Comparative Kinetic Study and Microwaves Non-Thermal Effects on the Formation of Poly(amic acid) 4,4′-(Hexafluoroisopropylidene)diphthalic Anhydride (6FDA) and 4,4′-(Hexafluoroisopropylidene)bis(p-phenyleneoxy)dianiline (BAPHF). Reaction Activated by Microwave, Ultrasound and Conventional Heating
Abstract
:1. Introduction
2. Experimental
2.1. Materials
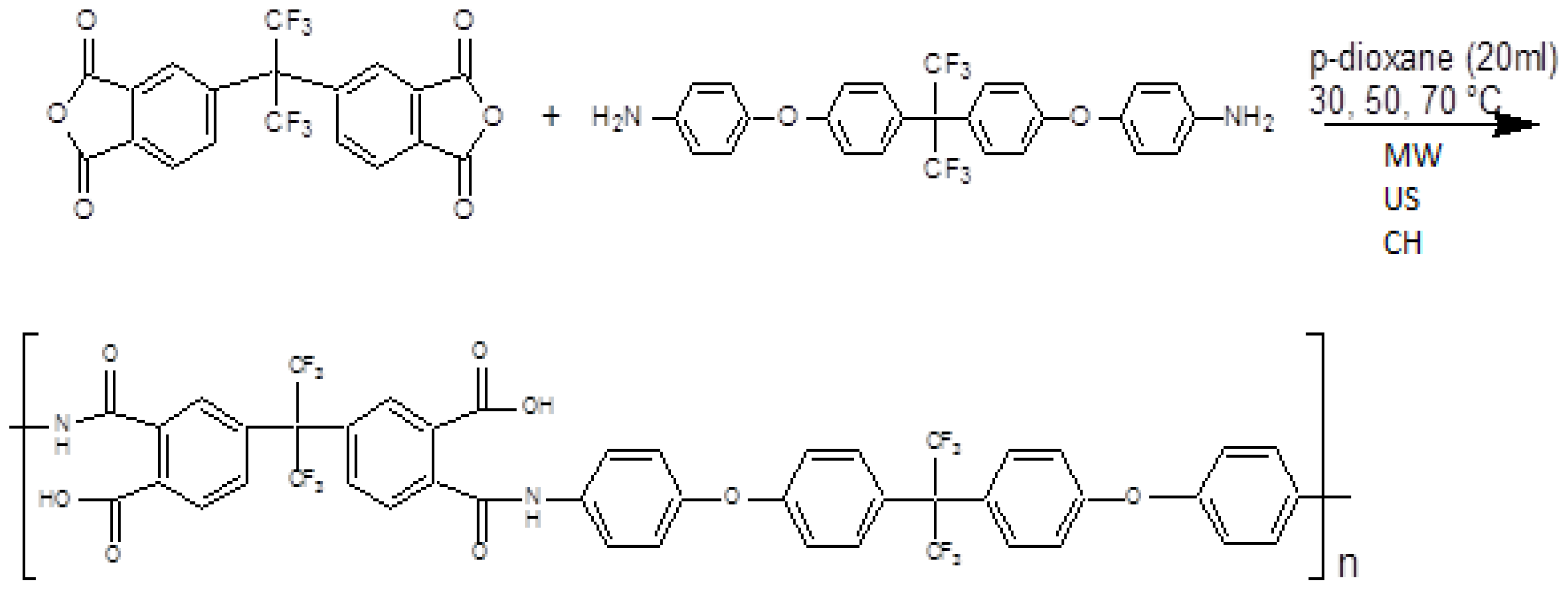
2.2. General Procedure of Polymerization Reaction
2.3. Methods of Polymer Characterization
- Fourier transform infrared spectra (FTIR) of our polymer samples were recorded in a FTIR-Spectrum 400 Perkin-Elmer instrument.
- The solubility of poly(amic acid) samples was studied in nine different solvents dissolving approximately 10 mg polymer sample in three drops of each solvent at constant temperature of 25 °C.
3. Results and Discussion
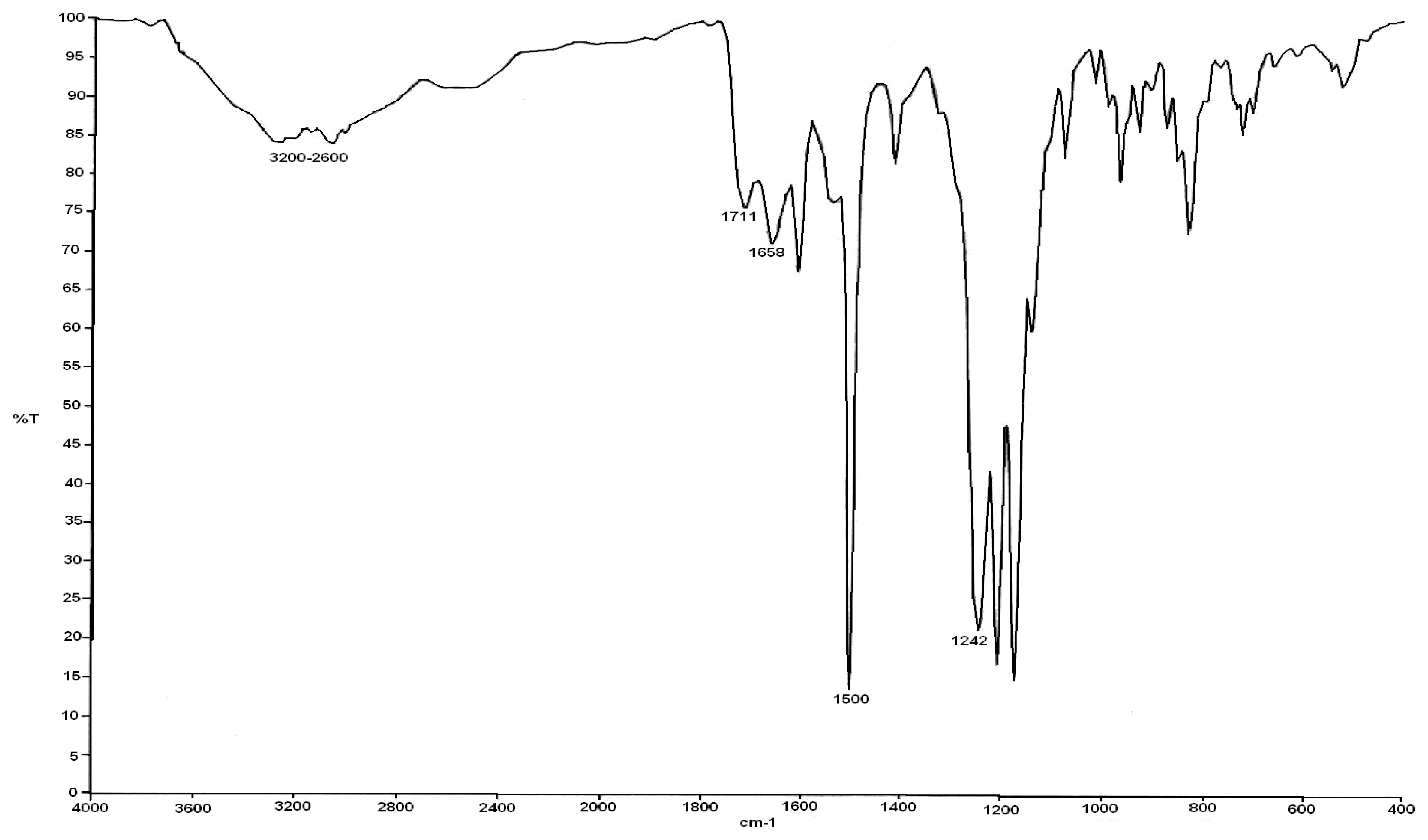
3.1. FTIR Poly (Amic Acid) Characterization
3.2. Solubility
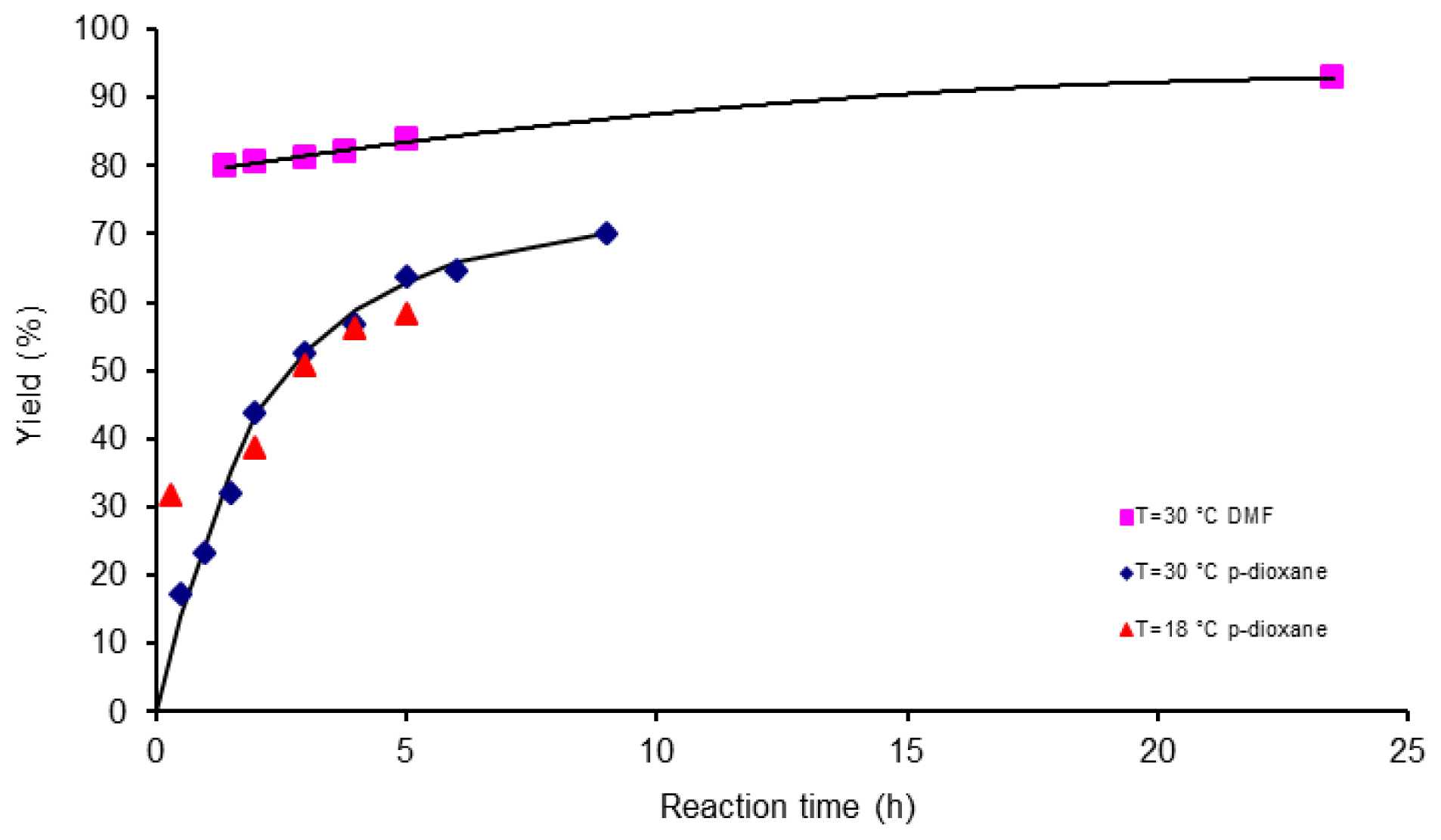
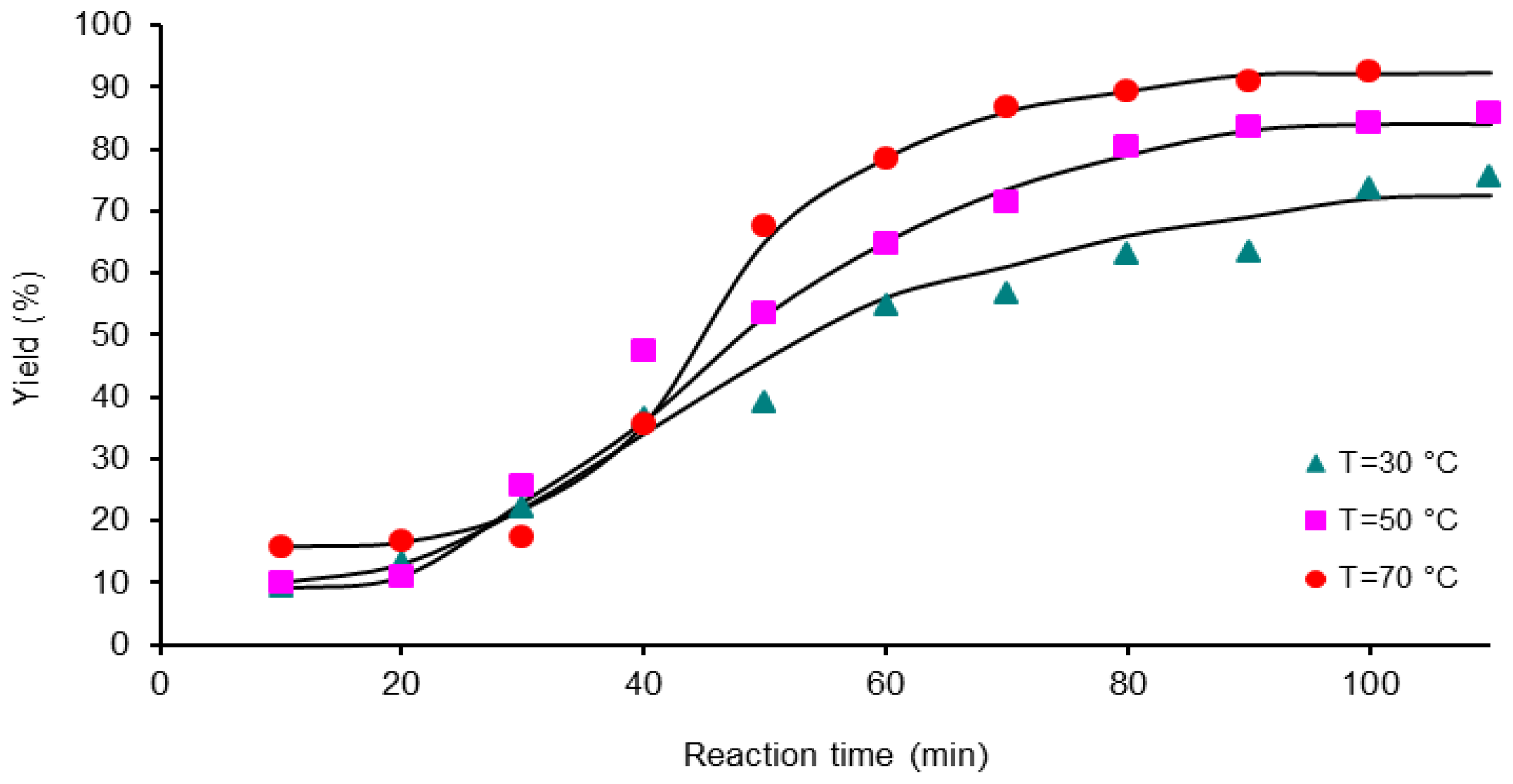
3.3. Polymerization
4. Conclusions
Acknowledgements
References
- Ba, C; Langer, J; Economy, J. Chemical modification of P84 copolyimide membranes by polyethylenimine for nanofiltration. J. Membr. Sci 2009, 327, 49–58. [Google Scholar]
- Hosseini, SS; Chung, TS. Carbon membranes from blends of PBI and polyimides for N2/CH4 and CO2/CH4 separation and hydrogen purification. J. Membr. Sci 2009, 328, 174–185. [Google Scholar]
- Hu, Z; Yin, Y; Okamoto, KI; Moriyama, Y; Morikawa, A. Synthesis and characterization of sulfonated polyimides derived from 2,2 -bis(4-sulfophenyl)-4,4′-oxydianiline as polymer electrolyte membranes for fuel cell applications. J. Membr. Sci 2009, 329, 146–152. [Google Scholar]
