Development of a Human Physiologically Based Pharmacokinetic (PBPK) Toolkit for Environmental Pollutants
Abstract
:1. Introduction
2. Methods
2.1. Model Structure and Physiological Parameters
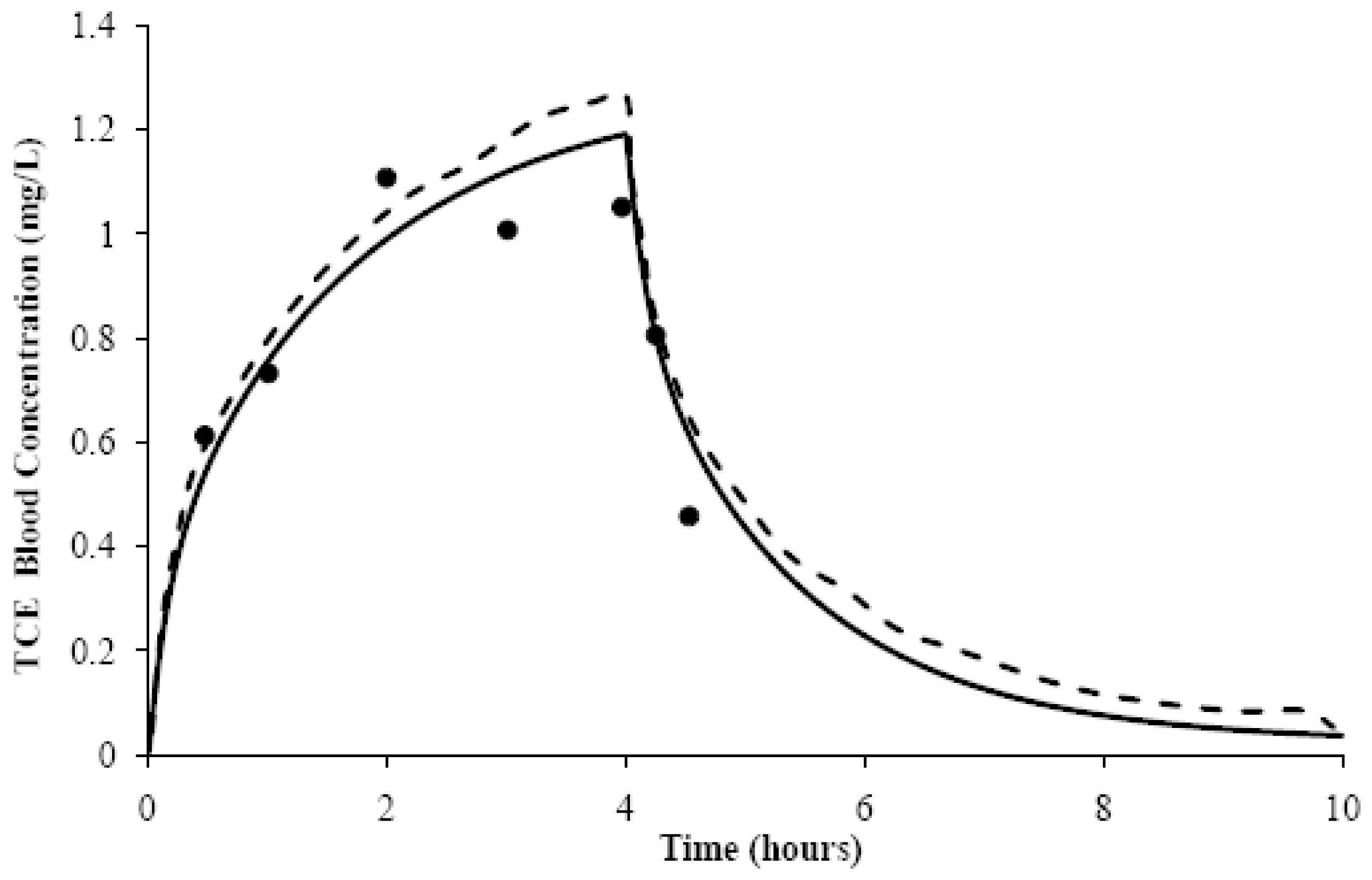
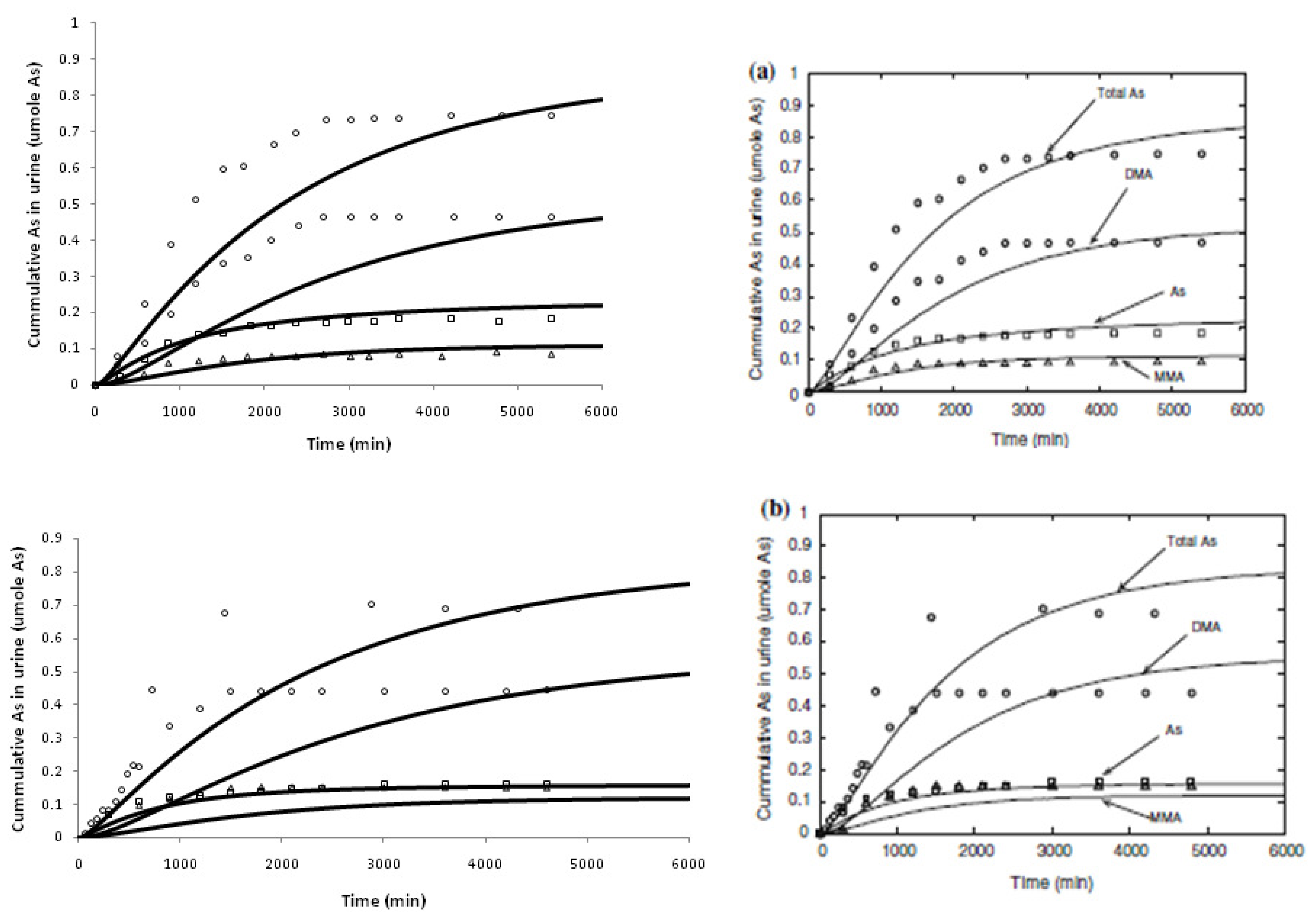
2.2. Model Evaluation
3. Model Applications
4. Results and Discussion
Acknowledgements
References
- Reddy, M.; Yang, R.S.; Andersen, M.E.; Clewell, H.J., III. Physiologically Based Pharmacokinetic Modeling: Science and Applications; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Zhao, P.; Zhang, L.; Grillo, J.A.; Liu, Q.; Bullock, J.M.; Moon, Y.J.; Song, P.; Brar, S.S.; Madabushi, R.; Wu, T.C.; et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin. Pharmacol. Ther 2011, 89, 259–267. [Google Scholar]
- Thompson, C.M.; Sonawane, B.; Barton, H.A.; DeWoskin, R.S.; Lipscomb, J.C.; Schlosser, P.; Chiu, W.A.; Krishnan, K. Approaches for applications of physiologically based pharmacokinetic models in risk assessment. J. Toxicol. Environ. Health B Crit. Rev 2008, 11, 519–547. [Google Scholar]
- Valcke, M.; Krishnan, K. Evaluation of the impact of the exposure route on the human kinetic adjustment factor. Regul. Toxicol. Pharmacol 2011, 59, 258–269. [Google Scholar]
- Krishnan, K.; Carrier, R. Approaches for evaluating the relevance of multiroute exposures in establishing guideline values for drinking water contaminants. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev 2008, 26, 300–316. [Google Scholar]
- Tardif, R.; Charest-Tardif, G.; Brodeur, J.; Krishnan, K. Physiologically based pharmacokinetic modeling of a ternary mixture of alkyl benzenes in rats and humans. Toxicol. Appl. Pharmacol 1997, 144, 120–134. [Google Scholar]
- Timchalk, C.; Nolan, R.J.; Mendrala, A.L.; Dittenber, D.A.; Brzak, K.A; Mattsson, J.L. A physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol. Sci. 2002, 66, 34–53. [Google Scholar]
