Abstract
Antimicrobial peptides constitute a diverse class of naturally occurring antimicrobial molecules which have activity against a wide range of pathogenic microorganisms. Antimicrobial peptides are exciting leads in the development of novel biocidal agents at a time when classical antibiotics are under intense pressure from emerging resistance, and the global industry in antibiotic research and development stagnates. This review will examine the potential of antimicrobial peptides, both natural and synthetic, as novel biocidal agents in the battle against multi-drug resistant pathogen infections.
1. Introduction
1.1. Antimicrobial Peptides
Despite continuing efforts, the increasing prevalence of resistance among pathogenic bacteria to common antibiotics has become one of the most significant concerns in modern medicine. With significantly reduced investment in antimicrobials research and development among major pharmaceutical companies, novel alternatives to existing treatment strategies are not being produced at a sufficient rate to keep pace with the emergence of resistance and the supply pipeline runs perilously close to drying up [1]. Incidences of hospital-acquired and community-acquired antibiotic resistant Staphylococcus aureus infections have risen dramatically in recent years [2], with almost 50% of hospital acquired Staphylococcus aureus infections classified as methicillin resistant and 30% of enterococci exhibiting vancomycin resistance [3]. In 2010, the Infectious Diseases Society of America launched its 10 × 20 initiative, calling for a global commitment to new antibacterial drug development, with the goal of ten new antibiotic agents by the year 2020 [4]. In addition, continued extensive use of the limited classes of effective antibiotics currently available threatens to call time on the ‘antibiotic era’. In the past decade, the crisis of antimicrobial resistance has worsened significantly and there exists an urgent requirement for new antimicrobial agents with activity against multidrug resistant pathogens [5].
One area of antimicrobial drug research that does shows significant promise is in the discovery and development of antimicrobial peptides (AMPs). Antimicrobial peptides in nature serve as important defensive weapons throughout the animal and plant kingdoms against a broad spectrum of bacterial and fungal pathogens [6]. Sources range from single celled microorganisms, such as bacteria themselves (bacteriocins) [7], to invertebrates [8]. As well as having a direct effect on the microorganism, antimicrobial peptides have been proven to promote the accumulation of immune cells including macrophages, neutrophils and lymphocytes [9]; neutralize lipopolysaccharide endotoxin derived from Gram-negative bacteria [10]; aid wound repair; stimulate angiogenesis [11] and control the actions of the innate and adaptive immune response with little or no resistance development reported. Antimicrobial peptides exert their microbicidal effect via disruption of the microbial cell membrane together with intracellular action [12,13].
Antimicrobial peptides are short (typically ranging from 12–100 amino acid residues in length), exhibit rapid and efficient antimicrobial toxicity against a range of pathogens [14,15] and constitute critical effector molecules in the innate immune system of both prokaryotic and eukaryotic organisms [16]. To date, over 1700 endogenous antimicrobial peptides have been isolated with many more synthetic analogues reported in the literature [17]. Structure activity relationship analyses have yielded vital information relating to the structural features of effective antimicrobial peptides, indicating that antimicrobial activity is governed primarily by charge and hydrophobicity [18], and that the initial target is the negatively charged bacterial cell membrane [19]. These studies have also facilitated the design of ultrashort, highly active antimicrobial peptide scaffolds [20,21] which may be prepared via established facile, solid phase synthetic protocols at lower costs compared with their natural antimicrobial peptide counterparts [22,23].