- Sun, H; Huo, H; Nie, H; Yang, S; Fan, L. Phenylethynyl terminated oligoimides derived from 3,3′,4,4′-diphenylsulfonetetracarboxylic dianhydride and their adhesive properties. Eur. Polym. J 2009, 45, 1169–1178. [Google Scholar]
- Zachmann, H; Heinker, S; Braun, A; Mudryi, AV; Gremenok, VF; Ivaniukovich, AV; Yakushev, MV. Characterisation of Cu(In,Ga)Se2-based thin film solar cells on polyimide. Thin Solid Films 2009, 517, 2209–2212. [Google Scholar]
- Wang, J; Hu, Y; Li, B; Gui, Z; Chen, Z. Preparation of polyacrylamide and gamma-zirconium phosphate nanocomposites by intercalative polymerization. Ultrason. Sonochem 2004, 11, 301–306. [Google Scholar]
- Martins, MAP; Pereira, C; Cunico, W; Moura, S; Rosa, FA; Peres, RL; Machado, P; Zanatta, N; Bonacorso, HG. Ultrasound promoted synthesis of 5-hydroxy-5-trihalomethyl-4,5- dihydroisoxazoles and β-enamino trihalomethyl ketones in water. Ultrason. Sonochem 2006, 13, 364–370. [Google Scholar]
- Ragaini, V; Guaita, C; Pirola, C. The beneficial influence of ultrasound in the polymerization of ɛ-caprolactam to polyamide-6 (Nylon 6). Part I: Primary experimental results. Ultrason. Sonochem 2007, 14, 680–688. [Google Scholar]
- Ragaini, V; Pirola, C; Rocco, G; Guaita, C. The beneficial influence of ultrasound in the polymerization of ɛ-caprolactam to polyamide-6 (Nylon 6). Part II: Additional experiment to understand the “pre-sonication effect”. Ultrason. Sonochem 2007, 14, 689–694. [Google Scholar]
- Teo, BM; Prescot, SW; Ashokkumar, M; Grieser, F. Ultrasound initiated miniemulsion polymerization of methacrylate monomers. Ultrason. Sonochem 2008, 15, 89–94. [Google Scholar]
- Tang, QL; Wang, KW; Zhu, YJ; Chen, F. Single-step rapid microwave-assisted synthesis of polyacrylamide–calcium phosphate nanocomposites in aqueous solution. Mater. Lett 2009, 63, 1332–1334. [Google Scholar]