- Macey, R.I.; Oster, G.F. Berkeley Madonna, Version 8.3.9; University of California at Berkeley: Berkeley, CA, USA, 2006. [Google Scholar]
- Ruiz, P.; Mumtaz, M; Osterloh, J.; Fisher, J.; Fowler, B.A. Interpreting NHANES biomonitoring data, cadmium. Toxicol. Lett. 2010, 198, 44–48. [Google Scholar]
- Ruiz, P.; Fowler, B.A.; Osterloh, J.D.; Fisher, J.; Mumtaz, M. Physiologically based pharmacokinetic (PBPK) tool kit for environmental pollutants—metals. SAR QSAR Environ. Res 2010, 21, 603–618. [Google Scholar]
- Mumtaz, M.; Ray, M.; Crowell, S.R.; Keys, D.; Fisher, J.; Ruiz, P. Translational research to develop a human PBPK models tool-kit—Volatile Organic Compounds (VOCs). J. Toxicol. Environ. Health B Crit. Rev. 2011, 74, 1431–1446. [Google Scholar]
- El-Masri, H.A.; Kenyon, E.M. Development of a human physiologically based pharmacokinetic (PBPK) model for inorganic arsenic and its mono- and di-methylated metabolites. J. Pharmacokinet. Pharmacodyn 2008, 35, 31–68. [Google Scholar]
- Carrier, G; Brunet, R.C.; Caza, M.; Bouchard, M. A toxicokinetic model for predicting the tissue distribution and elimination of organic and inorganic mercury following exposure to methyl mercury in animals and humans. I. Development and validation of the model using experimental data in rats. Toxicol. Appl. Pharmacol. 2001, 171, 38–49. [Google Scholar]
- Carrier, G.; Bouchard, M.; Brunet, R.C.; Caza, M. A toxicokinetic model for predicting the tissue distribution and elimination of organic and inorganic mercury following exposure to methyl mercury in animals and humans. II. Application and validation of the model in humans. Toxicol. Appl. Pharmacol 2001, 171, 50–60. [Google Scholar]
- Choudhury, H.; Harvey, T.; Thayer, W.C.; Lockwood, T.F.; Stiteler, W.M.; Goodrum, P.E.; Hassett, J.M.; Diamond, G.L. Urinary cadmium elimination as a biomarker of exposure for evaluating a cadmium dietary exposure—biokinetics model. J. Toxicol. Environ. Health A 2001, 63, 321–350. [Google Scholar]