1.2. Cationic Antimicrobial Peptides
The majority of antimicrobial peptides are cationic with more than a thousand characterized and are thus termed cationic antimicrobial peptides (CAPs) [24]. Naturally derived cationic antimicrobial peptides typically consist of a net positive charge between +2 and +9, due to the presence of few or no acidic residues, such as glutamate or aspartate and a high number of cationic amino acids such as lysine or arginine and/or histidine [25]. Hydrophobic residues, including tryptophan and branched amino acids such as valine, form 30–50% of the total peptide structure and serve a vital role in allowing a typical amphiphilic structure to form upon interaction with membranes [26]. This characteristic together with the presence of dense areas of high positive charge allow cationic antimicrobial peptides to exert their antimicrobial effect. Alteration of this net charge to hydrophobic ratio can vary the activity and spectrum of the peptide against a host of microorganisms. The increase in activity that can be therapeutically achieved by tapering the lipophilic:charge ratio is provided by the example of glycopeptides. Glycopeptides, for example vancomycin, represent one of the last line of effective antibiotics against Methicillin resistant Staphylococcus aureus. Lipoglycopeptide derivatives of vancomycin in development include oritavancin and dalbavancin which possess increased activity against vancomycin-resistant strains [27]. Slight modifications in the balance of hydrophobicity and charge can give rise to marked changes in antimicrobial selectivity/activity [28]. Cationic antimicrobial peptides, as a class of compounds, have broad structural diversity and antimicrobial spectrum influenced primarily by the amino acids that constitute the primary sequence of the peptide [29].
Although the secondary structures that antimicrobial peptides adopt may vary between classes they share the same characteristic of developing an amphipathic structure and being cationic under physiological conditions [30,31]. Secondary structures include amphiphilic β-sheet structures containing two or three stabilizing disulphide bonds, often with a short α-helical segment and/or two to four β-strands. These disulphide bridges form as a result of a cysteine-rich primary sequence. Examples of these peptides in nature include several classes of mammalian host defense peptides including α-defensin and β-defensin [32]. Linear peptides are unordered in hydrophilic solutions but form amphipathic α-helices upon contact with cell membranes and in a hydrophobic environment [33].
These peptides are of particular interest as one face of the helix structure contains a majority of hydrophobic residues whereas the opposite face contains mainly polar amino acids allowing efficient solubilization of microbial membranes [26]. Amphipathic α-helices lack cysteine and are thus unable to form disulphide bridges [34]. Examples in nature include mellitin derived from honeybee venom [35], magainin obtained from the skin secretions of the frog species Xenopus laevis [36] and the cecropins, a group of the dipteran insect defense peptides [37]. Cyclic peptides are a less prevalent structural class of cationic antimicrobial peptides that contain β-turn influenced by a single disulphide bond and include a dodecapeptide from bovine neutrophils [38].
Extended structures with a high regularity of one or two amino acid residues, such as proline, glycine, histidine or tryptophan, make up the remainder of the four major structural varieties [15,39]. Extended cationic peptides have no defined or typical structure due to the existence of novel folds. Examples in nature include the porcine derived tritrpticin [40] and the bovine neutrophil peptide indolicidin [41]. Indolicidin is a linear antimicrobial peptide consisting of a 13 amino acid structure high in tryptophan residues (~40%) [42]. Both these peptides form boat-like structures in the presence of cell membranes as the high tryptophan content interacts with the hydrophobic layer of the membrane with the remaining cationic arginine and lysine residues orientated towards the aqueous environment [43]. The cationic AMP mimetic tyrocidin was the first commercially available antibiotic. However major issues with toxicity toward human blood and reproductive cells lead to its withdrawal from the market [44]. The polypeptide bacitracin has had a more successful introduction clinically incorporated with both neomycin and polymyxin B in the topical product Neosporin® licensed for the topical treatment of a variety of localized skin and eye infections [45].
1.3. Anionic Antimicrobial Peptides
Despite the vast majority of antimicrobial peptides being cationic in nature, a significant number of anionic AMPs have been reported; serving as important weapons in the eukaryotic innate immune response [46–48]. Peptides that are anionic in nature tend to be rich in glutamic and aspartic acids and include the amphibian peptide Maximin-H5 and Dermcidin, a peptide derived from human sweat [49,50]. Anionic antimicrobial peptides commonly consist of 5 to 70 amino acid residues, possessing a net charge of −1 or −2 although structural characterization demonstrated that the truncated form of bovine peptide B, termed enkelytin, can possess a net charge as high as −7 [48,51]. Although less common, anionic peptides of 300 residues in length and net charge −20 have been reported [52–54]. Similarly to their cationic counterparts, anionic antimicrobial peptides can adopt varying amphiphilic structures such as the α-helix and the β-sheet conformations with interaction with the microbial membrane key to activity.