- Sen, G; Kumar, R; Ghosh, S; Pal, S. A novel polymeric flocculant based on polyacrylamide grafted carboxymethylstarch. Carbohydr. Polym 2009, 77, 822–831. [Google Scholar]
- Mallakpour, S; Dinari, M. Soluble new optically active polyamides derived from 5-(4-methyl-2-phthalimidylpentanoylamino)isophthalic acid and different diisocyanates under microwave irradiation in molten ionic liquid. J. Appl. Polym. Sci 2009, 112, 244–253. [Google Scholar]
- Mallakpour, S; Rafiee, Z. Expeditious synthesis of novel aromatic polyamides from 5-[3-phenyl- 2-(9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximido) propanoylamino] isophthalic acid and various diamines using microwave-assisted polycondensation. React. Funct. Polym 2009, 69, 252–258. [Google Scholar]
- Carretero, P; Sandin, R; Mercier, R; Lozano, AE; Campa, JG; De Abajo, J. Microwave-Induced Synthesis of Aromatic Polyamides by the Phosphorylation Reaction. Aust. J. Chem 2009, 62, 250–253. [Google Scholar]
- Costa, C; Santos, AF; Fortuny, M; Araújo, PHH; Sayer, C. Kinetic advantages of using microwaves in the emulsion polymerization of MMA. Mater. Sci. Eng 2009, 29, 415–419. [Google Scholar]
- Imai, Y; Nemoto, H; Kakimoto, MA. A new facile and rapid synthesis of aliphatic polypyromellitimides by microwave-assisted polycondensation of salt monomers composed of aliphatic diamines and pyromellitic acid. J. Polym. Sci. Pol. Chem 1996, 34, 701–704. [Google Scholar]
- Imai, Y. Recent advances in synthesis of high-temperature aromatic polymers. React. Funct. Polym 1996, 30, 3–15. [Google Scholar]
- Lu, JM; Ji, SJ; Chen, NY; Sung, ZR; Zhu, XL; Shi, WP; Wang, ZG. Third-order nonlinear optical properties of polyureas and polyimide synthesized by microwave irradiation. J. Appl. Polym. Sci 2003, 89, 2611–2617. [Google Scholar]
