A Supramolecular Sensing Platform for Phosphate Anions and an Anthrax Biomarker in a Microfluidic Device
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication of the Sensing Platform and Anion Detection
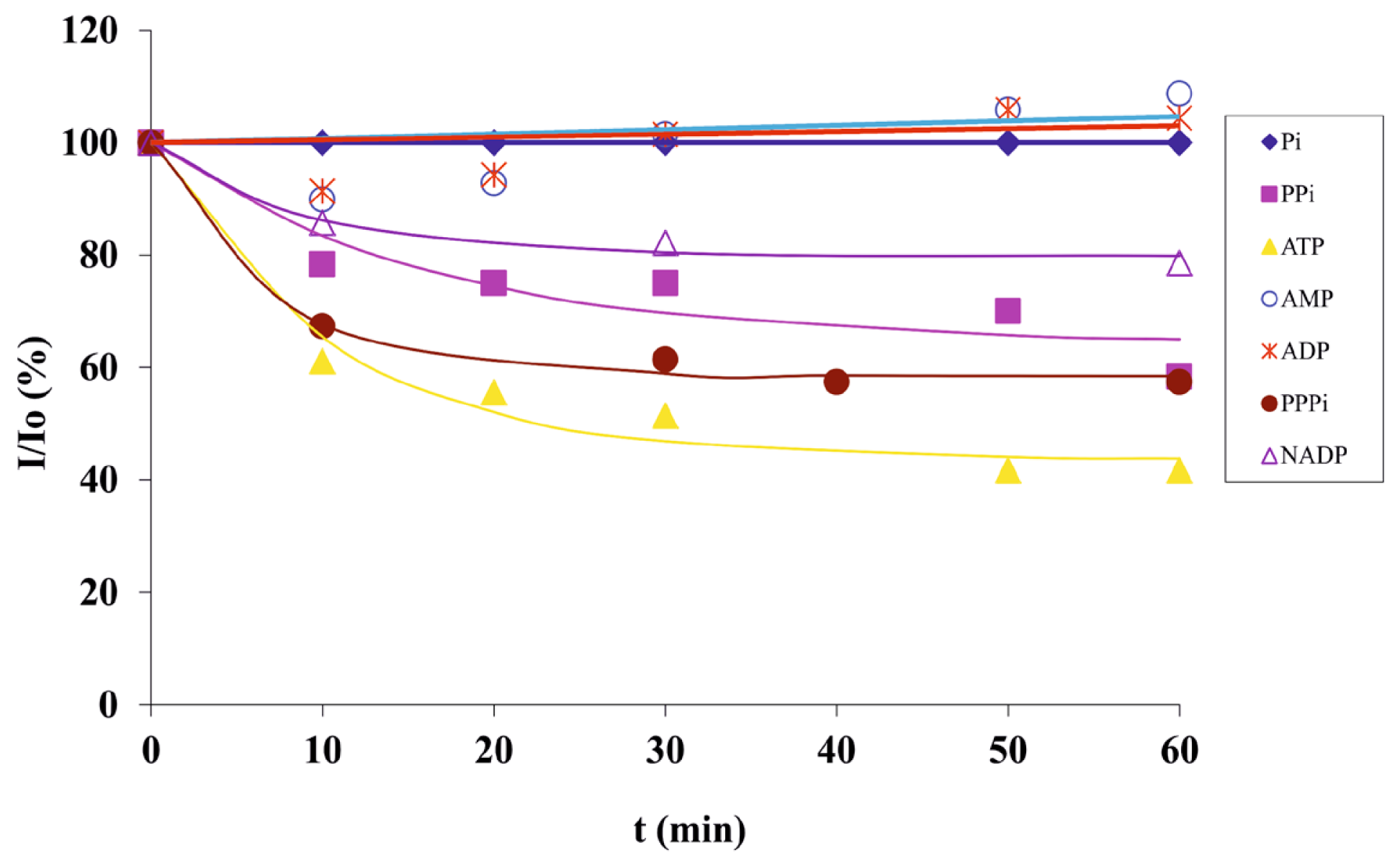
2.2. Sensing of Biologically Relevant Phosphates
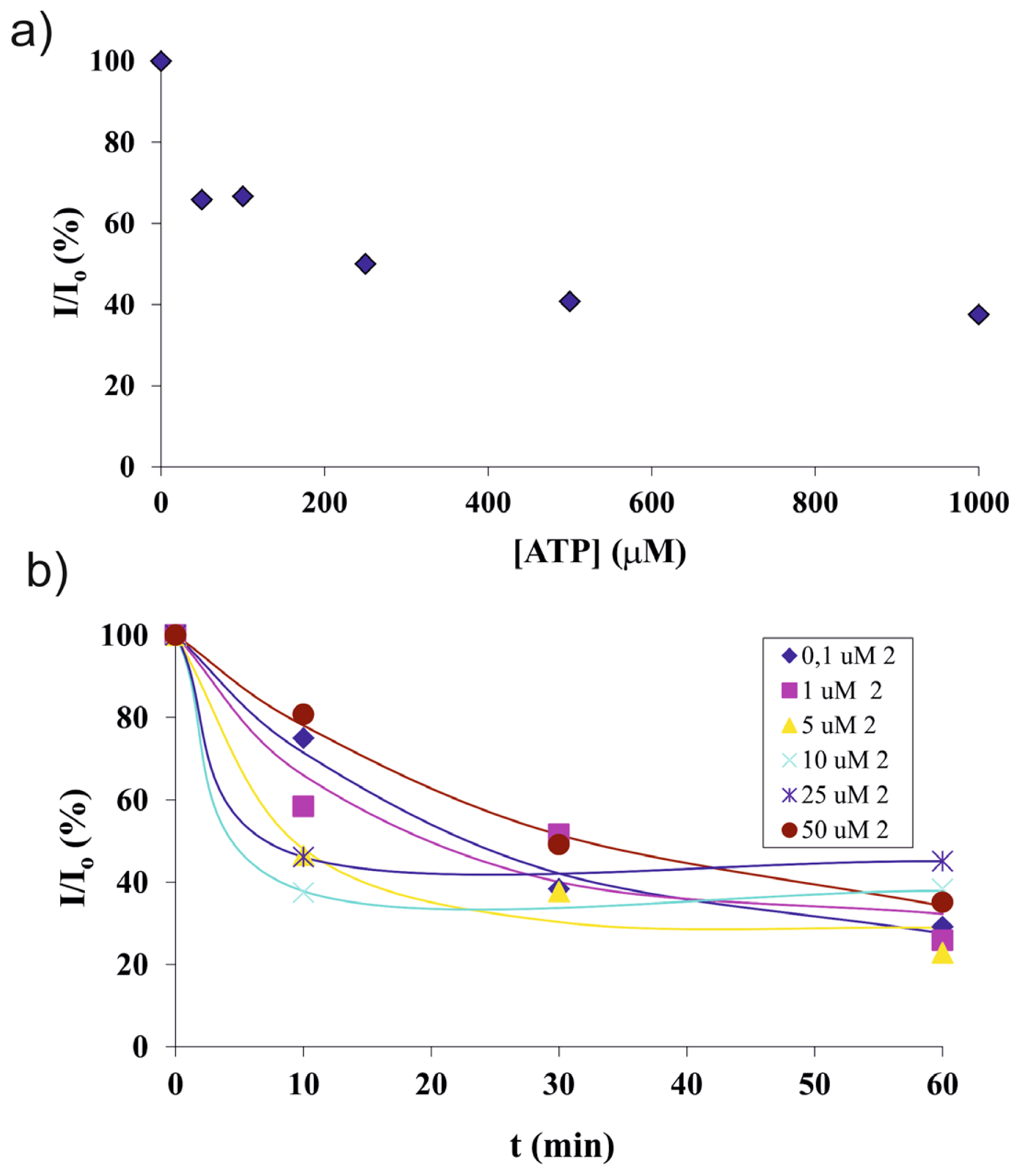
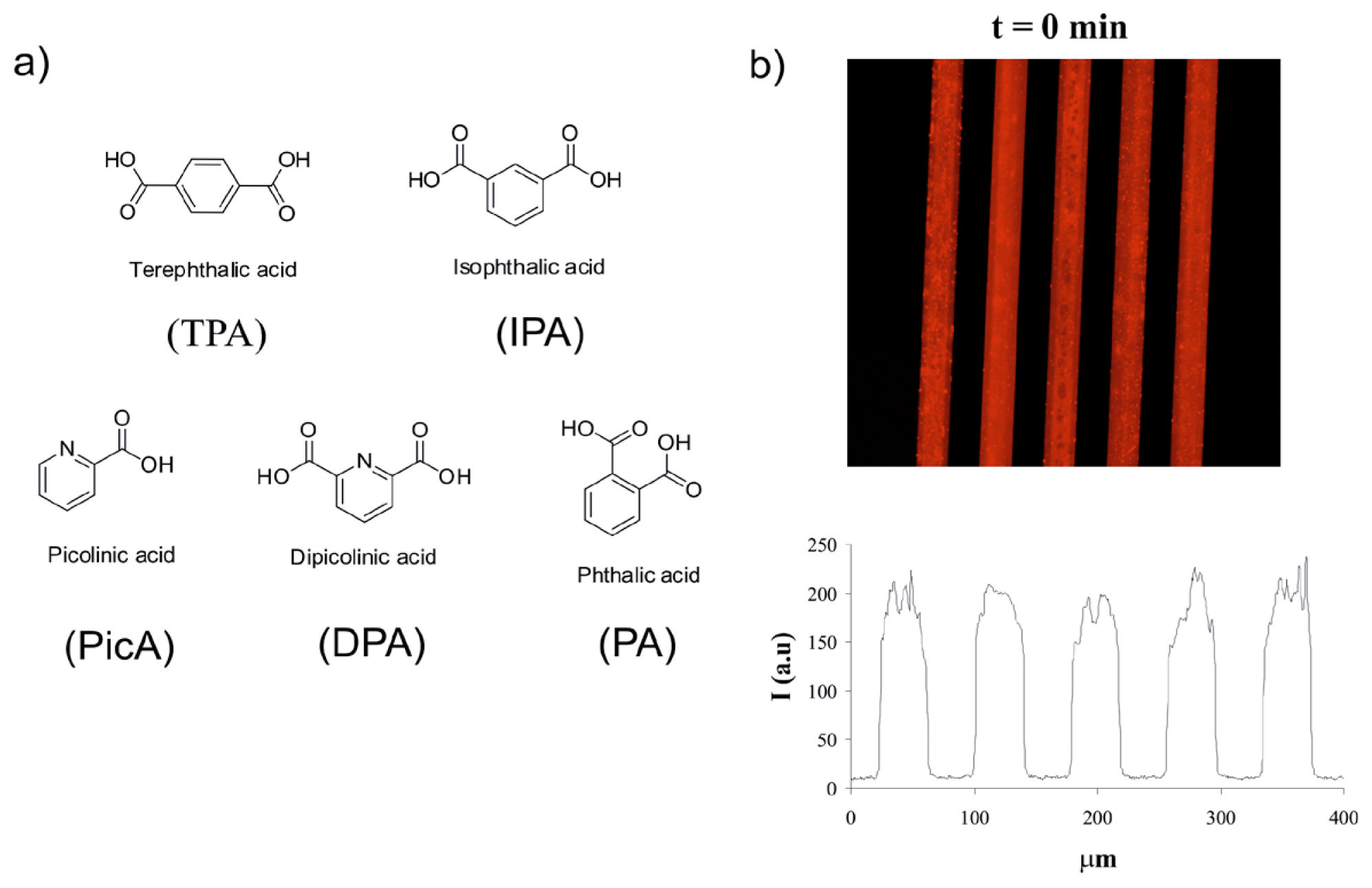
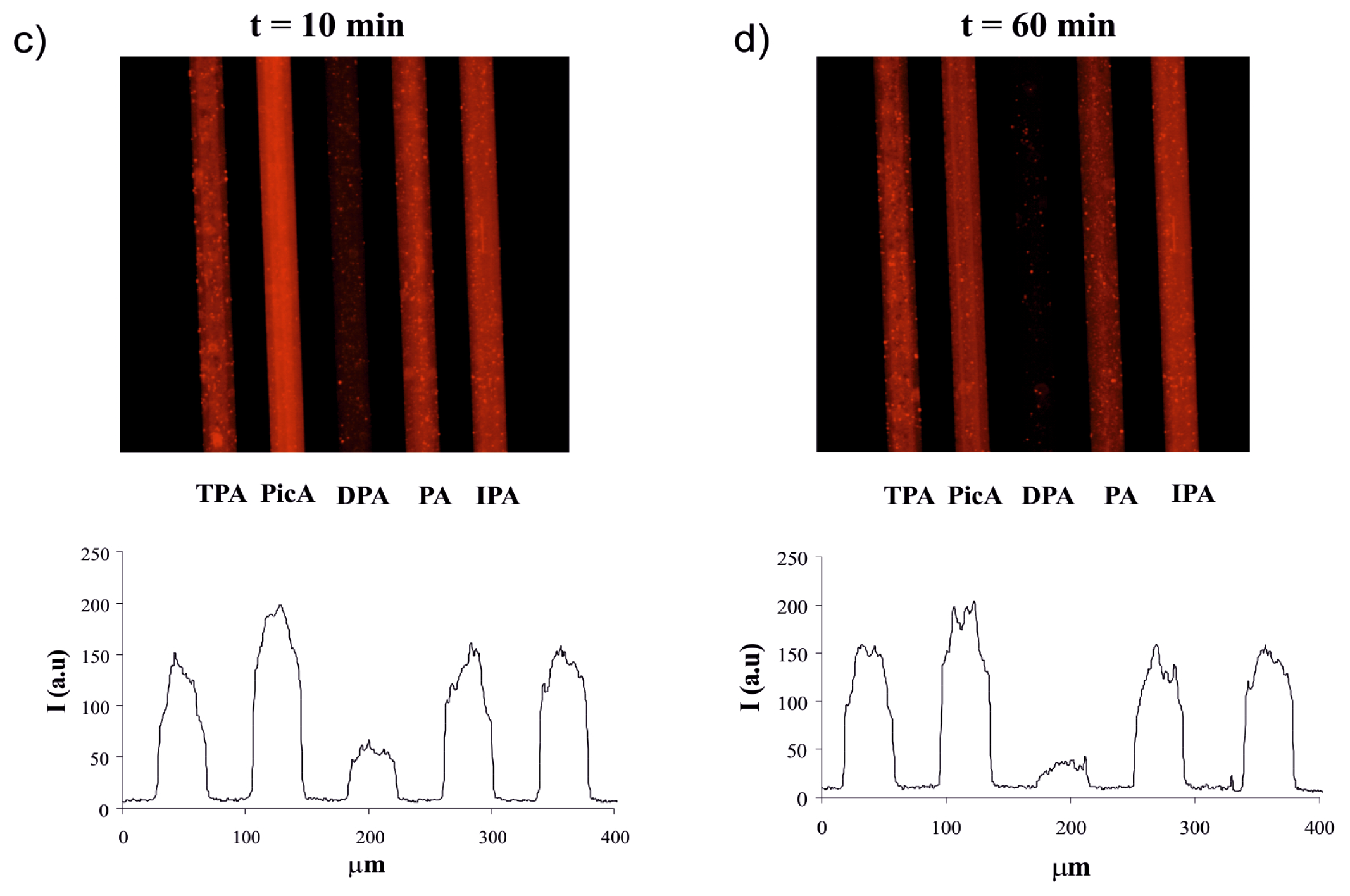
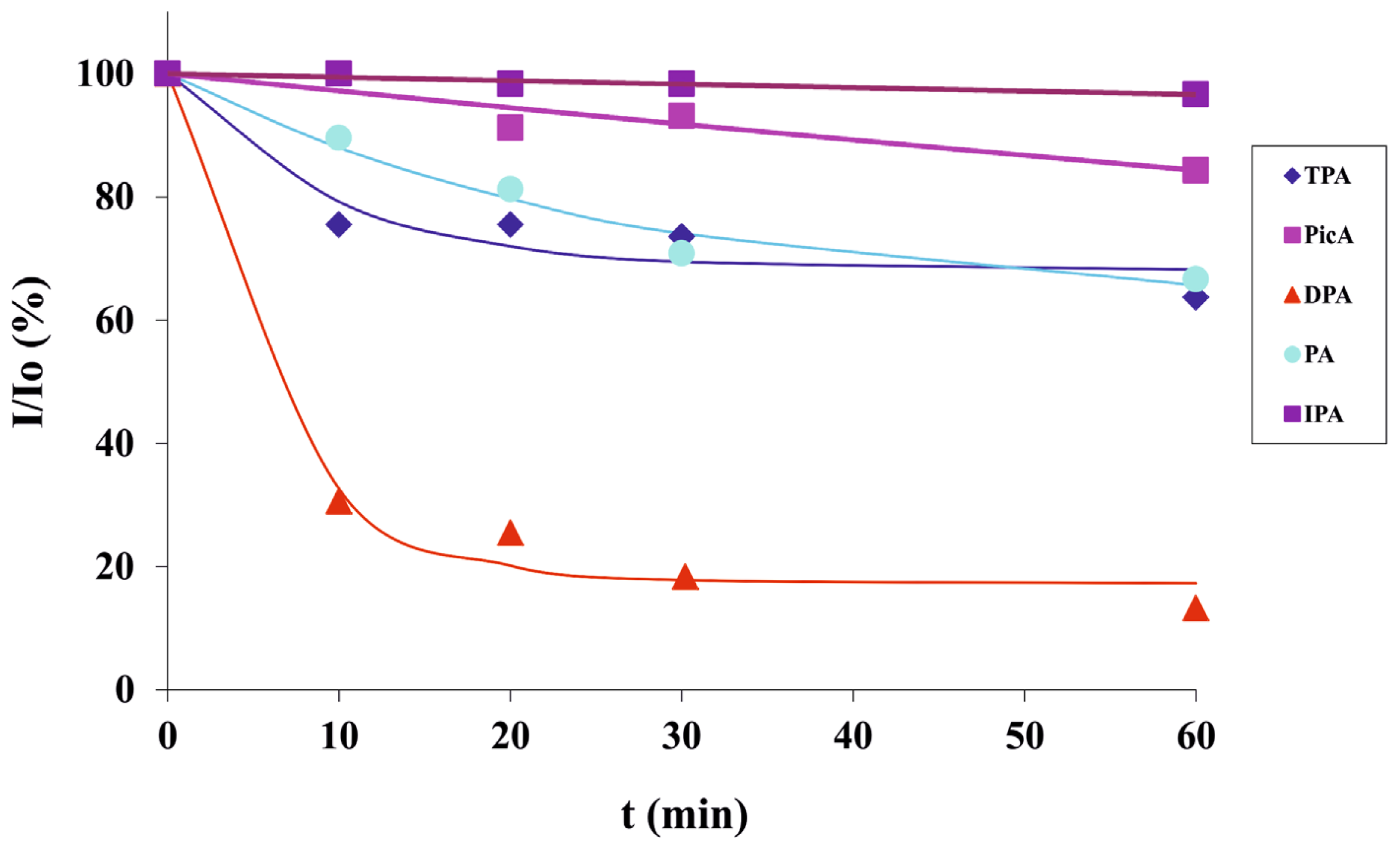
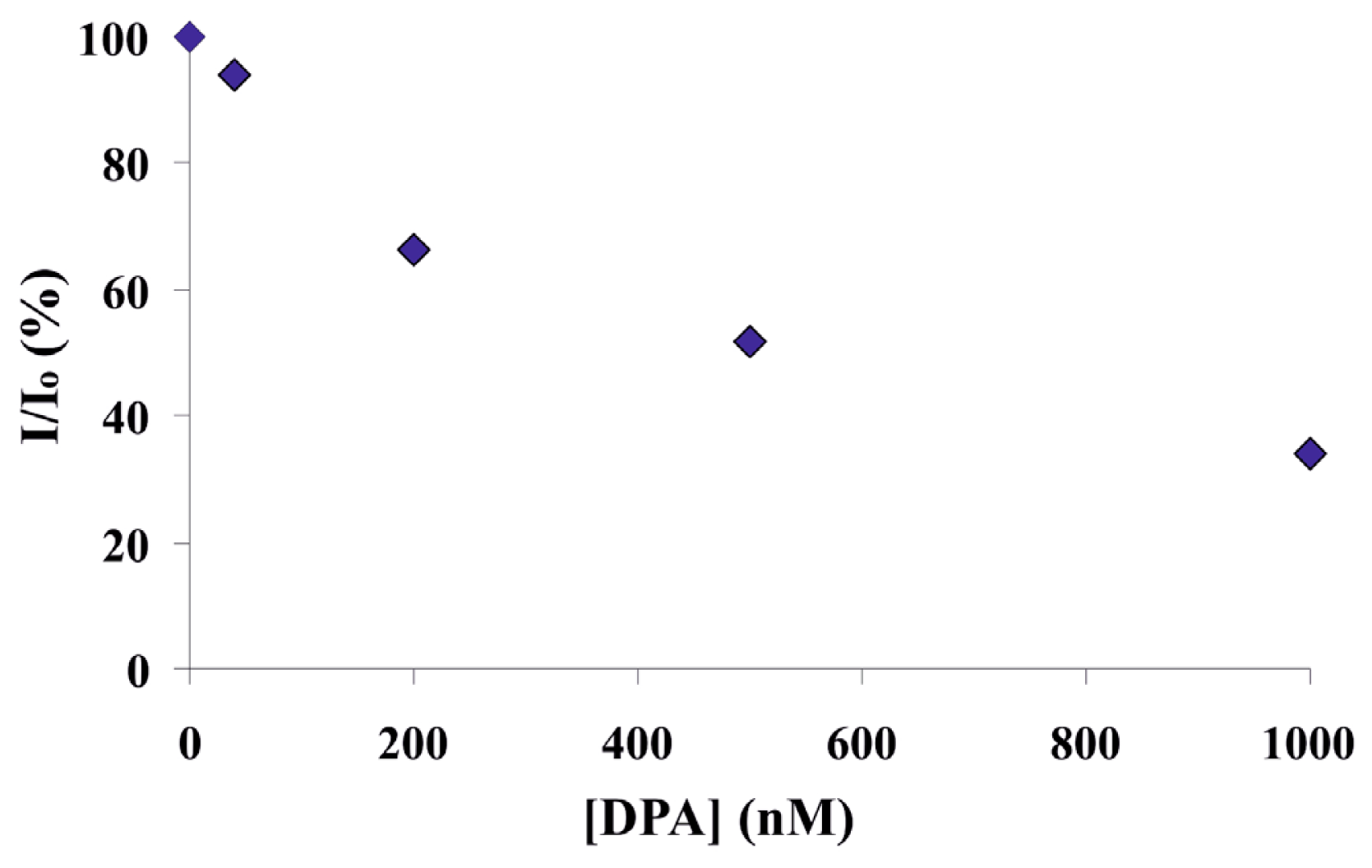
2.3. Screening of an Anthrax Biomarker and Potentially Interfering Anions
3. Experimental Section
3.1. Compounds