A disadvantage of many anionic antimicrobial peptides is that they often require cations, for example zinc (Zn2+), as cofactors for biocidal activity [55]. This may be why they are generally located at epithelia; sites were both ionic secretion and microbial susceptibility are highest. Anionic peptides, for example surfactant-associated anionic peptides present in pulmonary tissue, have been shown to possess increased potency against both Gram-positive and Gram-negative bacteria in the presence of synergistic cationic antimicrobial peptides and Zn2+ [56,57]. These cationic moieties act as a cationic linkage between the anionic antimicrobial peptide and the anionic microbial cell membrane. This allows transport of the anionic peptide to intracellular targets without damaging to the structure of the microbial membrane [55,58]. Anionic peptides target ribosomes within the cell inhibiting ribonulease activity, thus resulting in microbial cell death [59,60].
1.4. Amphibian Antimicrobial Peptides
Amphibian derived peptides are excellent examples of naturally occurring, structurally diverse peptides with high antimicrobial potency. These compounds are released in skin secretions often at high concentrations and their production reflects the evolution of amphibians to their humid habitat, an environment also suitable for the growth and proliferation of opportunistic pathogenic bacteria and fungi [61]. Such peptides are also beginning to show promise against cancer, as anti-tumor compounds [62] and also possess anti-viral activity, with potential benefits against HIV [63]. They also show activity against eukaryotic cells and therefore provide a means by which amphibians may be protected from predation [64]. The potential therefore exists that amphibian derived antimicrobial peptides may also be cytotoxic to humans. Such peptides are released naturally in response to injury or stress via contraction of myocytes surrounding the glands [65] and this may be replicated in the laboratory through the use of mild electrical stimulation [66] or injecting norepinephrine into the dorsal sac [67].
Structural characterization has facilitated their synthetic production via solid phase peptide synthesis (SPPS). In frogs, variations in the sequence and spectrum of activity of these peptides are considerable with hundreds of characterized peptides providing protection against a vast range of bacterial and fungal species [61,68,69]. The majority of antimicrobial peptides from frogs are cationic due to a high presence of lysine, with at least 50% of amino acids being hydrophobic of which leucine is most prevalent and an amphipathic α-helix secondary structure predominates at the cell membrane interface [61,70]. Relevant genera of frog studied include Bombina [71], Xenopus [36], Rana [72], Phyllomedusa [73] and Litoria [74]. The species Bombina maxima, commonly known as the Chinese red belly toad has as many as forty genes linked to the production of different antimicrobial peptides [75]. Research conducted by Lai et al. showed that Bombina maxima produced a group of peptides called maximins that demonstrated minimum inhibitory concentration (MIC) values in the μg/mL range against a broad spectrum of microbial and fungal pathogens including Staphylococcus aureus, Escherichia coli, Bacillus dysenteriae, Klebsiella pneumoniae and Candida albicans [76]. Both maximin-3 and maximin-4 were the most potent peptides tested with maximin-4 having the lowest MIC value of 2.7 μg/mL against Staphylococcus aureus. Maximin-4 consists of twenty seven amino acids and has the potential to be produced synthetically via SPPS. Dermaseptins are a family of linear lysine-rich cationic antimicrobial peptides derived from the genus Phyllomedusa. They consist of between twenty eight and thirty four amino acid residues and have been shown to inhibit the growth of a wide variety of Gram-positive and Gram-negative bacteria, fungi, protozoa and yeast [73,77].
1.5. Rational Design and Selection of an Antimicrobial Peptide Motif
The diverse range of antimicrobial peptides available in nature provides a vast scope for the design of novel and improved synthetic variations. Structure and its effect on the hydrophobic:charge ratio is more important with regard to antimicrobial activity than size and the manipulation of primary amino acid sequence can improve factors such as specificity, toxicity and stability [12,78]. Ultrashort antimicrobial peptides consist of approximately four or five amino acids residues, with amino acid selection fulfilling the minimum range of functionalities required for effective antimicrobial activity. These functionalities include a charged moiety such as arginine and a lipophilic unit, most commonly tryptophan, forming an antimicrobial pharmacophore with the correct balance between charge and lipophilicity [79].
