Synthesis and Bio-Activity Evaluation of Scutellarein as a Potent Agent for the Therapy of Ischemic Cerebrovascular Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
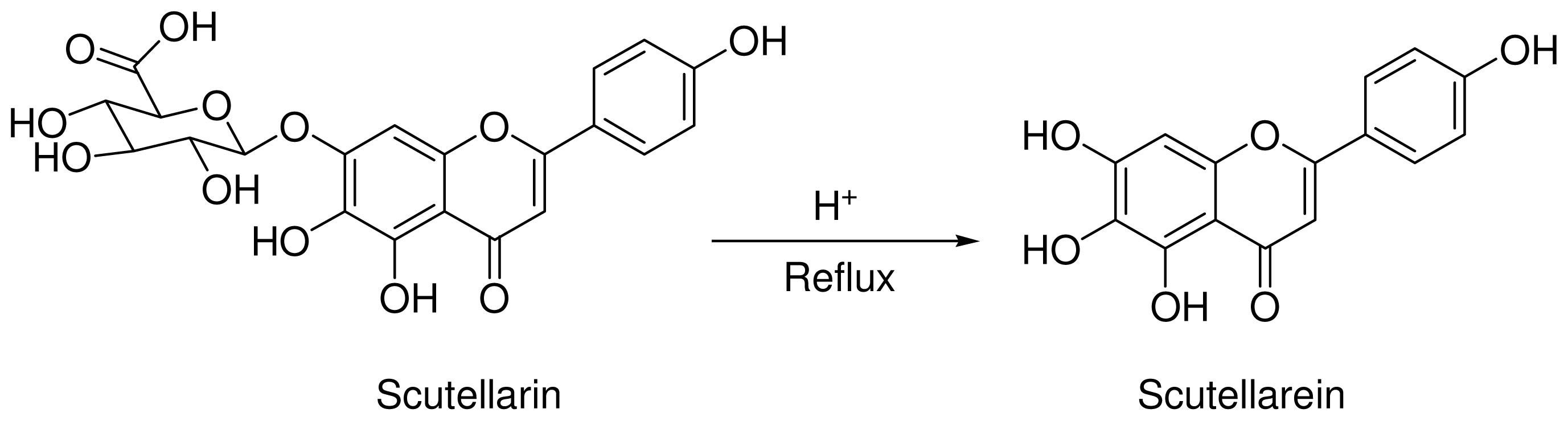
2.2. General Procedure for the Synthesis of Scutellarein
2.3. DPPH Radical-Scavenging Activity Assay
2.4. ABTS+• Radical-Scavenging Activity Assay
2.5. •OH Radical-Scavenging Activity Assay
2.6. MTT Assay for PC12 Cell Survival
3. Results and Discussion
3.1. Optimization of Reaction Conditions for the Synthesis of Scutellarein
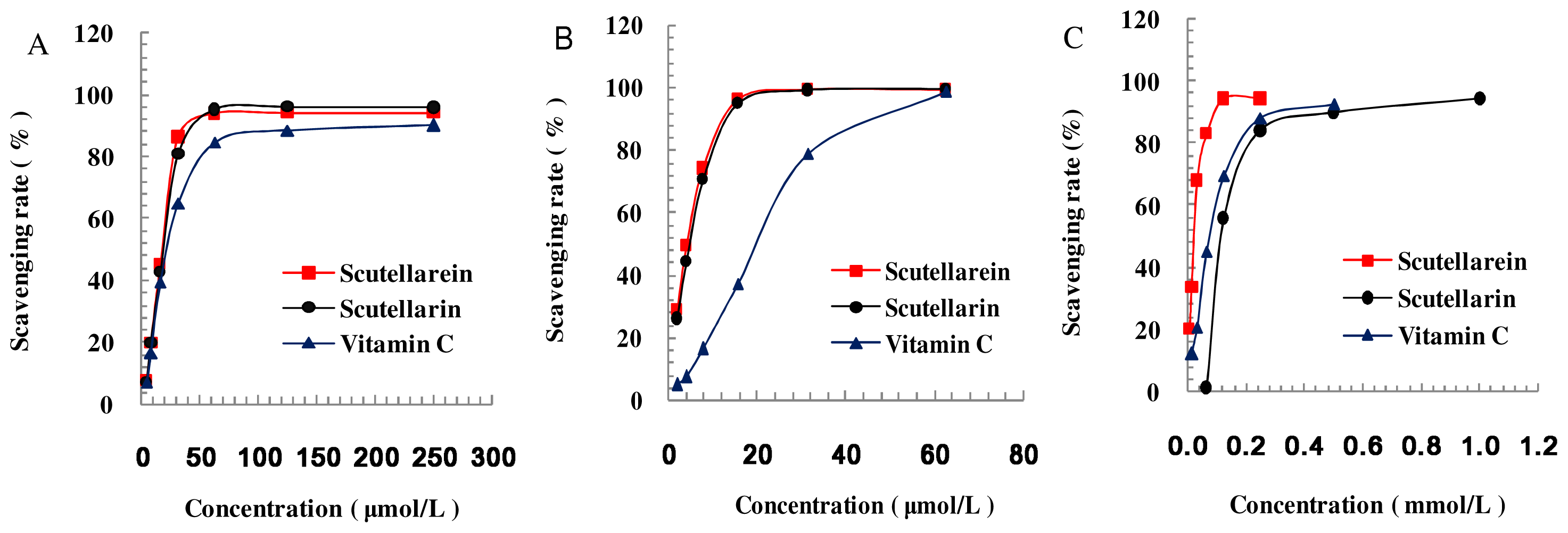
3.2. DPPH, ABTS+•, •OH Radical-Scavenging Activity
3.3. Protective Effect on H2O2-Induced Cytotoxicity in PC12 Cells
4. Conclusion
Acknowledgments
References
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 16121623. [Google Scholar]
- Cuzzocrea, S.; Riley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev 2001, 53, 135159. [Google Scholar]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med 2009, 7, 97107. [Google Scholar]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys 2010, 501, 2330. [Google Scholar]
- Xu, J.M.; Huang, Z.H. DengZhan XiXin capsules in the treatment of cerebrovascular disorders in 74 patients. Chin. J. New Drugs Clin. Rem 1991, 10, 260263. [Google Scholar]
- Chen, X.X.; He, B. Effects of breviscapine on the changes in antioxi-dant enzyme activity induced by cerebral ischemia reperfusion in rats. J. Chin. Pharm. Sci 1998, 7, 9193. [Google Scholar]
- Liu, Y.M.; Lin, A.H.; Chen, H.; Zeng, F.D. Study on pharmacokinetics of scutellarin in rabbits. Acta Pharm. Sin 2003, 38, 775778. [Google Scholar]
- Pan, Z.W.; Feng, T.M.; Shan, L.C.; Cai, B.Z.; Chu, W.F.; Niu, H.L.; Lu, Y.J.; Yang, B.F. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother. Res 2008, 22, 14281433. [Google Scholar]
