A Facile and Convenient Synthesis of some Novel Hydrazones, Schiff’s Base and Pyrazoles Incorporating Thieno[2,3-b]thiophenes
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
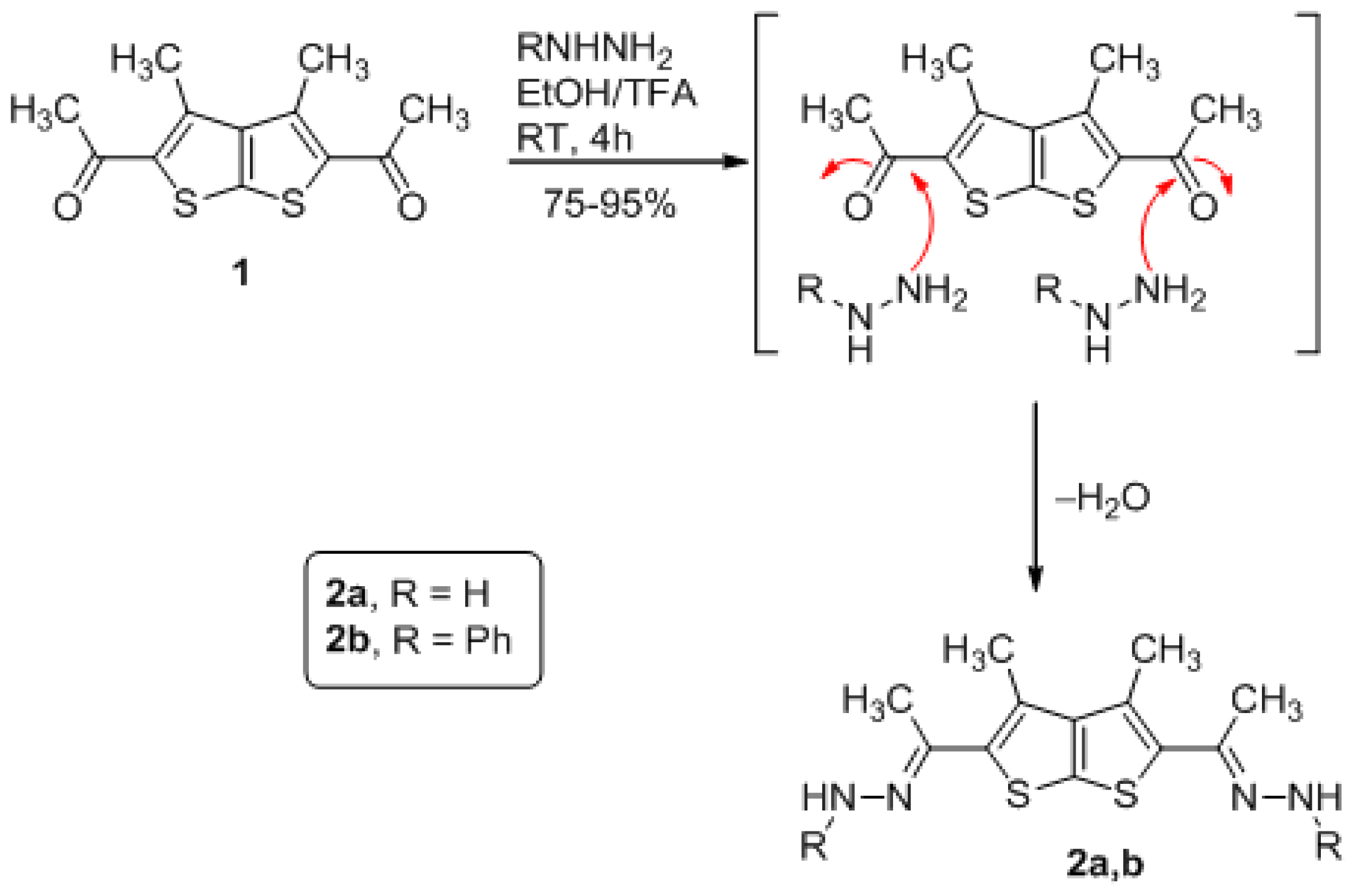
3.1. General Method for Preparation of Compounds Derivatives 2a,b (GP1)
(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(ethan-1-yl-1-ylidene) bis (hydrazine) (2a)
(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(ethan-1-yl-1-ylidene)bis(1-phenylhydrazine) (2b)
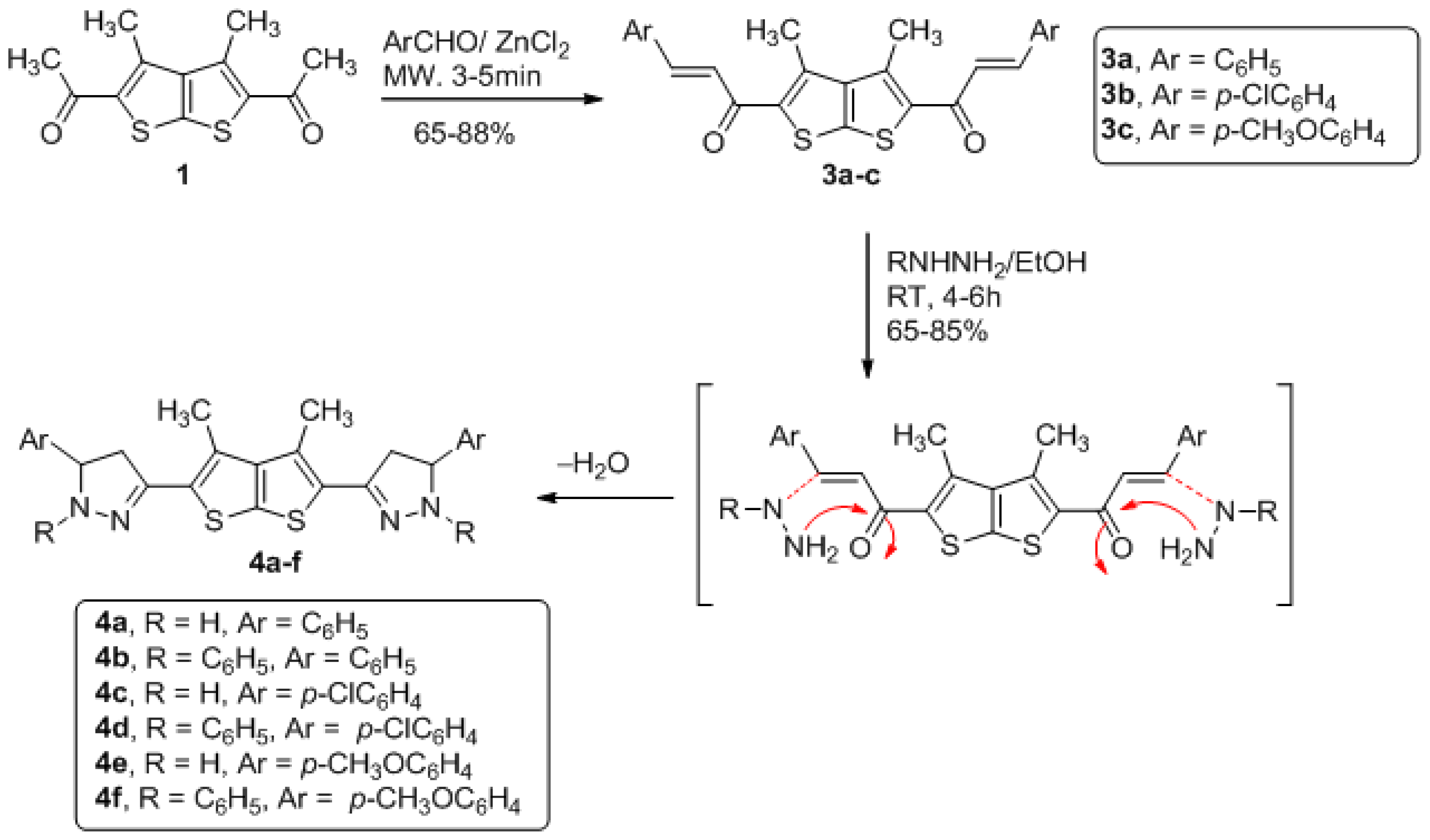
3.2. General Method for Preparation of Compounds Derivatives 3a–3c (GP2)
1,1′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(3-phenylprop-2-en-1-one) (3a)
1,1′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(3-(4-chlorophenyl)prop-2- en-1-one) (3b)
1,1′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(3-(4-methoxyphenyl)prop-2-en-1-one) (3c)
3.3. General Method for Preparation of Compounds Derivatives 4a–f (GP3)
3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis (5-phenyl-4,5-dihydro-1H-pyrazole) (4a)
3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(1,5-diphenyl-4,5-dihydro-1H-pyrazole) (4b)
3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-(4-chlorophenyl)-4,5-dihydro-1H-pyrazole) (4c)
3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-(4-chlorophenyl)-1-phenyl-4,5-dihydro-1Hpyrazole) (4d)