Strom et al. have proven that tryptophan provides better bulk and lipophilicity than tyrosine and a minimum of two bulk and two charged residues are required to be present to give activity against staphylococci, with Escherichia coli requiring an additional bulk tryptophan. Arginine also acts a better source of charge than lysine within the minimum motif [78]. However arginine is difficult to work with in terms of SPPS [80,81]. The obvious advantage to the use of ultrashort antimicrobial peptide is the large reduction in cost in synthesizing these molecules relative to synthetic variants of naturally occurring antimicrobial peptides. The attachment of an acyl chain to an active or inert ultrashort cationic peptide also potentially leads to an increased action against microorganisms in a similar way to native cationic antimicrobials [82]. Small alterations of the total methylene units present can also modify the spectrum of activity for the antimicrobial, the cell specificity and therefore levels of hemolysis [83]. Previous studies by Makovitski et al. reported however that peptides containing four amino acids would find it difficult to form a characterized, stable amphipathic structure, therefore questions still relate to their mode of action. Reported results also show that the balance of hydrophobicity and charge play an important role just as in the larger antimicrobial peptides. By altering both, the amino acid residues and the acyl chain length, it was found that increasing hydrophobicity and/or charge is not necessarily indicative to increasing antimicrobial function [84].
Host and microbial proteases and the unfavorable pharmacokinetics they bring present a major barrier to the use of peptides as antimicrobials in vivo, with many peptides limited to use as topical applications. Human chymotrypsin-like enzymes function at basic amino acid residues [85], therefore antimicrobial peptides are a viable target due to the requirement of basic residues to be present for antimicrobial activity. Simple α-helical or linear structures in particular are susceptible to proteolysis by a range of microbial proteases [86]. As proteases will only recognize natural L-amino acid residues, a switch to the use of D-amino acids at susceptible points will render the peptide partially or totally resistant to proteolysis without loss of antimicrobial activity [87]. L-stereoisomers also tend to be more hemolytic than their D counterparts [88]. Antimicrobial peptides are expensive to synthesize and costs are increased further by the use of d-enantiomers thus a more viable alternative is to select unnatural amino acids, such as ornithine. The use of ornithine as a charged moiety is preferable as the use of a non-coded amino acid provides stability against proteases [78]. Examples of synthetic harnessing of ornithine’s properties are demonstrated by Bisht et al. They demonstrated the potent action of ultrashort tetrapeptides with two ornithines representing the charged portion and two tryptophans providing bulk and hydrophobicity [21]. Modification occurred with conjugation of cinnamic groups to the Nα-amino terminus of ornithine. The incorporation of ornithine into the lipopeptide scaffold has also been tested in the synthetic peptide MSI-843 [89]. Containing six ornithine residues out of a total ten and a conjugated octanyl terminus, excellent activity was shown against Gram-positive Staphylococcus aureus; Gram-negative Pseudomonas aeruginosa and Escherichia coli and the fungus Candida albicans.