- Diamond, G.L.; Thayer, W.C.; Choudhury, H. Pharmacokinetics/pharmacodynamics (PK/PD) modeling of risks of kidney toxicity from exposure to cadmium: estimates of dietary risks in the U.S. population. J. Toxicol. Environ. Health A 2003, 66, 2141–2164. [Google Scholar]
- Kjellström, T.; Nordberg, G.F. A kinetic model of cadmium metabolism in the human being. Environ. Res 1978, 16, 248–269. [Google Scholar]
- Yokley, K.; Tran, H.T.; Pekari, K.; Rappaport, S.; Riihimaki, V.; Rothman, N.; Waidyanatha, S.; Schlosser, P.M. Physiologically-based pharmacokinetic modeling of benzene in humans: a Bayesian approach. Risk Anal 2006, 26, 925–943. [Google Scholar]
- Thrall, K.D.; Vucelick, M.E.; Gies, R.A.; Zangar, R.C.; Weitz, K.K.; Poet, T.S.; Springer, D.L.; Grant, D.M.; Benson, J.M. Comparative metabolism of carbon tetrachloride in rats, mice, and hamsters using gas uptake and PBPK modeling. J. Toxicol. Environ. Health A 2000, 60, 531–548. [Google Scholar]
- David, R.M.; Clewell, H.J.; Gentry, P.R.; Covington, T.R.; Morgott, D.A.; Marino, D.J. Revised assessment of cancer risk to dichloromethane II: Application of probabilistic methods to cancer risk determinations. Regul. Toxicol. Pharmacol 2006, 45, 55–65. [Google Scholar]
- Covington, T.R.; Gentry, P.R.; van Landingham, C.B.; Andersen, M.E.; Kester, J.E.; Clewell, H.J. The use of Markov Chain Monte Carlo Uncertainty Analysis to support a public health goal for perchloroethylene. Regul. Toxicol. Pharmacol 2007, 47, 1–18. [Google Scholar]
- Fisher, J.; Mahle, D.; Abbas, R. A human physiologically based pharmacokinetic model for trichloroethylene and its metabolites, trichoroacetic acid and free trichloroethanol. Toxicol. Appl. Pharmacol 1998, 152, 339–359. [Google Scholar]
- Clewell, H.J.; Gentry, P.R.; Gearhart, J.M.; Allen, B.C.; Andersen, M.E. Comparison of cancer risk estimates for vinyl chloride using animal and human data with a PBPK model. Sci. Total Environ 2001, 274, 37–66. [Google Scholar]
- Grab It! ™ XP2; Datatrend Software: Raleigh, NC, USA, 2001.