3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-(4-methoxyphenyl)-4,5-dihydro-1Hpyrazole)( 4e)
3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-(4-methoxyphenyl)-1-phenyl-4,5-dihydro-1Hpyrazole)( 4f)
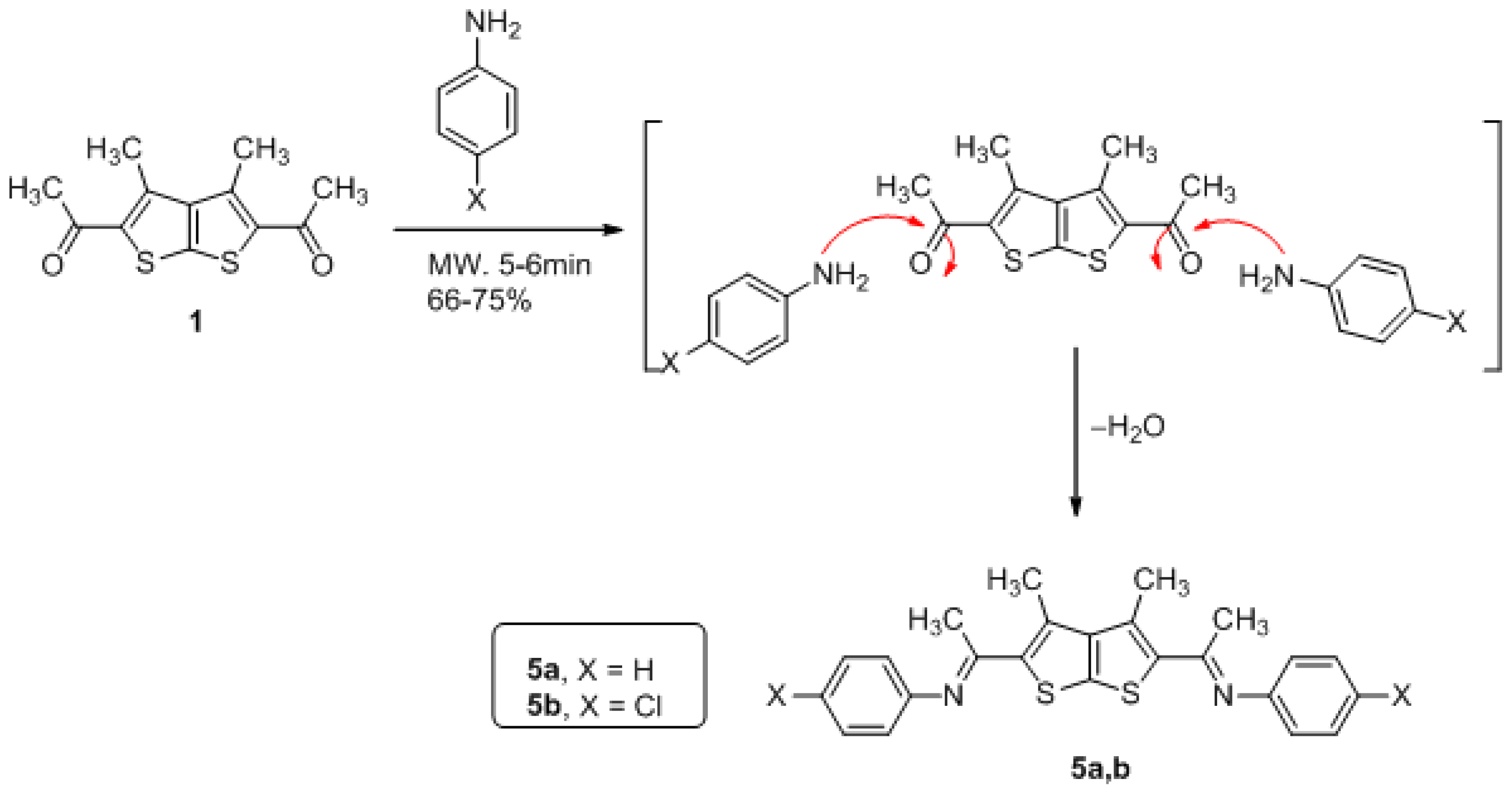
3.4. General Method for Preparation of Compounds Derivatives 5a,b (GP4)
(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(ethan-1-yl-1-ylidene)bis(4-chloroaniline)(5b)
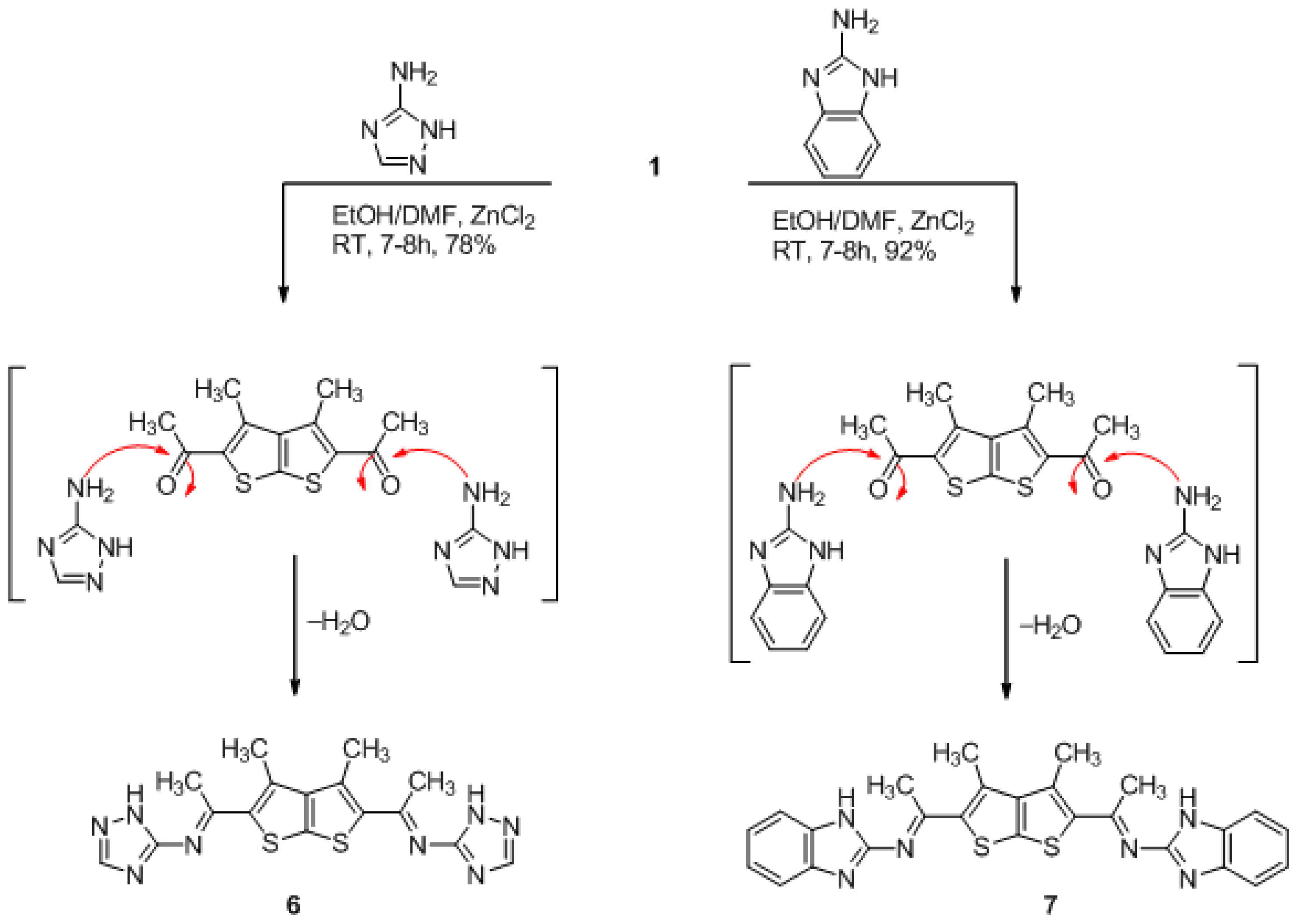
(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(ethan-1-yl-1-ylidene)bis(1H-1,2,4-triazol-5-amine) (6)
(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(ethan-1-yl-1-ylidene)bis(1H-benzo[d]imidazol-2- amine) (7)
4. Conclusions
Acknowledgments
References
- Litvinov, V.P.; Gol’dfarb, Y.L. Advanced Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: New York, NY, USA, 1976; Volume 19, p. 123. [Google Scholar]
- King, W.J.; Nord, F.F. Studies in the thiophene series. V. Wolff-Kishner reductions. J. Org. Chem 1949, 14, 638–642. [Google Scholar]
- Wu, C.; Decker, E.R.; Blok, N.; Bui, H.; You, T.J.; Wang, J.; Bourgoyne, A.R.; Knowles, V.; Berens, K.L.; Holland, G.W.; Brock, T.A.; Dixon, R.A.F. Discovery, modeling, and human pharmacokinetics of N-(2-acetyl-4,6-dimethylphenyl)-3-(3,4-dimethylisoxazol-5-ylsulfamoyl) thiophene-2-carboxamide (TBC3711), a second generation, ETA selective, and orally bioavailable endothelin antagonist. J. Med. Chem 2004, 47, 1969–1986. [Google Scholar]