1.6. Ultrashort Cationic Antimicrobial Peptides
Ultrashort cationic antimicrobial peptides consist of approximately four or five amino acids residues, with amino acid selection fulfilling the minimum range of properties required for effective antimicrobial activity to take place. The use of ultrashort cationic antimicrobial peptides is advantageous in terms of manufacture with reduced production costs and synthesis times. Their production correlates to a trend amongst peptide companies that has seen an increase in the production of these smaller peptides in relation to larger, more expensive natural peptides [90,91]. Haug and colleagues discovered, using lactoferrin derivatives, that a minimum motif existed for antibacterial action against many forms of bacteria including strains of Staphylococcus aureus resistant to common antibiotic regimens [79,92–97]. Of high importance to their activity is the overall balance between lipophilicity and charge with the presence of two units of bulk and two cationic charges mandatory for membrane interaction and antimicrobial activity [16,78]. Naturally occurring antimicrobial peptides have a largely lipophilic character with the primary sequence consisting of 30–50% bulky hydrophobic residues such as tryptophan [26]. Tetra and pentapeptides possess a relatively small pharmacophore with limited bulk, thus in order to produce a favorable increase in the lipophilicity to charge ratio the amino terminus is acylated with a variety of hydrocarbon moieties [98]. With this knowledge Haug et al. produced a series of smaller dipeptides of a general formula XRY where; X represented a variant of a bulky amino acid with a lipophilic side chain, for example cyclohexylalanine; R represented arginine, the charged moiety; Y represented a C-terminal ester or a lipophilic amide derivative [79]. Both X and Y provided bulk to the short pharmacophore. A 2,5,7-tri-tert-butyl-tryptophan-arginine-benzyl amide was shown to be the most active peptide in the study. This structure correlates with the minimum requirement for two units of bulk, provided by the tryptophan derivative and benzene ring of the benzyl amide and two units of charge imparted by arginine’s guanidino group and the free Nα-amino terminus. Central to this theory Bisht et al. produced a series of ornithine and tryptophan containing tetrapeptides and evaluated them against planktonic forms of various Gram-positive and Gram-negative bacteria [21]. To increase hydrophobicity cinnamic acid and its derivatives were attached to the amino terminus of the peptide motif. These terminal carboxylic acids included cinnamic acid, 3,4-dimethoxycinnamic acid, 4-hydroxy cinnamic acid and 3-(4-hydroxyphenyl) propionic acid.
1.7. Lipopeptides
Lipopeptides are essentially a peptide attached to a lipid moiety. Such a peptide may include an antimicrobial peptide. Native lipopeptides are formed in bacteria and fungi non-ribosomally by cultivation on a range of carbon sources to form complex cyclic structures [99]. They normally consist of a sequence of six or seven amino acids with a N-terminal fatty acid moiety attached. They are active against a range of multi-resistant bacteria and fungi [100]. Antimicrobial lipopeptides are primarily bacterial compounds synthesized via non-ribosomal biosynthetic pathways and comprise a peptidyl portion conjugated to a fatty acid to form an acylated peptide [99]. Many naturally occurring lipopeptides are cyclized and may also contain unnatural amino acids that confer stability against proteolytic degradation. Lipopeptides may comprise an anionic (e.g., Daptomycin, surfactin) or cationic (e.g., polymyxin B, colistin) peptide motif, which dictates the spectrum of activity [99]. Fatty acids, fatty amines, alcohols and gylceryl esters have all been shown to exhibit varying degrees of antimicrobial activity [101,102], whilst acylation of peptide scaffolds has been demonstrated to significantly improve antimicrobial activity [99,103]. For example when the natural occurring polymyxin is coupled to a fatty acid tail, removal of this tail leads to a decrease in antimicrobial action [104]. The conjugation of a fatty acid moiety at the N-terminus can also compensate for a loss of hydrophobicity within the peptidic chain based on amino acid residue selection [105]. Therefore, the combination of an optimized peptidyl scaffold and N-terminal acyl substituent (i.e., fatty acid) with inherent antimicrobial activities represents an approach to the development potent antimicrobial agents, whereby spectrum of activity may be modulated via modification of the N-terminal substituent, whilst circumventing the commercial barriers associated with the manufacture of natural antimicrobial peptides in high yields.
1.8. Licensed and Commercially Available Lipopeptides
A variety of anionic and cationic lipopeptides are currently licensed for commercial use, thus highlighting the clinical potential of these antimicrobials. These include daptomycin, polymyxin B and polymyxin E (colistin).