- Zhang, H.Y.; Ping, Q.N.; Guo, J.X.; Cao, F. Pharmacokinetics of breviscapine and its β-cyclodextrin complex in rats. Acta Pharm. Sin 2005, 40, 563567. [Google Scholar]
- Cao, F.; Guo, J.X.; Ping, Q.N.; Shao, Y.; Liang, J. Ester prodrug of scutellarin: Synthesis, physicochemical property and degradation. Acta Pharm. Sin 2006, 41, 595602. [Google Scholar]
- Ge, Q.H.; Zhou, Z.; Zhi, X.J.; Ma, L.L.; Chen, X.H. Pharmacokinetics and absolute bioavailability of breviscapine in beagle dogs. Chin. J. Pharm 2003, 34, 618632. [Google Scholar]
- Jiang, X.H.; Li, S.H.; Lan, K.; Yang, J.Y.; Zhou, J. Study on the pharmacokinetics of scutellarin in dogs. Acta Pharm. Sin 2003, 38, 371373. [Google Scholar]
- Zhang, J.L.; Che, Q.M.; Li, S.Z.; Zhou, T.H. Study on metabolism of scutellarin in rats by HPLC-MS and HPLC-NMR. J. Asian Nat. Prod. Res 2003, 5, 249256. [Google Scholar]
- Che, Q.M.; Pan, L.Y.; Chen, Y.; He, H. Study on pharmacokinetics of scutellarein in rats. Chin. Pharm. J 2007, 42, 14181421. [Google Scholar]
- Ju, W.Z.; Zhang, J.; Tan, H.S.; Jiang, M.; Chen, M.; Xiong, N.N. Determination of scutellarin in human plasma by LC-MS method and its clinical pharmacokinetics in Chinese healthy volunteers. Chin. J. Clin. Pharmacol. Ther 2005, 10, 298301. [Google Scholar]
- Song, Y.; Zhang, H.M.; Ma, J.J.; Che, Q.M.; Li, C.L. Protection of scutellarein on cerebral ischemia in rats. Chin. J. New Drug 2009, 18, 20612064. [Google Scholar]
- Farkas, L.; Mezey-Vándor, G.; Nórdj, M. Die sythese des scutellarins, plantaginins, scutellarein-7-β-rutinosids und die erste herstellung des isoscutellareins. Chem. Bet 1974, 107, 38783882. [Google Scholar]
- Cui, J.M.; Fang, G.; Duan, Y.B. Total synthesis of scutellarin-7-O-glucuronide. J. Asian Nat. Prod. Res 2005, 7, 655660. [Google Scholar]
- Che, Q.M. A method to synthesis scutellarein. CN Patent 1683357A[P], 2005. [Google Scholar]
- Wang, H.; Gao, D.X.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem 2008, 106, 888895. [Google Scholar]