- Li, N; Lu, J; Yao, S. Synthesis and optical properties of a new series of side-chain poly(amic acid)s with p-π conjugation. Macromol. Chem. Phys 2005, 206, 559–565. [Google Scholar]
- Wu, G; Yao, CH; Qiu, F. Synthesis, characterization and non-linear optical properties of polyimide and polyimide/silica hybrid materials. Iran. Polym. J 2010, 19, 651–660. [Google Scholar]
- Mallakpour, S; Shahmohammadi, MH. Synthesis of new optically active poly(amide-imide)s derived from N,N′-(pyromellitoyl)-bis-S-valine diacid chloride and aromatic diamines under microwave irradiation and classical heating. Iran. Polym. J 2005, 14, 473–483. [Google Scholar]
- Mallakpour, S; Hajipour, AR; Khoee, S. Microwave-assisted polycondensation of ′4,4 - (hexafluoroisopropylidene)-N,N′-bis(phthaloyl-l-leucine) diacid chloride with aromatic diols. J. Appl. Polym. Sci 2000, 77, 3003–3009. [Google Scholar]
- Mallakpour, S; Kowsari, E. Soluble novel optically active poly(amide–imide)s derived from N,N′-(4,4′-oxydiphthaloyl)-bis-l-leucine diacid chloride and various aromatic diamines: Synthesis and characterization. J. Appl. Polym. Sci 2005, 96, 435–442. [Google Scholar]
- Mallakpour, S; Kowsari, E. Preparation and characterization of new optically active poly(amideimide) s derived from N,N-(4,4′-Oxydiphthaloyl)-bis-(s)-(+)-valine diacid chloride and aromatic diamines. Polym. Eng. Sci 2006, 46, 558–665. [Google Scholar]
- Mallakpour, S; Kowsari, E. Polycondensation reaction of N,N′-(4,4′-Oxydiphthaloyl)-bis-l-isoleucine diacid chloride with aromatic diamines. Iran. Polym. J 2005, 14, 799–806. [Google Scholar]
