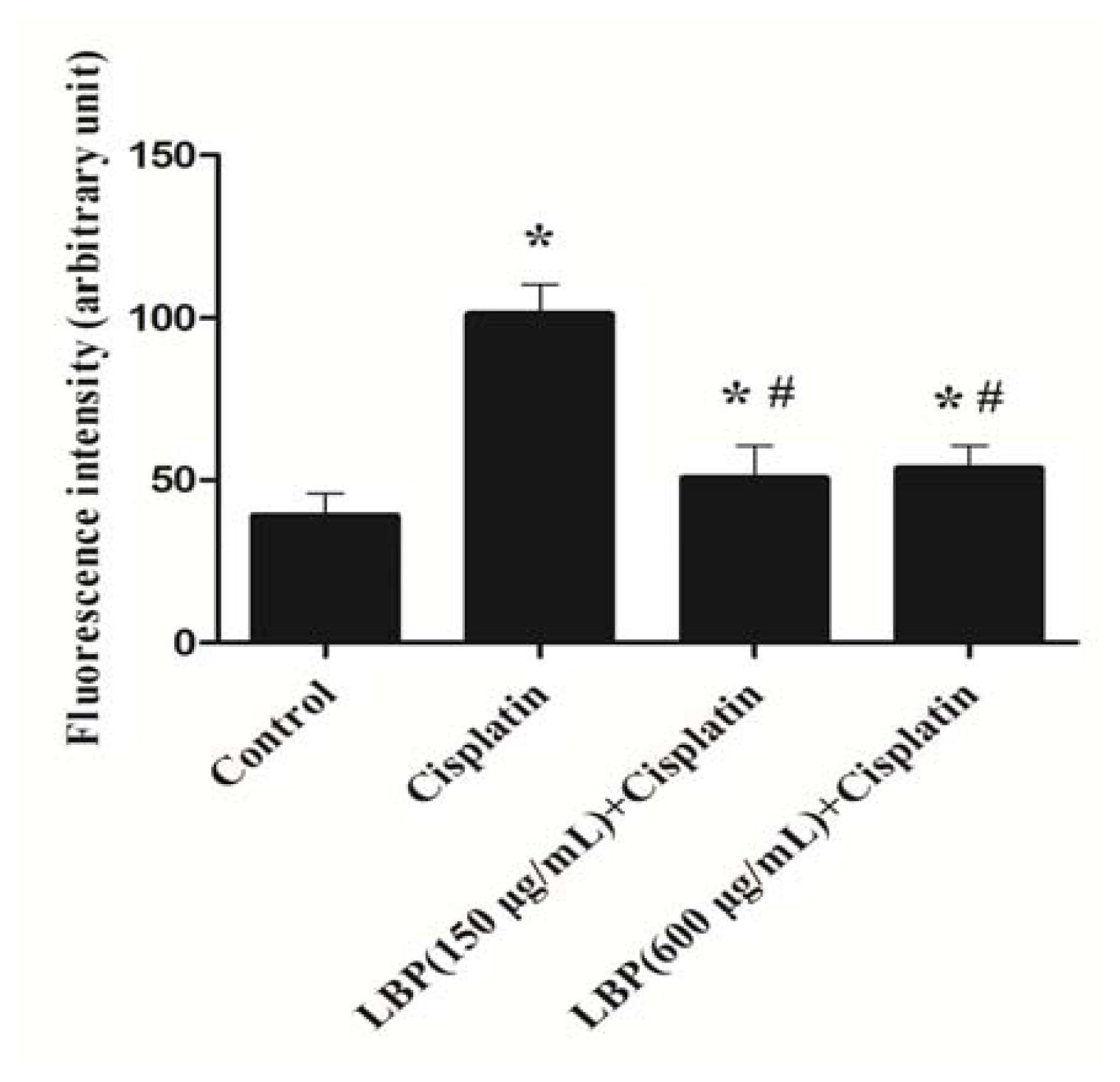
2.2. LBP Reduced the Generation of ROS in Hair Cells
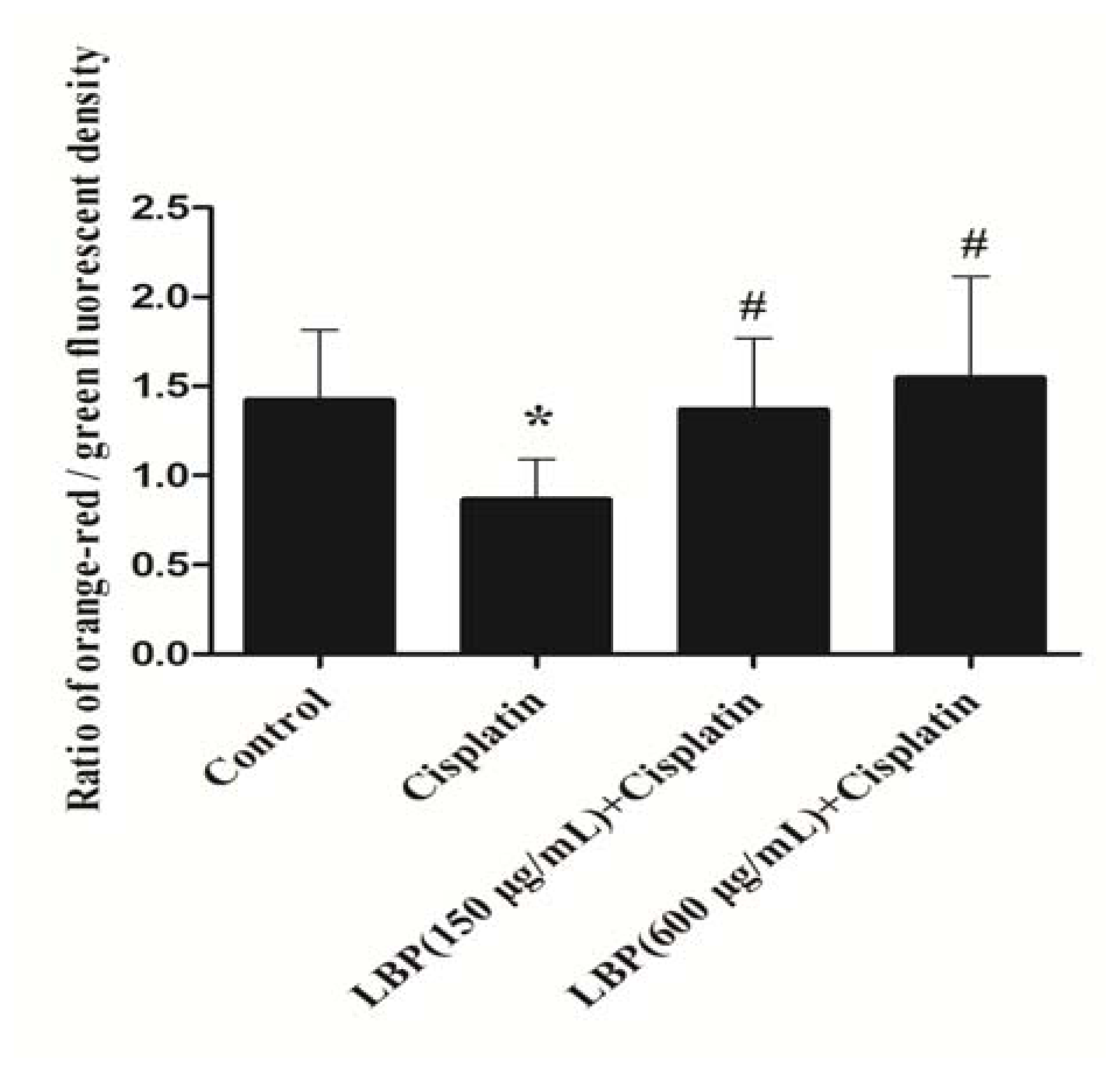
2.3. Effects of LBP on Mitochondrial ΔΨm
2.4. Discussion
3. Experimental Section
3.1. LBP Extract Processing
3.2. Animals and Dissection
3.3. Organ of Corti Culture
3.4. Cisplatin and LBP Treatment
3.5. Assessment of Hair Cells Death
3.6. ROS Levels in Organ of Corti Explant
3.7. Measurement of Mitochondrial Membrane Potential (ΔΨm)
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflicts of InterestThe authors declare no conflicts of interest.