- Liu, Z.Q. Chemical methods to evaluate antioxidant ability. Chem. Rev 2010, 110, 56755691. [Google Scholar]
- Ji, B.S.; Gao, Y. Protective effect of trihexyphenidyl on hydrogen peroxide-induced oxidative damage in PC12 cells. Neurosci. Lett 2008, 437, 5054. [Google Scholar]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 24242428. [Google Scholar]
- Pavlica, S.; Gebbardt, R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci 2010, 86, 7986. [Google Scholar]
- Hong, H.; Liu, G.Q. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci 2004, 74, 29592973. [Google Scholar]
| Run | Reaction conditions | Yield (%) |
|---|---|---|
| 1 | 1.0 mol/L H2SO4 in water, 90 °C, 6~24 h | No product |
| 2 | 2.0 mol/L H2SO4 in water, 90 °C, 6~24 h | No product |
| 3 | 3.0 mol/L H2SO4 in water, 90 °C, 6~24 h | No product |
| 4 | 0.5 mol/L H2SO4 in 70% ethanol, 90 °C, 6~24 h | No product |
| 5 | 0.5 mol/L H2SO4 in 80% ethanol, 90 °C, 6~24 h | No product |
| 6 | 0.5 mol/L H2SO4 in 90% ethanol, 90 °C, 24 h | 2.1 |
| 7 | 1.0 mol/L H2SO4 in 90% ethanol, 90 °C, 24 h | 5.3 |
| 8 | 2.0 mol/L H2SO4 in 90% ethanol, 90 °C, 24 h | 8.5 |
| 9 | 3.0 mol/L H2SO4 in 90% ethanol, 90 °C, 24 h | 10.0 |
| 10 | 3.0 mol/L H2SO4 in 90% ethanol, 90 °C, 48 h | 12.1 |
| 11 | 3.0 mol/L H2SO4 in 90% ethanol, 100 °C, 48 h | 15.2 |
| 12 | 3.0 mol/L H2SO4 in 90% ethanol, 120 °C, 48 h | 17.3 |
| Compounds | IC50 | ||
|---|---|---|---|
| DPPH (μmol/L) | ABTS+• (μmol/L) | •OH (mmol/L) | |
| Scutellarein | 16.84 | 3.00 | 0.31 |
| Scutellarin | 17.56 | 3.53 | 3.19 |
| Vitamin C | 24.81 | 12.56 | 1.12 |
| Drug (μmol/L) | Coincubation | Preincubation for 30 min | Preincubation for 8 h | |||
|---|---|---|---|---|---|---|
| A517 | Inhibiting Rate (%) | A517 | Inhibiting Rate (%) | A517 | Inhibiting Rate (%) | |
| Normal | 0.550 ± 0.004 | — | 0.589 ± 0.003 | — | 0.624 ± 0.004 | — |