- Faghihi, K; Zamani, K; Mirsamie, A; Mallakpour, S. Facile synthesis of novel optically active poly(amide-imide)s containing N,N′-(pyromellitoyl)-bis-l-phenylalanine diacid chloride and 5,5-disubstituted hydantoin derivatives under microwave irradiation. J. Appl. Polym. Sci 2004, 91, 516–524. [Google Scholar]
- Mendoza, H; Palacios, J; López, JG; Alvarez, C. Microwave assisted polycondensation of polyimides by [4,4′-(hexafluoroisopropylidene)diphthalic anhydride, pyromellitic dianhydride] and [2,4,6-trimethyl-m-phenylenediamine]. Power, time, and solvent effect. J. Appl. Polym. Sci 2010, 116, 2816–2824. [Google Scholar]
- Kappe, CO. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed 2004, 43, 6250–6284. [Google Scholar]
- Shimizu, H; Yoshimura, Y; Hinou, H; Nishimura, SI. A new glycosylation method part 3: Study of microwave effects at low temperatures to control reaction pathways and reduce byproducts. Tetrahedron 2008, 64, 10091–10096. [Google Scholar]
- Loupy, A. Solvent-free microwave organic synthesis as an efficient procedure for green chemistry. C. R. Chim 2004, 7, 103–112. [Google Scholar]
- Lidström, P; Tierney, J; Wathey, B; Westman, J. Microwave assisted organic synthesis—a review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar]
- Loupy, A. Microwave in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Lewis, DA; Summers, JD; Ward, TC; McGrath, JE. Accelerated imidization reactions using microwave radiation. J. Polym. Sci. Pol. Chem 1992, 30, 1647–1653. [Google Scholar]
- Li, Q; Yang, X; Chen, W; Yi, C; Xu, Z. Preparation of poly(amic acid) and polyimide via microwave assisted polycondansation of aromatic dianhydrides and diamines. Macromol. Symp 2008, 261, 148–156. [Google Scholar]
- Tang, X; Lu, J; Zhang, Z; Zhu, X; Wang, L; Li, N; Sun, Z. Polycondensation of sodium tetrazodiphenyl naphthionate and pyromellitic dianhydride under microwave irradiation and the performance of the third-order nonlinear optics. J. Appl. Polym. Sci 2003, 88, 1121–1128. [Google Scholar]