- Clewell, H.J.; Gentry, P.R.; Covington, T.R.; Gearhart, J.M. Development of a physiologically based pharmacokinetic model of trichloroethylene and its metabolites for use in risk assessment. Environ. Health Perspect 2000, 108, 283–305. [Google Scholar]
- Clewell, H.J.; Gentry, P.R.; Kester, J.E.; Andersen, M.E. Evaluation of physiologically based pharmacokinetic models in risk assessment: an example with perchloroethylene. Crit. Rev. Toxicol 2005, 35, 413–433. [Google Scholar]
- Brown, R.P.; Delp, M.C.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar]
- Cowles, A.L.; Borgstedt, H.H.; Gillies, A.J. Tissue weights and rates of blood flow in man for the prediction of anesthetic uptake and distribution. Anesthesiology 1971, 3, 523–526. [Google Scholar]
- Corley, R.A.; Gordon, S.M.; Wallace, L.A. Physiologically based pharmacokinetic modeling of the temperature-dependent dermal absorption of chloroform by humans following bath water exposures. Toxicol. Sci 2000, 53, 13–23. [Google Scholar]
- Poet, T.S.; Weitz, K.K.; Gies, R.A. PBPK Modeling of the percutaneous absorption of perchloroethylene from a soil matrix in rats and humans. Toxicol. Sci 2002, 67, 17–31. [Google Scholar]
- Poet, T.S.; Weitz, K.K.; Gies, R.A.; Edwards, J.A.; Thrall, K.D.; Corley, R.A.; Tanojo, H.; Hui, X.; Maibach, H.I.; Wester, R.C. Assessment of the percutaneous absorption of trichloroethylene in rats and humans using MX/MX real-time breath analysis and physiologically based pharmacokinetic modeling. Toxicol. Sci 2000, 56, 42–51. [Google Scholar]
- Reitz, R.H.; Gargas, M.L.; Andersen, M.E.; Provan, W.F.; Green, T. Predicting cancer risk from vinyl chloride exposure with a physiologically based pharmacokinetic model. Toxicol. Appl. Pharmacol 1996, 137, 253–267. [Google Scholar]
- Fisher, J.; Mahle, D.; Bankston, L.; Greene, R.; Gearhart, J. Lactational transfer of volatile chemicals in breast milk. Am. Ind. Hyg. Assoc. J 1997, 58, 425–531. [Google Scholar]
- Brown, E.A.; Shelley, M.L.; Fisher, J.W. A pharmacokinetic study of occupational and environmental benzene exposure with regard to gender. Risk Anal 1998, 18, 205–213. [Google Scholar]
- Delic, J.I.; Lilly, P.D.; MacDonald, A.J.; Loizou, G.D. The utility of PBPK in the safety assessment of chloroform and carbon tetrachloride. Regul. Toxicol. Pharmacol 2000, 32, 144–155. [Google Scholar]
- Thomas, R.S.; Bigelow, P.L.; Keefe, T.J.; Yang, R.S.H. Variability in biological exposure indices using physiologically based pharmacokinetic modeling and Monte Carlo Simulation. Am. Ind. Hyg. Assoc. J 1996, 57, 23–32. [Google Scholar]
- Morgan, M.G.; Henrion, M. The propagation and analysis of uncertainty. In Uncertainty: A Guide to Dealing with Uncertainty and Quantitative Risk and Policy Analysis; Cambridge University Press: Cambridge, UK, 1990; pp. 172–219. [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Available Online: http://www.cdc.gov/exposurereport accessed on 18 June 2011.