1.8.1. Daptomycin
Daptomycin is an anionic lipopeptide that is produced naturally by Streptomyces roseosporus (Actinobacteria). Daptomycin is a cyclic depsipeptide consisting of thirteen amino acids, which includes three D-amino acid residues (D-asparagine, D-alanine, and D-serine) linked to a hydrocarbon tail ten carbons in length derived from decanoic acid (Figure 1). Synthetic production of lipopeptides has led to the approval of the lipopeptide daptomycin which gained US and EU product licenses in 2003 and 2006 respectively for skin and soft tissue infections [106]. More recently it has been passed for use in the US for staphylococcal bacteremia and right sided endocarditis [107]. Daptomycin is also available in the UK as an injection and is commercially available as the brand Cubicin® with further research showing possible use in the treatment of enterococcal bacteremia caused by Enterococcus faecium and Enterococcus faecalis [108]. Daptomycin, itself anionic, is dependent on the presence of calcium cations for antimicrobial activity. Electrostatic interactions allow daptomycin to alter to an active conformation. Increased amphiphilicity and integration of daptomycin into bacterial membranes occurs via the acyl chains [109–113]. This results in the formation of micelle-like structures with the lipid tails of daptomycin pointing inwards and the anionic side groups held together by calcium ions [114]. At bacterial membranes these micelles dissociate with daptomycin oligomerising in the membrane creating a potassium efflux ultimately resulting in; membrane disruption; cessation of the synthesis of the macromolecules Deoxyribonucleic acid (DNA), Ribonucleic acid (RNA) and protein synthesis; eventually leading to cell death. Unlike β-lactams this process is cell lysis independent with only the cell membrane, not the cell wall, disrupted [115]. Generally, daptomycin is well tolerated, although myopathy has been reported in some patients [116].
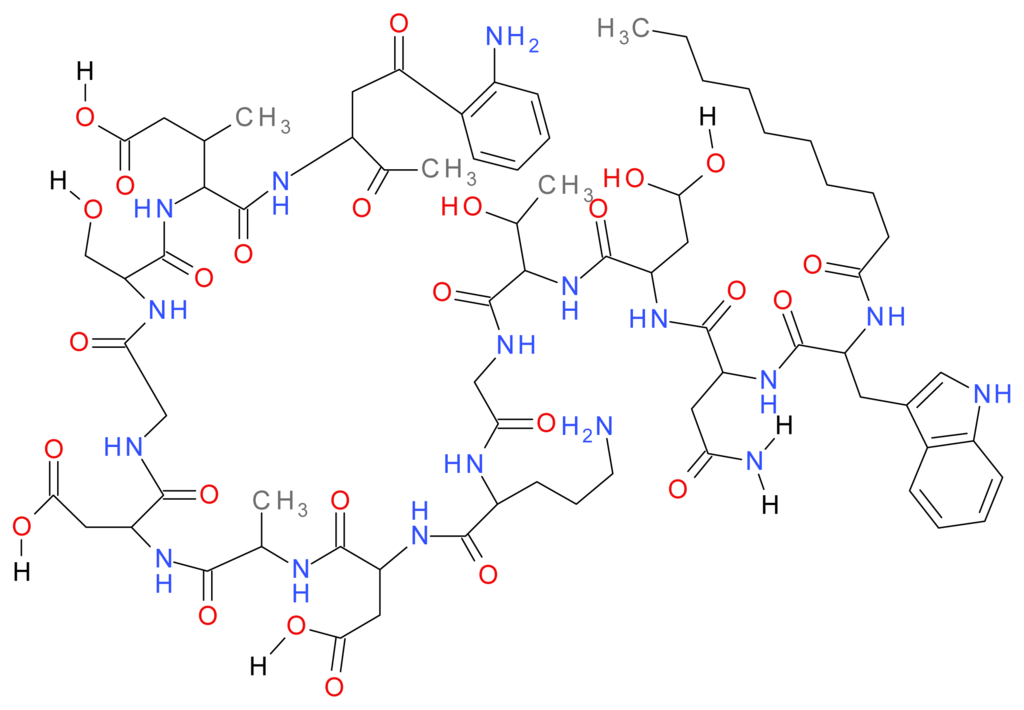
Figure 1.
The structure of Daptomycin. Adapted from Steenbergen et al. 2005 [113].