3.2. Microchip Fabrication Procedure
3.3. Surface Modification of the Microchips
3.4. Assembly of the Sensing Layer on the Microchip Surface
3.5. Microfluidic Fluorescent Sensing Experiments
4. Conclusions
Acknowledgments
References
- Jenkins, A.L.; Uy, O.M.; Murray, G.M. Polymer-Based Lanthanide Luminescent Sensor for Detection of the Hydrolysis Product of the Nerve Agent Soman in Water. Anal. Chem 1999, 71, 373–378. [Google Scholar]
- Puopolo, P.R.; Chamberlin, P.; Flood, J.G. Detection and confirmation of cocaine and cocaethylene in serum emergency toxicology specimens. Clin. Chem 1992, 38, 1838–1842. [Google Scholar]
- van der Schalie, W.H; Shedd, T.R.; Widder, M.W.; Brennan, L.M. Response characteristics of an aquatic biomonitor used for rapid toxicity detection. J. Appl. Toxicol. 2004, 24, 387–394. [Google Scholar]
- Rega, M.F.; Reed, J.C.; Pellecchia, M. Robust lanthanide-based assays for the detection of anti-apoptotic Bcl-2-family protein antagonists. Bioorg. Chem 2007, 35, 113–120. [Google Scholar]
- Hajduk, P.J.; Gerfin, T.; Boehlen, J.M.; Haberli, M.; Marek, D.; Fesik, S.W. High-Throughput Nuclear Magnetic Resonance-Based Screening. J. Med. Chem 1999, 42, 2315–2317. [Google Scholar]