- Doré, K.; Dubus, S.; Ho, H.A.; Lévesque, I.; Brunette, M.; Corbeil, G.; Boissinot, M.; Boivin, G.; Bergeron, M.G.; Boudreau, D.; Leclerc, M. Fluorescent polymeric transducer for the rapid, simple, and specific detection of nucleic acids at the zeptomole level. J. Am. Chem. Soc 2004, 126, 4240–4244. [Google Scholar]
- Rost, C.; Karg, S.; Riess, W.; Loi, M.A.; Murgia, M.; Muccini, M. Ambipolar light-emitting organic field-effect transistor. Appl. Phys. Lett 2004, 85, 1613–1615. [Google Scholar]
- Vriezema, D.M.; Hoogboom, J.; Velonia, K.; Takazawa, K.; Christianen, P.C.M.; Maan, J.C.; Rowan, A.E.; Nolte, R.J.M. Vesicles and polymerized vesicles from thiophene-containing rod-coil block copolymers. Angew. Chem. Int. Ed 2003, 42, 772–776. [Google Scholar]
- Yu, H.; Pullen, A.E.; Büchel, M.G.; Swager, T.M. Charge-specific interactions in segmented conducting polymers: An approach to selective ionoresistive responses. Angew. Chem. Int. Ed 2004, 43, 3700–3703. [Google Scholar]
- Mabkhot, Y.N.; Kheder, N.A.; Al-Majid, A.M. Facile and convenient synthesis of new thieno[2,3-b]thiophene derivatives. Molecules 2010, 15, 9418–9426. [Google Scholar]