| H2O2 | 0.353 ± 0.006 ## | — | 0.319 ± 0.0123 ## | — | 0.375 ± 0.015 ## | — |
| Scutellarin/100 | 0.433 ± 0.009 ** | 40.78 | 0.440 ± 0.009 ** | 44.81 | 0.482 ± 0.002 ** | 42.97 |
| Scutellarein/100 | 0.540 ± 0.038 ** | 94.92 | 0.500 ± 0.040 ** | 67.04 | 0.458 ± 0.013 ** | 33.33 |
| Scutellarein/10 | 0.422 ± 0.007 ** | 35.32 | 0.395 ± 0.019 ** | 28.12 | 0.414 ± 0.002 ** | 15.96 |
| Scutellarein/1 | 0.362 ± 0.002 | 4.57 | 0.336 ± 0.007 * | 6.10 | 0.383 ± 0.010 | — |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qian, L.-H.; Li, N.-G.; Tang, Y.-P.; Zhang, L.; Tang, H.; Wang, Z.-J.; Liu, L.; Song, S.-L.; Guo, J.-M.; Ding, A.-W. Synthesis and Bio-Activity Evaluation of Scutellarein as a Potent Agent for the Therapy of Ischemic Cerebrovascular Disease. Int. J. Mol. Sci. 2011, 12, 8208-8216. https://doi.org/10.3390/ijms12118208
Qian L-H, Li N-G, Tang Y-P, Zhang L, Tang H, Wang Z-J, Liu L, Song S-L, Guo J-M, Ding A-W. Synthesis and Bio-Activity Evaluation of Scutellarein as a Potent Agent for the Therapy of Ischemic Cerebrovascular Disease. International Journal of Molecular Sciences. 2011; 12(11):8208-8216. https://doi.org/10.3390/ijms12118208
Chicago/Turabian StyleQian, Li-Hua, Nian-Guang Li, Yu-Ping Tang, Li Zhang, Hao Tang, Zhen-Jiang Wang, Li Liu, Shu-Lin Song, Jian-Ming Guo, and An-Wei Ding. 2011. "Synthesis and Bio-Activity Evaluation of Scutellarein as a Potent Agent for the Therapy of Ischemic Cerebrovascular Disease" International Journal of Molecular Sciences 12, no. 11: 8208-8216. https://doi.org/10.3390/ijms12118208
APA StyleQian, L.-H., Li, N.-G., Tang, Y.-P., Zhang, L., Tang, H., Wang, Z.-J., Liu, L., Song, S.-L., Guo, J.-M., & Ding, A.-W. (2011). Synthesis and Bio-Activity Evaluation of Scutellarein as a Potent Agent for the Therapy of Ischemic Cerebrovascular Disease. International Journal of Molecular Sciences, 12(11), 8208-8216. https://doi.org/10.3390/ijms12118208