- Huestis, M.A; Smith, ML. Modern analytical technologies for the detection of drug abuse and doping. 2006, 3, 49–57. [Google Scholar]
- Fu, E.; Chinowsky, T.; Nelson, K.; Johnston, K.; Edwards, T.; Helton, K.; Grow, M.; Miller, J.W.; Yager, P. SPR imaging-based salivary diagnostics system for the detection of small molecule analytes. Ann. NY Acad. Sci 2007, 1098, 335–344. [Google Scholar]
- Smith, A.M.; Dave, S.; Nie, S.; True, L.; Gao, X. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev. Mol. Diagn 2006, 6, 232–244. [Google Scholar]
- Haas, P.; Then, P.; Wild, A.; Grange, W.; Zorman, S.; Hegner, M.; Calame, M.; Aebi, U.; Flammer, J.; Hecht, B. Fast quantitative single-molecule detection at ultralow concentrations. Anal. Chem 2010, 82, 6299–6302. [Google Scholar]
- Lester, E.; Ponce, A. An anthrax “smoke” detector. Online monitoring of aerosolized bacterial spores. IEEE Eng. Med. Biol 2002, 38–42. [Google Scholar]
- Lester, E.; Ponce, A. A second-generation anthrax “smoke” detector. IEEE Eng. Med. Biol 2004, 130–135. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic and Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Narayanaswamy, R.; Wolfbeis, O.S. (Eds.) Springer Series on Chemical Sensors and Biosensors; Springer: Berlin, Germany, 2004; Volume 1.