- Albano, C; Parra, C; Gonzalez, G. Comparison between different synthesis methods of PMMA/HA using ultrasonic radiation. Macromol. Symp 2010, 290, 95–106. [Google Scholar]
- Teo, BM; Prescott, SW; Price, GJ; Grieser, F; Ashokkumar, M. Synthesis of temperature responsive poly(N-isopropylacrylamide) using ultrasound irradiation. J. Phys. Chem. B 2010, 114, 3178–3184. [Google Scholar]
- Kim, YJ; Glass, TE; Lyle, GD; McGrath, JE. Kinetic and mechanistic investigations of the formation of polyimides under homogeneous conditions. Macromolecules 1993, 26, 1344–1358. [Google Scholar]
- Mark, HF. Encyclopedia of Polymer Science and Technology, Synthesis of Polyimides; John Wiley & Sons: New York, NY, USA, 2004; pp. 6275–6285. [Google Scholar]
- Palacios, J; Valverde, C. Microwave initiated emulsion polymerization of styrene: Reaction conditions. New Polym. Mater 1996, 5, 93–101. [Google Scholar]
- Chia, HL; Jacob, J; Boey, FYC. The microwave radiation effect on the polymerization of styrene. J. Polym. Sci. Pol. Chem 1996, 34, 2087–2094. [Google Scholar]
- de la Hoz, A; Diaz, A; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev 2005, 34, 164–178. [Google Scholar]
- Kuznetsov, AA; Tsegelskaya, AY; Buzin, PV; Yablokova, MY; Semenova, GK. High temperature polyimide synthesis in “Active” medium: Reactivity leveling of the high and the low basic diamines. High Perform Polym 2007, 19, 711–721. [Google Scholar]
- Lu, H; Zhou, J; He, T. Diffusion-limited kinetics modeling of one-step polyimide formation. J. Appl. Polym. Sci 2001, 79, 2052–2059. [Google Scholar]