References
- Zuur, C.L.; Simis, Y.J.; Verkaik, R.S.; Schornagel, J.H.; Balm, A.J.; Dreschler, W.A.; Rasch, C.R. Hearing loss due to concurrent daily low-dose cisplatin chemoradiation for locally advanced head and neck cancer. Radiother. Oncol 2008, 89, 38–43. [Google Scholar]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H., Jr; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci 2007, 334, 115–124. [Google Scholar]
- Kim, H.J.; Lee, J.H.; Kim, S.J.; Oh, G.S.; Moon, H.D.; Kwon, K.B.; Park, C.; Park, B.H.; Lee, H.K.; Chung, S.Y.; et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J. Neurosci 2010, 30, 3933–3946. [Google Scholar]
- Ravi, R.; Somani, S.M.; Rybak, L.P. Mechanism of cisplatin ototoxicity: Antioxidant system. Pharmacol. Toxicol 1995, 76, 386–394. [Google Scholar]
- Minami, S.B.; Sha, S.H.; Schacht, J. Antioxidant protection in a new animal model of cisplatin-induced ototoxicity. Hear. Res 2004, 198, 137–143. [Google Scholar]
- Kopke, R.D.; Liu, W.; Gabaizadeh, R.; Jacono, A.; Feghali, J.; Spray, D.; Garcia, P.; Steinman, H.; Malgrange, B.; Ruben, R.J. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol 1997, 18, 559–571. [Google Scholar]
- Chen, Z.; Tan, B.K.H.; Chan, S.H. Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int. Immunopharmacol 2008, 8, 1663–1671. [Google Scholar]
- Miao, Y.; Xiao, B.; Jiang, Z.; Guo, Y.; Mao, F.; Zhao, J.; Huang, X.; Guo, J. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med. Oncol 2010, 27, 785–790. [Google Scholar]
- Gan, L.; Hua, Z.S.; Liang, Y.X.; Bi, X.H. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int. Immunopharmacol 2004, 4, 563–569. [Google Scholar]
- Li, X.M.; Ma, Y.L.; Liu, X.J. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J. Ethnopharmacol 2007, 111, 504–511. [Google Scholar]
- Zhao, R.; Li, Q.W.; Li, J.; Zhang, T. Protective effect of Lycium barbarum polysaccharide 4 on kidneys in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol 2009, 87, 711–719. [Google Scholar]
- Niu, A.J.; Wu, J.M.; Yu, D.H.; Wang, R. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Int. J. Biol. Macromol 2008, 42, 447–449. [Google Scholar]
- Cheng, D.; Kong, H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 2011, 16, 2542–2550. [Google Scholar]
- Lin, C.L.; Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int. J. Biol. Macromol 2009, 45, 146–151. [Google Scholar]
- Hong, B.N.; You, Y.O.; Kang, T.H. Curculigo orchioides, natural compounds for the treatment of noise-induced hearing loss in mice. Arch. Pharm. Res 2011, 34, 653–659. [Google Scholar]
- Huang, X.; Whitworth, C.A.; Rybak, L.P. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol. Neurotol 2007, 28, 828–833. [Google Scholar]
- Choi, H.S.; Park, K.J.; Hwang, S.C.; Park, H.Y.; Kim, Y.S.; Park, K. The role of peroxiredoxin III in the ototoxic drug-induced mitochondrial apoptosis of cochlear hair cells. Acta Otolaryngol 2008, 128, 944–951. [Google Scholar]
- Jeong, H.J.; Choi, Y.; Kim, M.H.; Kang, I.C.; Lee, J.H.; Park, C.; Park, R.; Kim, H.M. Rosmarinic acid, active component of Dansam-Eum attenuates ototoxicity of cochlear hair cells through blockage of caspase-1 activity. PLoS One 2011, 6, e18815. [Google Scholar]
- Lu, M.; Gong, X. Upstream reactive oxidative species (ROS) signals in exogenous oxidative stress-induced mitochondrial dysfunction. Cell Biol. Int 2009, 33, 658–664. [Google Scholar]
- Ma, Q.; Fang, H.; Shang, W.; Liu, L.; Xu, Z.; Ye, T.; Wang, X.; Zheng, M.; Chen, Q.; Cheng, H. Superoxide flashes: Early mitochondrial signals for oxidative stress-induced apoptosis. J. Biol. Chem 2011, 286, 27573–27581. [Google Scholar]