- Martinez–Manez, R.; Sancenon, F. New advances in fluorogenic anion chemosensors. J. Fluoresc 2005, 15, 267–285. [Google Scholar]
- Martinez–Manez, R.; Sancenon, F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev 2003, 103, 4419–4476. [Google Scholar]
- Niikura, K.; Metzger, A.; Anslyn, E.V. Chemosensor ensemble with selectivity for inositol-trisphosphate. J. Am. Chem. Soc 1998, 120, 8533–8534. [Google Scholar]
- Metzger, A.; Anslyn, E.V. A Chemosensor for Citrate in Beverages. Chem. Int. Ed 1998, 37, 649–652. [Google Scholar]
- Murray, N.S.; Jarvis, S.P.; Gunnlaugsson, T. Luminescent self-assembly formation on a gold surface observed by reversible “off–on” switching of Eu(III) emission. Chem. Commun 2009, 33, 4959–4961. [Google Scholar]
- Massue, J.; Quinn, S.J.; Gunnlaugsson, T. Lanthanide luminescent displacement assays: the sensing of phosphate anions using Eu(III)-cyclen-conjugated gold nanoparticles in aqueous solution. J. Am. Chem. Soc 2008, 130, 6900–6901. [Google Scholar]
- Cable, M.L.; Kirby, J.P.; Sorasaenee, K.; Gray, H.B.; Ponce, A. bacterial spore detection by [Tb3+(macrocycle)(dipicolinate)] luminescence. J. Am. Chem. Soc 2007, 129, 1474–1475. [Google Scholar]
- Ai, K.; Zhang, B.; Lu, L. Europium-Based fluorescence nanoparticle sensor for rapid and ultrasensitive detection of an anthrax biomarker. Angew. Chem. Int. Ed 2009, 48, 304–308. [Google Scholar]
- Yilmaz, M.D.; Hsu, S.; Reinhoudt, D.N.; Velders, A.H.; Huskens, J. Ratiometric fluorescent detection of an anthrax biomarker at molecular printboards. Angew. Chem. Int. Ed 2010, 49, 5938–5941. [Google Scholar]
- Leonard, J.P.; Nolan, C.B.; Stomeo, F.; Gunnlaugsson, T. Photochemistry and photophysics of coordination compounds: lanthanides. Top. Curr. Chem 2007, 281, 1–43. [Google Scholar]
- Leonard, J.P.; Jensen, P.; McCabe, T.; O’Brien, J.E.; Peacock, R.D.; Kruger, P.E.; Gunnlaugsson, T. Self-assembly of chiral luminescent lanthanide coordination bundles. J. Am. Chem. Soc 2007, 129, 10986–10987. [Google Scholar]
- Gunnlaugsson, T.; Stomeo, F. Recent advances in the formation of luminescent lanthanide architectures and self-assemblies from structurally defined ligands. Org. Biomol. Chem 2007, 5, 1999–2009. [Google Scholar]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev 2009, 109, 4283–4374. [Google Scholar]
- Crooks, R.M.; Ricco, A.J. New Organic Materials Suitable for Use in Chemical Sensor Arrays. Acc. Chem. Res 1998, 31, 219–227. [Google Scholar]
- Kaifer, A.E. Functionalized Self-Assembled Monolayers Containing Preformed Binding Sites. Isr. J. Chem 1996, 36, 389–397. [Google Scholar]
- Zimmerman, R.; Basabe–Desmonts, L.; van der Baan, F.; Reinhoudt, D.N.; Crego-Calama, M. A combinatorial approach to surface-confined cation sensors in water. J. Mater. Chem 2005, 15, 2772–2777. [Google Scholar]
- Kim, Y.R.; Kim, H.J.; Kim, J.S.; Kim, H. Rhodamine-Based “Turn-On” Fluorescent chemodosimeter for Cu(II) on ultrathin platinum films as molecular switches. Adv. Mater 2008, 20, 4428–4432. [Google Scholar]
- Flink, S.; van Veggel, F.C.J.M.; Reinhoudt, D.N. Sensor functionalities in self-assembled monolayers. Adv. Mater 2000, 12, 1315–1328. [Google Scholar]
- van der Veen, N.J.; Flink, S.; Deij, M.A.; Egberink, R.J.M.; van Veggel, F.C.J.M.; Reinhoudt, D.N. Monolayers of a Na+ selective fluoroionophore on glass: connecting the fields of monolayers and optical detection of metal ions. J. Am. Chem. Soc 2000, 122, 6112–6113. [Google Scholar]
- Crego-Calama, M.; Reinhoudt, D.N. New materials for metal ion sensing by self-assembled monolayers on glass. Adv. Mater 2001, 13, 1171–1174. [Google Scholar]