- Yilmaz, T; Guclu, H; Ozarlan, O; Yildiz, E; Kuyulu, A; Ekinci, E; Gungor, A. Kinetic investigations of formation of polyimides containing arylene sulfone ether linkages by potentiometric titration and their characterization. J. Polym. Sci. Pol. Chem 1997, 35, 2981–2990. [Google Scholar]
- Perreux, L; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium and mechanistic considerations. Tetrahedron 2001, 57, 9199–9223. [Google Scholar]
- Li, H; Liao, L; Liu, L. Kinetic investigation into the non-thermal microwave effect on the ring-opening polymerization of ɛ-caprolactone. Macromol. Rapid Commun 2007, 28, 411–416. [Google Scholar]

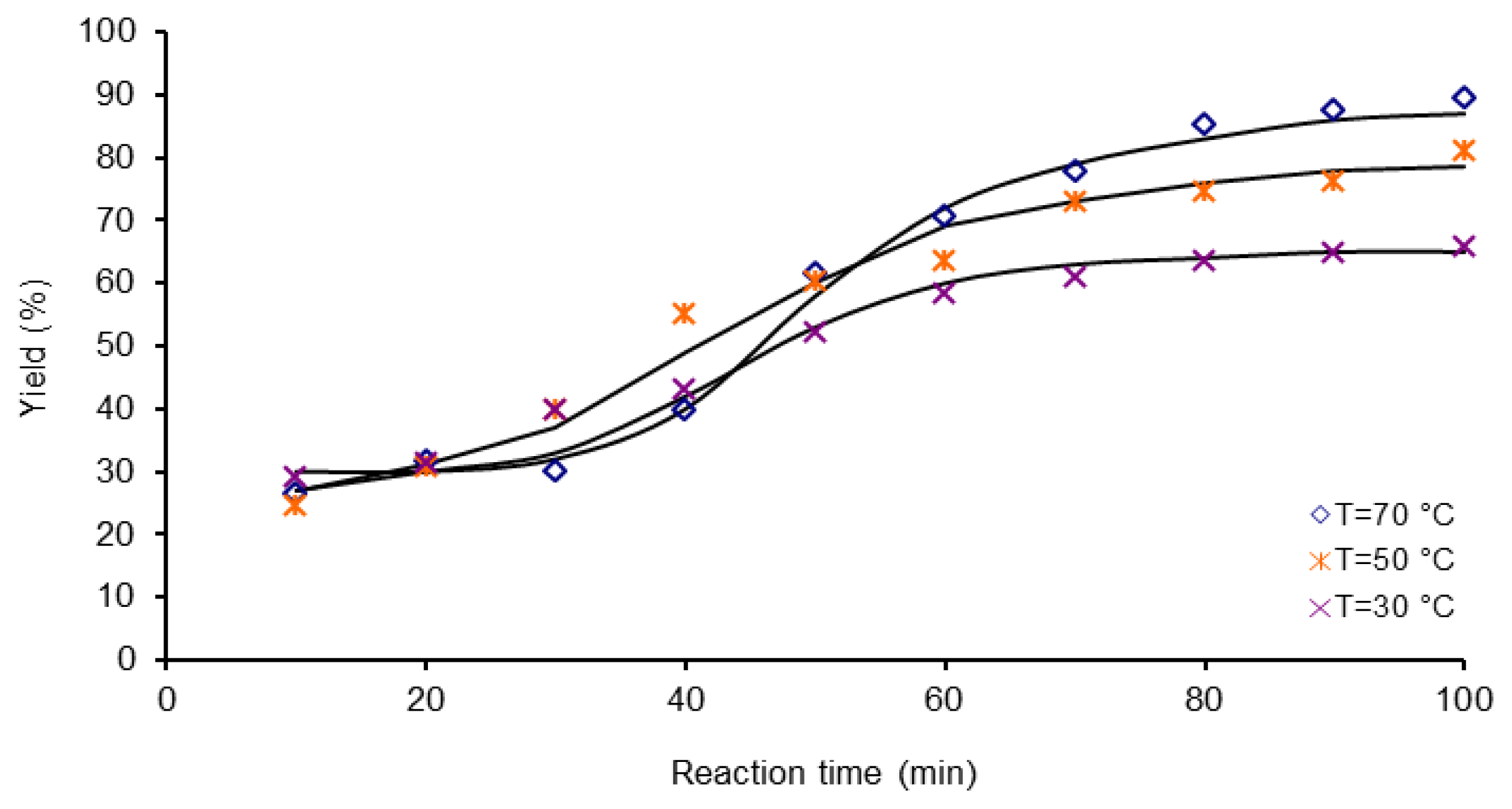

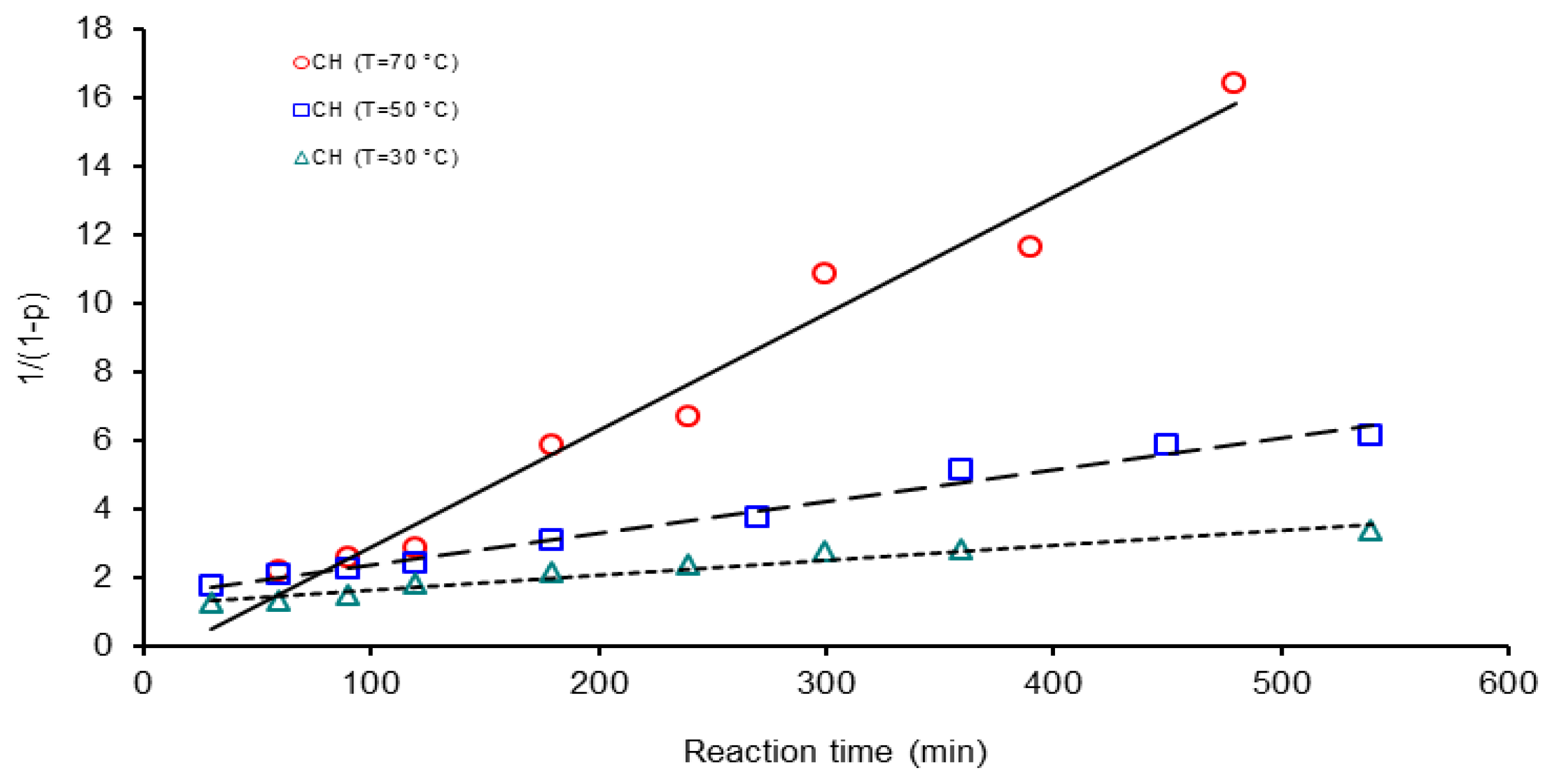
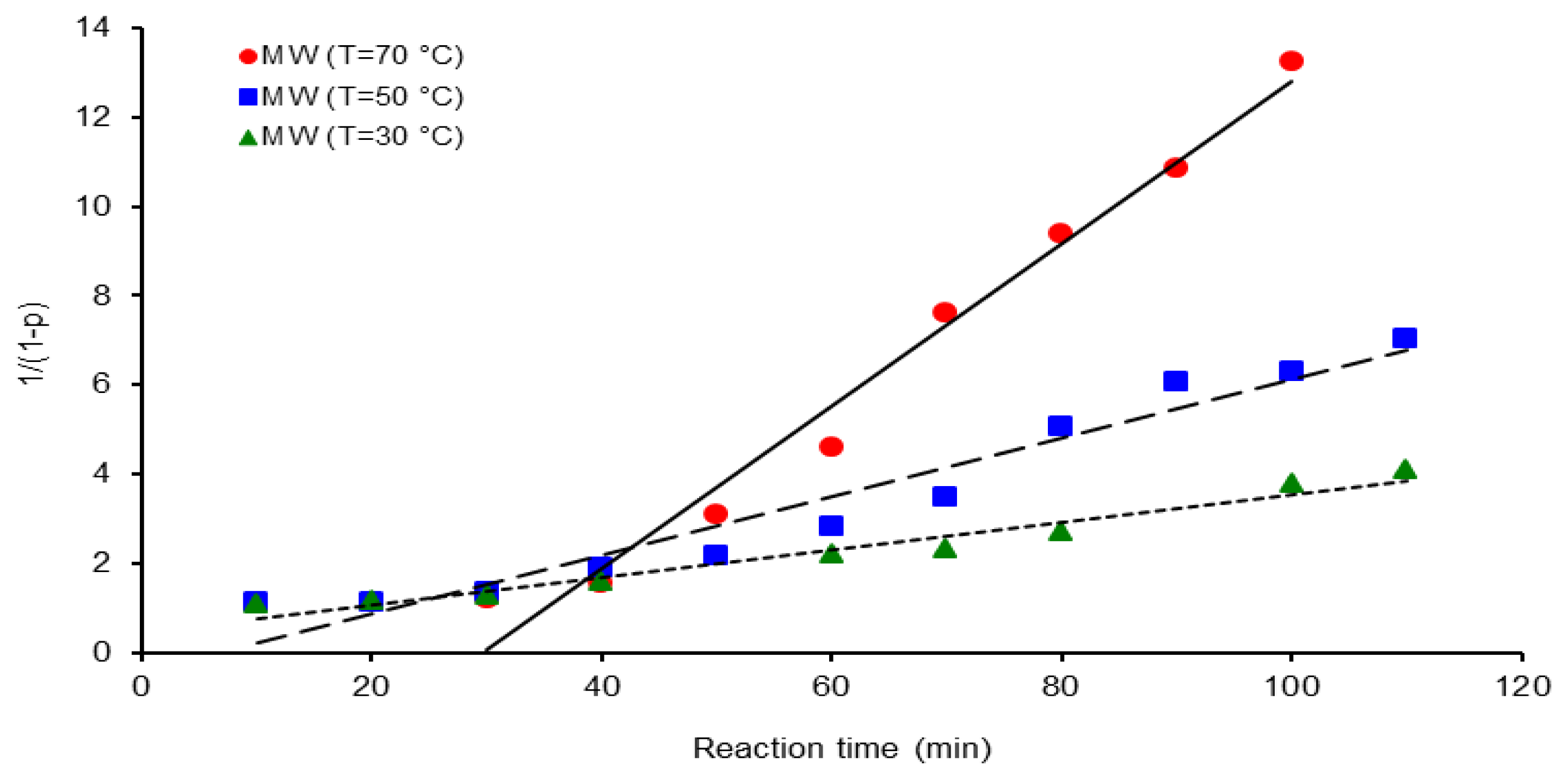
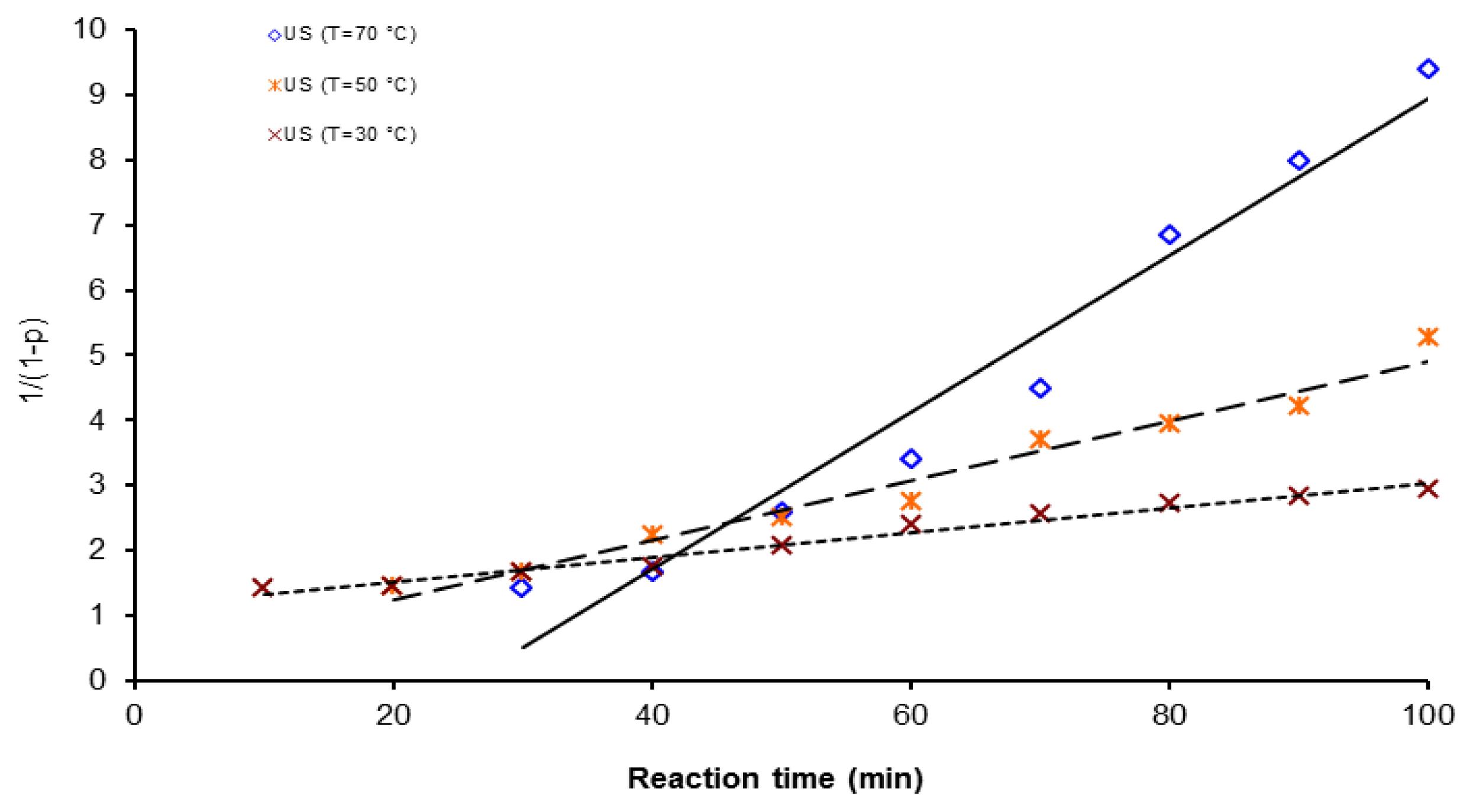
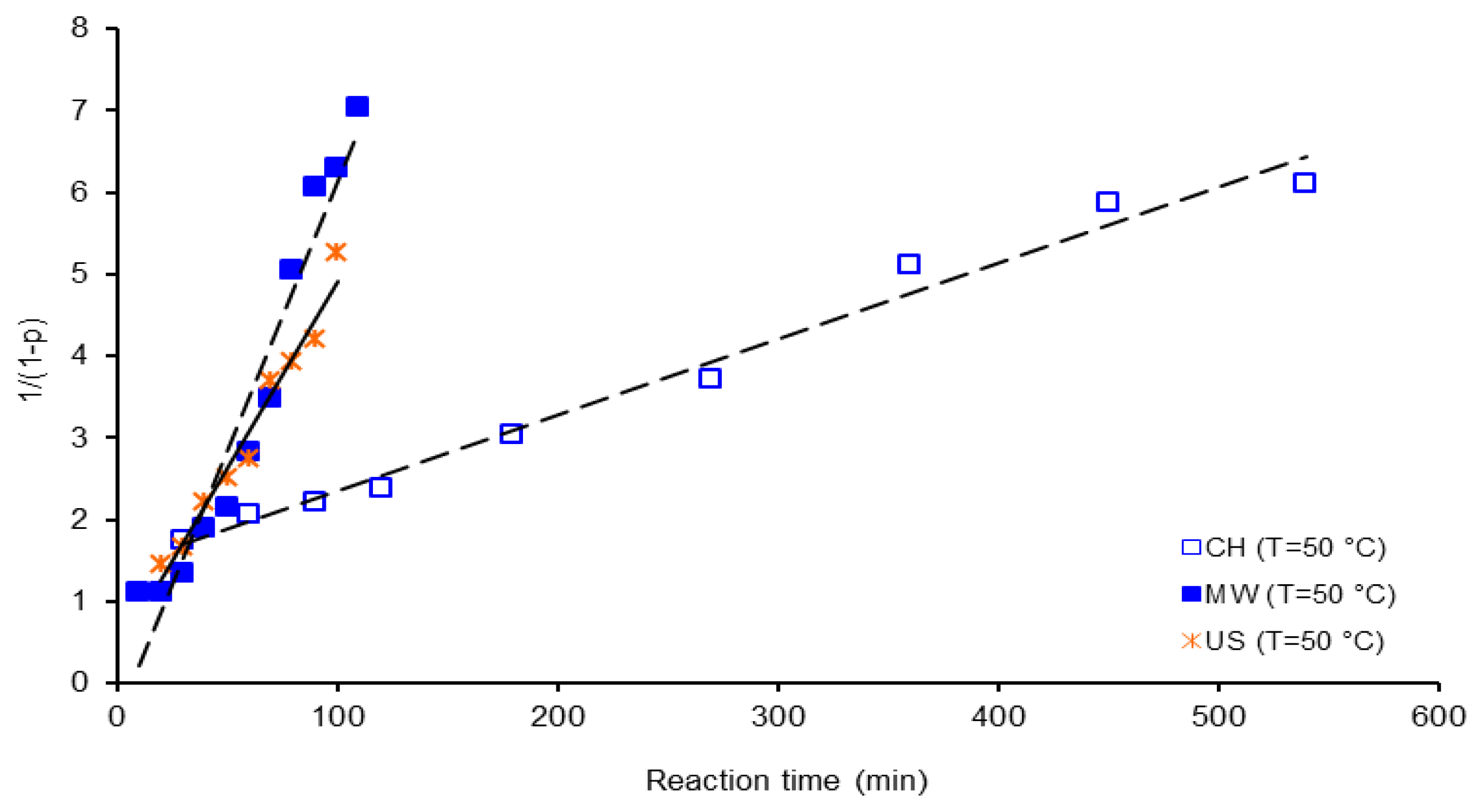
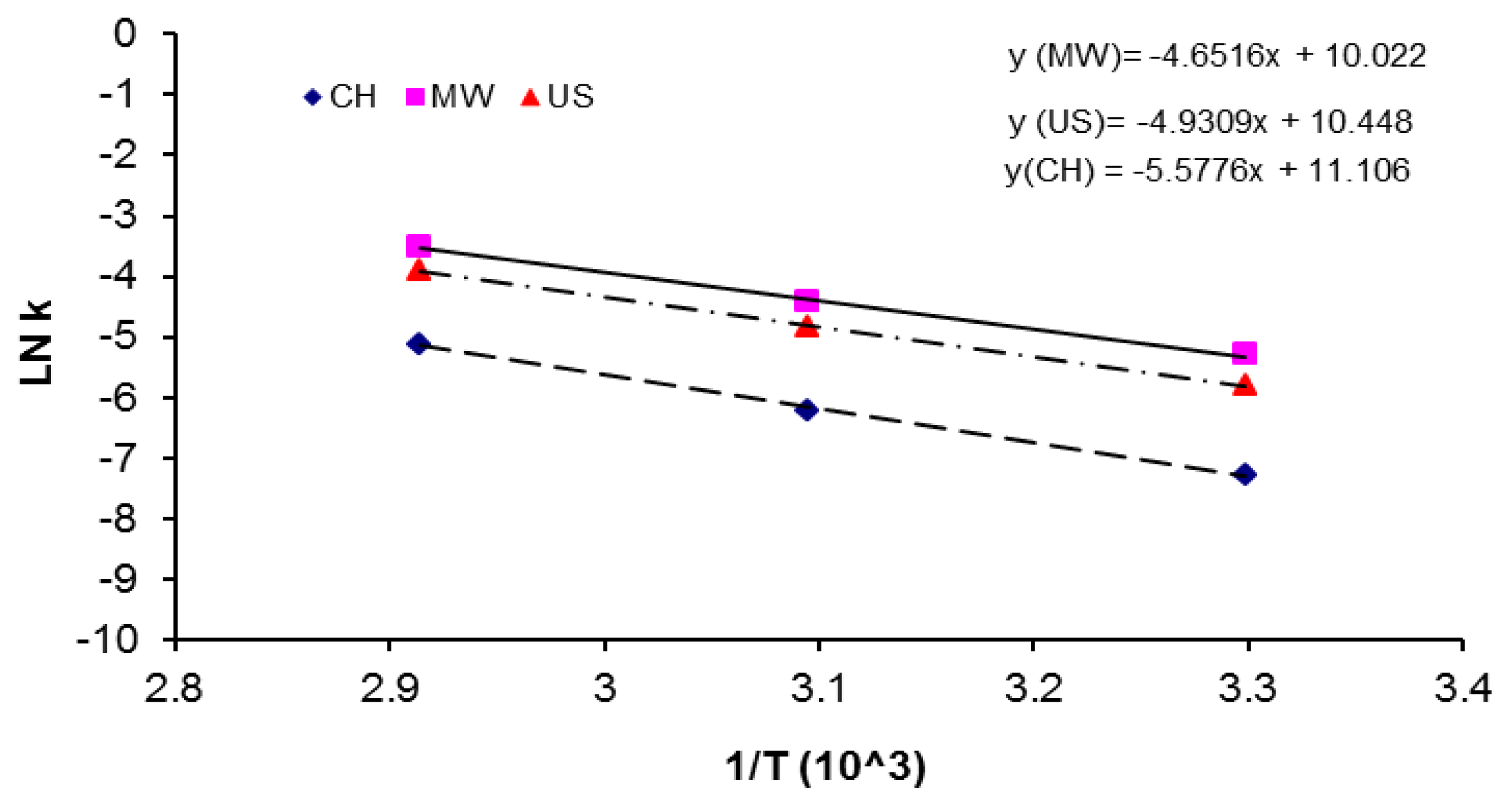
| Activation Method | T(°C) | k × 103 (L mol−1 s−1) | ΔGa(KJ/mol) | Δ * | A(L mol−1 s−1) | Induction time (min) |
|---|---|---|---|---|---|---|
| CH | 30 | 0.7 | 46.37 | 7.7 | 6.6 × 104 | 250.0 |
| 50 | 2.0 | 166.6 | ||||
| 70 | 6.0 | 16.6 | ||||
| US | 30 | 3.0 | 40.99 | 2.3 | 3.4 × 104 | 58.3 |
| 50 | 8.0 | 6.66 | ||||
| 70 | 2.0 | 21.1 | ||||
| MW | 30 | 5.0 | 38.67 | - | 2.2 × 104 | 16.6 |
| 50 | 12 | 3.3 | ||||
| 70 | 30 | 30.0 |
| Anhydride | Amine | Solvent | k × 103 (L mol−1 s−1) * |
|---|---|---|---|
| Phthalic anhydride | 4-Phenoxyaniline | THF | 0.3 a |
| Phthalic anhydride | 4-Phenoxyaniline | Acetonitrile | 0.1 a |
| Phthalic anhydride | Aniline | THF | 0.59 b |
| Tetrahydrophthalic anhydride | 4-Phenoxyaniline | Acetonitrile | 7.5 a |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tellez, H.M.; Alquisira, J.P.; Alonso, C.R.; Cortés, J.G.L.; Toledano, C.A. Comparative Kinetic Study and Microwaves Non-Thermal Effects on the Formation of Poly(amic acid) 4,4′-(Hexafluoroisopropylidene)diphthalic Anhydride (6FDA) and 4,4′-(Hexafluoroisopropylidene)bis(p-phenyleneoxy)dianiline (BAPHF). Reaction Activated by Microwave, Ultrasound and Conventional Heating. Int. J. Mol. Sci. 2011, 12, 6703-6721. https://doi.org/10.3390/ijms12106703