- Mabkhot, Y.N. synthesis and chemical characterisation of new bis-thieno[2,3-b]thiophene derivatives. Molecules 2010, 15, 3329–3337. [Google Scholar]
- Mabkhot, Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing Sulphur. Molecules 2009, 14, 1904–1914. [Google Scholar]
- Sabir, H.M.; Sangvikar, Y.S.; Ghadigaonkar, S.G.; Ashraf, M.; Meetsma, A. Oxa-bridged cyclophanes featuring thieno[2,3-b]thiophene and C2-symmetric binol or bis-naphthol rings: Synthesis, structures, and conformational studies. Tetrahedron 2008, 64, 8837–8842. [Google Scholar]
- Sabir, H.M.; Ashraf, M.; Hariharasubrahmanian, H.; Kelloggb, R.M.; Meetsma, A. Donor-acceptor thieno[2,3-b]thiophene systems: Synthesis and structural study of 3-anisyl-4-pyridyl(pyridinium) thieno[2,3-b]thiophenes. J. Mol. Struct. 2004, 689, 107–113. [Google Scholar]
- Bugge, A. preparation of some brominated thieno[2,3-b]thiophenes and thieno[3,2-b]thiophenes. Acta Chem. Scand 1969, 23, 2704–2710. [Google Scholar]
- Mashraqui, S.H.; Sangvikar, Y.S.; Ashraf, M.; Kumar, S.; Daub, E. Dipyridyl/pyridinium thieno[2,3-b]thiophenes as new atropisomeric systems. Synthesis, conformat-ional analysis and energy minimization. Tetrahedron 2005, 61, 3507–3513. [Google Scholar]