- Feng, X.; Xia, Q.; Yuan, L.; Yang, X.; Wang, K. Impaired mitochondrial function and oxidative stress in rat cortical neurons: implications for gadolinium-induced neurotoxicity. Neurotoxicology 2010, 31, 391–398. [Google Scholar]
- Chiu, T.L.; Su, C.C. Tanshinone IIA induces apoptosis in human lung cancer A549 cells through the induction of reactive oxygen species and decreasing the mitochondrial membrane potential. Int. J. Mol. Med 2010, 25, 231–236. [Google Scholar]
- Campbell, K.C.; Meech, R.P.; Rybak, L.P.; Hughes, L.F. The effect of d-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J. Am. Acad. Audiol 2003, 14, 144–156. [Google Scholar]
- Giordano, P.; Lorito, G.; Ciorba, A.; Martini, A.; Hatzopoulos, S. Protection against cisplatin ototoxicity in a Sprague-Dawley rat animal model. Acta Otorhinolaryngol. Ital 2006, 26, 198–207. [Google Scholar]
- Wang, J.; Lloyd-Faulconbridge, R.V.; Fetoni, A.; Guitton, M.J.; Pujol, R.; Puel, J.L. Local application of sodium thiosulfate prevents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology 2003, 45, 380–393. [Google Scholar]
- Dickey, D.T.; Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther 2005, 314, 1052–1058. [Google Scholar]
- Deng, H.B.; Cui, D.P.; Jiang, J.M.; Feng, Y.C.; Cai, N.S.; Li, D.D. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on non-enzyme glycation in d-galactose induced mouse aging model. Biomed. Environ. Sci 2003, 16, 267–275. [Google Scholar]
- Kim, C.H.; Kang, S.U.; Pyun, J.; Lee, M.H.; Hwang, H.S.; Lee, H. Epicatechin protects auditory cells against cisplatin-induced death. Apoptosis 2008, 13, 1184–1194. [Google Scholar]
- Zhang, M.; Liu, W.; Ding, D.; Salvi, R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience 2003, 120, 191–205. [Google Scholar]
- Image Pro-Plus, version 6.0; Media Cybernetics, Inc: Bethesda, MD, USA, 2006.
- Mathur, A.; Hong, Y.; Kemp, B.K.; Barrientos, A.A.; Erusalimsky, J.D. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc. Res 2000, 46, 126–138. [Google Scholar]
- Troiano, L.; Ferraresi, R.; Lugli, E.; Nemes, E.; Roat, E.; Nasi, M.; Pinti, M.; Cossarizza, A. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat. Protoc 2007, 2, 2719–2727. [Google Scholar]
- Lugli, E.; Troiano, L.; Ferraresi, R.; Roat, E.; Prada, N.; Nasi, M.; Pinti, M.; Cooper, E.L.; Cossarizza, A. Characterization of cells with different mitochondrial membrane potential during apoptosis. Cytometry A 2005, 68, 28–35. [Google Scholar]
- Stata Statistical Software, version 10; StataCorp LP: College Station, TX, USA, 2007.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Q.; Li, Y.; Hu, L.; Wang, D. Lycium barbarum Polysaccharides Attenuate Cisplatin-Induced Hair Cell Loss in Rat Cochlear Organotypic Cultures. Int. J. Mol. Sci. 2011, 12, 8982-8992. https://doi.org/10.3390/ijms12128982
Liu Q, Li Y, Hu L, Wang D. Lycium barbarum Polysaccharides Attenuate Cisplatin-Induced Hair Cell Loss in Rat Cochlear Organotypic Cultures. International Journal of Molecular Sciences. 2011; 12(12):8982-8992. https://doi.org/10.3390/ijms12128982
Chicago/Turabian StyleLiu, Quan, Yanqing Li, Li Hu, and Dehui Wang. 2011. "Lycium barbarum Polysaccharides Attenuate Cisplatin-Induced Hair Cell Loss in Rat Cochlear Organotypic Cultures" International Journal of Molecular Sciences 12, no. 12: 8982-8992. https://doi.org/10.3390/ijms12128982
APA StyleLiu, Q., Li, Y., Hu, L., & Wang, D. (2011). Lycium barbarum Polysaccharides Attenuate Cisplatin-Induced Hair Cell Loss in Rat Cochlear Organotypic Cultures. International Journal of Molecular Sciences, 12(12), 8982-8992. https://doi.org/10.3390/ijms12128982