- Basabe-Desmonts, L.; Beld, J.; Zimmerman, R.S.; Hernando, J.; Mela, P.; García-Parajó, M.F.; van Hulst, N.F.; van den Berg, A.; Reinhoudt, D.N.; Crego-Calama, M. A simple approach to sensor discovery and fabrication on self-assembled monolayers on glass. J. Am. Chem. Soc 2004, 126, 7293–7299. [Google Scholar]
- Rudzinski, C.M.; Young, A.M.; Nocera, D.G. A supramolecular microfluidic optical chemosensor. J. Am. Chem. Soc 2002, 124, 1723–1727. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed; W.H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Ojida, A.; Takashima, I.; Kohira, T.; Nonaka, H.; Hamachi, I. Turn-on fluorescence sensing of nucleside polyphosphates using a xanthene-based Zn(II) complex chemosensor. J. Am. Chem. Soc 2008, 130, 12095–12101. [Google Scholar]
- Beer, P.D.; Gale, P.A. Anion recognition and sensing: the state of the art and future perspectives. Angew. Chem. Int. Ed 2001, 40, 486–516. [Google Scholar]
- Li, Q.; Dasgupta, P.K.; Temkin, H. Airborne bacterial spore counts by terbium-enhanced luminescence detection: pitfalls and real values. Environ. Sci. Technol 2008, 42, 2799–2804. [Google Scholar]
- Henderson, D.A. The looming threat of bioterrorism. Science 1999, 283, 1279–1282. [Google Scholar]
- Enserink, M. Biodefense hampered by inadequate tests. Science 2001, 294, 1266–1267. [Google Scholar]
- Yung, P.T.; Lester, E.D.; Bearman, G.; Ponce, A. An automated front-end monitor for anthrax surveillance systems based on the rapid detection of airborne endospores. Biotechnol. Bioeng 2007, 98, 864–871. [Google Scholar]
- Bailey, G.F.; Karp, S.; Sacks, L.E. Ultraviolet-Absorption spectra of dry bacterial spores. J. Bacteriol 1965, 89, 984. [Google Scholar]
- Walt, D.R.; Franz, D.R. Biological warfare detection. Anal. Chem 2000, 72, 738a–746a. [Google Scholar]
- Rode, L.J.; Foster, J.W. Germination of bacterial spores by long-chain alkyl amines. Nature 1960, 188, 1132–1134. [Google Scholar]
- Rosen, D.L. Bacterial endospore detection using photoluminescence from terbium dipicolinate. Rev. Anal. Chem 1999, 18, 1–21. [Google Scholar]
- Mela, P.; Onclin, S.; Goedbloed, M.H.; Levi, S.; García-Parajó, M.F.; van Hulst, N.F.; Ravoo, B.J.; Reinhoudt, D.N.; van den Berg, A. Monolayer-functionalized microfluidics devices for optical sensing of acidity. Lab Chip 2005, 5, 163–170. [Google Scholar]
- Vilkner, T.; Janasek, D.; Manz, A. Micro total analysis systems. Recent developments. Anal. Chem 2004, 7612, 3373–3385. [Google Scholar]
- Andersson, H.; van den Berg, A. Microfluidic devices for cellomics: a review. Sens. Actuator. B 2003, 92, 315–325. [Google Scholar]
- Fan, H.Y.; Lu, F.Y.; Stump, A.; Reed, S.T.; Baer, T.; Schunk, R.; Perez–Luna, V.; Lopez, G.P.; Brinker, C.J. Rapid prototyping of patterned functional nanostructures. Nature 2000, 405, 56–60. [Google Scholar]
- Ludden, M.J.W.; Reinhoudt, D.N.; Huskens, J. Molecular printboards: versatile platforms for the creation and positioning of supramolecular assemblies and materials. Chem. Soc. Rev 2006, 35, 1122–1134. [Google Scholar]
- Metzger, A.; Lynch, V.M.; Anslyn, E.V. A synthetic receptor selective for citrate. Angew. Chem. Int. Ed 1997, 36, 862–865. [Google Scholar]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar]
- Shao, N.; Jin, J.; Wang, G.; Zhang, Y.; Yang, R.; Yuan, J. Europium(III) complex-based luminescent sensing probes for multi-phosphate anions: modulating selectivity by ligand choice. Chem. Commun 2008, 1127–1129. [Google Scholar]
- Charbonnière, L.J.; Schurhammer, R.; Mameri, S.; Wipff, G.; Ziessel, R.F. Photo-physical Impact of phosphorylated anions associated to lanthanide complexes in water. Inorg. Chem 2005, 44, 7151–7160. [Google Scholar]
- Shanbhag, S.M.; Choppin, G.R. Thermodynamics of Ln(III) complexation with AMP and ATP. Inorg. Chim. Acta 1987, 139, 119–120. [Google Scholar]