- Chou, C.H.S.; Holler, J.; de Rosa, C.T. Minimal risk levels (MRLs) for hazardous substances. J. Clean Technol. Environ. Toxicol. Occup. Med 1998, 7, 1–24. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Minimal risk levels for priority substances and guidance for derivation. Fed. Regist. 1996, 61, 33511–33520.
| AUCr | MSSD | |||
|---|---|---|---|---|
| VOCs | Generic Model | Original Model | Generic Model | Original Model |
| BENa | 0.9 | 1.6 | 0.0008 | 0.0009 |
| CCl4b | 2.5 | 1.9 | 0.4515 | 0.2344 |
| DCMc | 1.1 | 1.1 | 3.8214 | 1.1722 |
| PCEc | 0.6 | 0.8 | 0.0805 | 0.0164 |
| TCEc | 0.8 | 0.8 | 0.0095 | 0.0089 |
| VCb | 1.2 | 1.1 | 0.1875 | 0.1831 |
| BEN+ | CCl4+ | DCM+ | PCE+ | TCE+ | VC+ | ||
|---|---|---|---|---|---|---|---|
| MRL * | 0.003/0.0005 | 0.03/0.007 | 0.6/0.2 | 0.3/0.06 | 0.2/0.05 | 2/0.2 | none |
| Exposure Duration | Chronic | Intermediate | Acute | Chronic | Acute | Acute | ---- |
| PBPK MODEL | Blood Concentration (ng/mL) | ||||||
| Predicted Peak | 0.04 | 0.40 | 18.12 | 6.70 | 10.76 | 111.65 | ---- |
| NHANES ** | Blood Concentration (ng/mL) | ||||||
| 0.260 (0.210–0.320) | <LOD | <LOD | 0.140 (0.091–0.300) | <LOD | ND ** | ||
| Limit of Detection (LOD) | 0.024 | 0.005 | 0.07 | 0.048 | 0.012 | ND | |
| Age group (years) | Males | Females | ||||
|---|---|---|---|---|---|---|
| * Urinary Cd (μg/g creatinine) | Cd Intake GM (μg/day) | * Urinary Cd (μg/g creatinine) | Cd Intake GM (μg/day) | |||
| Measured | Predicted | Measured | Predicted | |||
| 6–11 | 0.088 (0.071−0.11) | 0.101 (0.071−0.11) | 15.0 | 0.088 (0.072−0.108) | 0.172 (0.152−0.188) | 13.5 |
| 12–19 | 0.074 (0.066−0.083) | 0.087 (0.078−0.095) | 19.7 | 0.103 (0.089−0.118) | 0.163 (0.136−0.190) | 15.1 |
| 20–39 | 0.125 (0.114−0.137) | 0.137 (0.082−0.190) | 22.4 | 0.179 (0.159−0.202) | 0.285 (0.182−0.386) | 16.2 |
| 40–59 | 0.208 (0.184−0.234) | 0.214 (0.188−0.241) | 22.1 | 0.342 (0.305−0.383) | 0.427 (0.377−0.477) | 16.5 |
| ≥60 | 0.366 (0.324−0.414) | 0.226 (0.221−0.232) | 17.6 | 0.507 (0.460−0.558) | 0.453 (0.447−0.459) | 14.4 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruiz, P.; Ray, M.; Fisher, J.; Mumtaz, M. Development of a Human Physiologically Based Pharmacokinetic (PBPK) Toolkit for Environmental Pollutants. Int. J. Mol. Sci. 2011, 12, 7469-7480. https://doi.org/10.3390/ijms12117469
Ruiz P, Ray M, Fisher J, Mumtaz M. Development of a Human Physiologically Based Pharmacokinetic (PBPK) Toolkit for Environmental Pollutants. International Journal of Molecular Sciences. 2011; 12(11):7469-7480. https://doi.org/10.3390/ijms12117469
Chicago/Turabian StyleRuiz, Patricia, Meredith Ray, Jeffrey Fisher, and Moiz Mumtaz. 2011. "Development of a Human Physiologically Based Pharmacokinetic (PBPK) Toolkit for Environmental Pollutants" International Journal of Molecular Sciences 12, no. 11: 7469-7480. https://doi.org/10.3390/ijms12117469
APA StyleRuiz, P., Ray, M., Fisher, J., & Mumtaz, M. (2011). Development of a Human Physiologically Based Pharmacokinetic (PBPK) Toolkit for Environmental Pollutants. International Journal of Molecular Sciences, 12(11), 7469-7480. https://doi.org/10.3390/ijms12117469