- Liu, M.-G.; Hu, Y.-G.; Ding, M.-W. New iminophosphorane-mediated synthesis of thieno[3′,2′:4,5]thieno[3,2-d]pyrimidin-4(3H)-ones and 5H-2,3-dithia-5,7-diaza-cyclopenta[c,d] indenes. Tetrahedron 2008, 64, 9052–9059. [Google Scholar]
- Wu, Y.X.; Cao, J.; Deng, H.Y.; Feng, J.X. Synthesis, complexation, and fluorescence behavior of 3,4-dimethylthieno[2,3-b]thiophene carrying two monoaza-15-crown-5 ether groups. Spectrochim. Acta Part A 2011, 82, 340–344. [Google Scholar]
- McCulloch, I.; Heeney, M.; Chabinyc, M.L.; DeLongchamp, D.; Kline, R.J.; Cölle, M.; Duffy, W.; Fischer, D.; Gundlach, D.; Hamadani, B.; et al. Semiconducting thienothiophene copolymers: Design, synthesis, morphology, and performance in thin-film organic transistors. Adv. Mater 2009, 21, 1091–1109. [Google Scholar]
- Sabir, H.M.; Sanghvikar, Y.; Ghadhigaonkar, S.; Kumar, S.; Meetsma, A.; Trân Huu Dâu, E. [3.3]Dithia-bridged cyclophanes featuring a thienothiophene ring: Synthesis, structures and conformational analysis. Beilstein. J. Org. Chem. 2009, 5, 1–8. [Google Scholar]
- Heeney, M.; Bailey, C.; Genevicius, K.; Shkunov, M.; Sparrowe, D.; Tierney, S.; McCulloch, I. Stable polythiophene semiconductors incorporating Thieno[2,3-b]thiophene. J. Am. Chem. Soc 2005, 127, 1078–1079. [Google Scholar]