- Kanekiyo, Y.; Naganawa, R.; Tao, H. Fluorescence detection of ATP based on the ATP-mediated aggregation of pyrene-appended boronic acid on a polycation. Chem Commun 2004, 1006–1007. [Google Scholar]
- Bazzicalupi, C.; Biagini, S.; Bencini, A.; Faggi, E.; Giorgi, C.; Matera, I.; Valtancoli, B. ATP Recognition and sensing with a phenanthroline-containing polyammonium receptor. Chem. Commun 2006, 4087–4089. [Google Scholar]
- Zyryanov, G.V.; Palacios, M.A.; Anzenbacher, P., Jr. Rational design of a fluorescence-turn-on sensor array for phosphates in blood serum. Angew. Chem. Int. Ed. 2007, 46, 7849–7852. [Google Scholar]
- Kirby, J.P.; Cable, M.L.; Levine, D.J.; Gray, H.B.; Ponce, A. Spectroscopic analysis of ligand binding to lanthanide-macrocycle platforms. Anal. Chem 2008, 80, 5750–5754. [Google Scholar]
- Wu, S.L.; Horrocks, W.D., Jr. General method for the determination of stability constants of lanthanide ion chelates by ligand-ligand competition: laser-excited Eu3+ luminescence excitation spectroscopy. Anal. Chem. 1996, 68, 394–401. [Google Scholar]
- Vaid, A.; Bishop, A.H. The destruction by microwave radiation of bacterial endospores and amplification of the released DNA. J. Appl. Microbiol 1998, 85, 115–122. [Google Scholar]
- Ashton, P.R.; Königer, R.; Stoddart, J.F.; Alker, D.; Harding, V.D. Amino acid derivatives of β-cyclodextrin. J. Org. Chem 1996, 61, 903–908. [Google Scholar]
- Hsu, S.H.; Yilmaz, M.D.; Blum, C.; Subramaniam, V.; Reinhoudt, D.N.; Velders, A.H.; Huskens, J. Expression of sensitized Eu3+ luminescence at a multivalent interface. J. Am. Chem. Soc 2009, 131, 12567–12569. [Google Scholar]
- Yuan, J.; Matsumoto, K. Fluorescence enhancement by electron-withdrawing groups on-Diketones in Eu(lIl)-diketonato-topo Ternary Complexes. Anal. Sci 1996, 12, 31–36. [Google Scholar]
- Gardeniers, J.G.E.; Oosterbroek, R.E.; van den Berg, A. Silicon and glass micromachining for μTAS. In Lab-on-A-Chip: Miniaturized Sytems for (Bio)Chemical Analysis and Synthesis; Oosterbroek, R.E., van den Berg, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 37–64. [Google Scholar]
- Wensink, H.; Elwenspoek, M.C. A closer look at the ductile–brittle transition in solid particle erosion. Wear 2002, 253, 1035–1043. [Google Scholar]
- Ludden, M.J.W.; Ling, X.Y.; Gang, T.; Bula, W.P.; Gardeniers, H.J.G.E.; Reinhoudt, D.N.; Huskens, J. Molecular printboards inside microfluidic devices: small molecule and antibody recognition. Chem. Eur. J 2008, 14, 136–142. [Google Scholar]



© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eker, B.; Yilmaz, M.D.; Schlautmann, S.; Gardeniers, J.G.E.; Huskens, J. A Supramolecular Sensing Platform for Phosphate Anions and an Anthrax Biomarker in a Microfluidic Device. Int. J. Mol. Sci. 2011, 12, 7335-7351. https://doi.org/10.3390/ijms12117335
Eker B, Yilmaz MD, Schlautmann S, Gardeniers JGE, Huskens J. A Supramolecular Sensing Platform for Phosphate Anions and an Anthrax Biomarker in a Microfluidic Device. International Journal of Molecular Sciences. 2011; 12(11):7335-7351. https://doi.org/10.3390/ijms12117335
Chicago/Turabian StyleEker, Bilge, Mahmut Deniz Yilmaz, Stefan Schlautmann, Johannes G. E. Gardeniers, and Jurriaan Huskens. 2011. "A Supramolecular Sensing Platform for Phosphate Anions and an Anthrax Biomarker in a Microfluidic Device" International Journal of Molecular Sciences 12, no. 11: 7335-7351. https://doi.org/10.3390/ijms12117335
APA StyleEker, B., Yilmaz, M. D., Schlautmann, S., Gardeniers, J. G. E., & Huskens, J. (2011). A Supramolecular Sensing Platform for Phosphate Anions and an Anthrax Biomarker in a Microfluidic Device. International Journal of Molecular Sciences, 12(11), 7335-7351. https://doi.org/10.3390/ijms12117335