Tellez HM, Alquisira JP, Alonso CR, Cortés JGL, Toledano CA. Comparative Kinetic Study and Microwaves Non-Thermal Effects on the Formation of Poly(amic acid) 4,4′-(Hexafluoroisopropylidene)diphthalic Anhydride (6FDA) and 4,4′-(Hexafluoroisopropylidene)bis(p-phenyleneoxy)dianiline (BAPHF). Reaction Activated by Microwave, Ultrasound and Conventional Heating. International Journal of Molecular Sciences. 2011; 12(10):6703-6721. https://doi.org/10.3390/ijms12106703
Chicago/Turabian StyleTellez, Hugo Mendoza, Joaquín Palacios Alquisira, Carlos Rius Alonso, José Guadalupe López Cortés, and Cecilio Alvarez Toledano. 2011. "Comparative Kinetic Study and Microwaves Non-Thermal Effects on the Formation of Poly(amic acid) 4,4′-(Hexafluoroisopropylidene)diphthalic Anhydride (6FDA) and 4,4′-(Hexafluoroisopropylidene)bis(p-phenyleneoxy)dianiline (BAPHF). Reaction Activated by Microwave, Ultrasound and Conventional Heating" International Journal of Molecular Sciences 12, no. 10: 6703-6721. https://doi.org/10.3390/ijms12106703
APA StyleTellez, H. M., Alquisira, J. P., Alonso, C. R., Cortés, J. G. L., & Toledano, C. A. (2011). Comparative Kinetic Study and Microwaves Non-Thermal Effects on the Formation of Poly(amic acid) 4,4′-(Hexafluoroisopropylidene)diphthalic Anhydride (6FDA) and 4,4′-(Hexafluoroisopropylidene)bis(p-phenyleneoxy)dianiline (BAPHF). Reaction Activated by Microwave, Ultrasound and Conventional Heating. International Journal of Molecular Sciences, 12(10), 6703-6721. https://doi.org/10.3390/ijms12106703