- Egbertson, M.S.; Cook, J.J.; Bednar, B.; Prugh, J.D.; Bednar, R.A.; Gaul, S.L.; Gould, R.J.; Hartman, G.D.; Homnick, C.F.; Holahan, M.A.; et al. Non-peptide GPIIb/IIIa inhibitors. 20. Centrally constrained thienothiophene α-sulfonamides are potent, long acting in vivo inhibitors of platelet aggregation. J. Med. Chem 1999, 42, 2409–2421. [Google Scholar]
- Mabkhot, Y.N.; Al-Majid, A.M.; Assem, B.; Alshahrani, S.; Siddiqui, Y. 1,1′-(3-Methyl-4- phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as building block in heterocyclic synthesis. Novel synthesis of some pyrazoles, and pyrimidines derivatives. Molecules 2011, 16, 6502–6511. [Google Scholar]
- Mabkhot, Y.N.; Al-Majid, A.M.; Alamary, A.S. Synthesis and chemical characterisation of some new diheteroaryl thienothiophene derivatives. Molecules 2011, 16, 7706–7714. [Google Scholar]
- Bougrin, K.; Loupy, A. Microwave-assisted solvent-free heterocyclic synthesis. J. Photochem. Photobiol. C Photochem. Rev 2005, 6, 139–167. [Google Scholar]
- Aggarwal, V.K.; de Vicente, J.; Bonnert, R.V. A novel one-pot method for the preparation of pyrazoles by 1,3-dipolar cycloadditions of diazo compounds generated in situ. J. Org. Chem 2003, 68, 5381–5383. [Google Scholar]
- Kost, A.N.; Grandberg, I.I. Progress in pyrazole chemistry. Adv. Heterocycl. Chem 1966, 6, 347–429. [Google Scholar]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; John Wiley & Sons: New York, NY, USA, 1984; Volume I. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nasser Mabkhot, Y.; Barakat, A.; Al-Majid, A.M.; Al-Othman, Z.A.; Alamary, A.S. A Facile and Convenient Synthesis of some Novel Hydrazones, Schiff’s Base and Pyrazoles Incorporating Thieno[2,3-b]thiophenes. Int. J. Mol. Sci. 2011, 12, 7824-7834. https://doi.org/10.3390/ijms12117824
Nasser Mabkhot Y, Barakat A, Al-Majid AM, Al-Othman ZA, Alamary AS. A Facile and Convenient Synthesis of some Novel Hydrazones, Schiff’s Base and Pyrazoles Incorporating Thieno[2,3-b]thiophenes. International Journal of Molecular Sciences. 2011; 12(11):7824-7834. https://doi.org/10.3390/ijms12117824
Chicago/Turabian StyleNasser Mabkhot, Yahia, Assem Barakat, Abdullah Mohammed Al-Majid, Zeid A. Al-Othman, and Abdullah Saleh Alamary. 2011. "A Facile and Convenient Synthesis of some Novel Hydrazones, Schiff’s Base and Pyrazoles Incorporating Thieno[2,3-b]thiophenes" International Journal of Molecular Sciences 12, no. 11: 7824-7834. https://doi.org/10.3390/ijms12117824
APA StyleNasser Mabkhot, Y., Barakat, A., Al-Majid, A. M., Al-Othman, Z. A., & Alamary, A. S. (2011). A Facile and Convenient Synthesis of some Novel Hydrazones, Schiff’s Base and Pyrazoles Incorporating Thieno[2,3-b]thiophenes. International Journal of Molecular Sciences, 12(11), 7824-7834. https://doi.org/10.3390/ijms12117824