1.8.2. Polymyxins (B and E)
Polymyxins are a group of cationic antimicrobials discovered in the late 1940s [117–119]. Polymyxin E was later identified in the 1950s [120]. Polymyxins are N-terminally fatty acylated cationic lipopeptides consisting of a cyclic peptide structure attached to a hydrocarbon tail. Removal of the fatty acid tail reduces the antimicrobial activity of polymyxin [104]. They are isolated naturally from the Gram-positive bacteria Bacillus polymyxia [121]. Only two polymyxins are commercially available, polymyxin B and polymyxin E (colistin) [122]. Polymyxin B is a cyclic heptapeptide with a tripeptide side chain that is acylated at its amino terminus by a fatty acid. Polymyxin B consists of the amino acids D-phenylalanine, l-threonine and l-α-γ-diaminobutyric acid [123,124]. Colistin has the same structure as polymyxin B but d-phenylalanine is replaced by d-leucine [124]. A range of topical and parenteral formulations are available indicated for the treatment of Gram-negative skin and eye infections, including those involving Pseudomonas aeruginosa, with a powdered nebulizer solution available to treat multidrug resistant Gram-negative infection in cystic fibrosis patients [125–127]. The mechanism of action of the polmyxins is similar to cationic antimicrobial peptides in general [128,129]. Interaction of the hydrophobic tail with lipopolysaccharides present on the outer membrane of Gram-negative bacteria creates a detergent-like effect compromising the integrity of the bacterial membrane [130]. Polymyxins may also displace magnesium and calcium ions from cationic binding sites present at the bacterial cell surface resulting in leakage of cell contents from the cytoplasm [131]. The membrane permeabilisation effect also has the benefit of making Gram-negative bacteria more susceptible to hydrophobic antimicrobials for example erythromycin [132–134]. Polymyxins also have the benefit of binding to and neutralizing Gram-negative endotoxins [135,136]. However the systemic use of polymyxins is limited with nephrotoxicity, ototoxicity and neurotoxicity common [137–139]. Colistin methanesulphonate, a prodrug of colistin, has shown increased use therapeutically due possessing a lower toxicity profile than colistin itself [140].
2. Mechanism of Action of Antimicrobial Peptides
2.1. Targeting of the Microbial Cell Membrane
As with the majority of antimicrobials, interaction with the cell membrane of microorganisms is fundamental to the mode of action of cationic antimicrobial peptides. The significant difference in the compositions of eukaryotic membranes in comparison to prokaryotic membranes highlights the important selectivity of cationic antimicrobials for bacterial cells. Phospatidylcholine, phosphatidylethanolamine, an analogue of phospatidylcholine, sphingomyelin together with the sterols, ergosterol and cholesterol are predominantly found in eukaryotes and normally have no net charge leading to an overall neutrally charged phospholipid bilayer. In comparison prokaryotic bacterial cytoplasmic membranes are negatively charged, with a high electrical potential gradient, contributed in part to the presence of acidic hydroxylated phospholipids such as phosphatidylglycerol, cardiolipin and phosphatidylserine [12]. Cationic antimicrobial peptides will therefore bind preferentially to the negatively charged phospholipid bilayer of bacterial cells [34,141]. This is advantageous with regard to reducing toxicity in any potential therapeutic environment. The lack of specific receptors will also make it difficult for bacteria to develop resistance to the peptide. Bacteria would have to alter the properties of their membrane as a whole rather than specific receptors. The overall positive charge associated with cationic antimicrobial peptides means that initial electrostatic interaction with the bacterial cell membrane will involve areas of dense anionic charge. Acidic polymers such as teichoic acids in Gram-positive [142] and phosphate groups present on lipopolysaccharides in Gram-negative bacteria [143] allow attachment of the peptide prior to formation of transmembrane pores and ultimately membrane permeabilization.
Fungal cell membranes tend to share similar properties to the zwitterionic eukaryotic membranes [144]. Peptides that primarily possess antifungal activity tend to consist of neutral amino acids with regions of high polarity suggesting that a unique structure-activity relationship exists [145]. Proof of this structure activity relationship and the importance of lipophilic moieties in the peptide structure were provided by Lopez-Garcia et al. [146]. They demonstrated that there was a direct correlation between the antifungal activity of peptides and the ability of peptides to form aggregative complexes with lipid formulations. Four main hypotheses exist as to how antimicrobial peptides achieve entry into the bacterial cell (Figure 2: part A–D). These are the toroidal pore, aggregate, barrel stave, and carpet models.
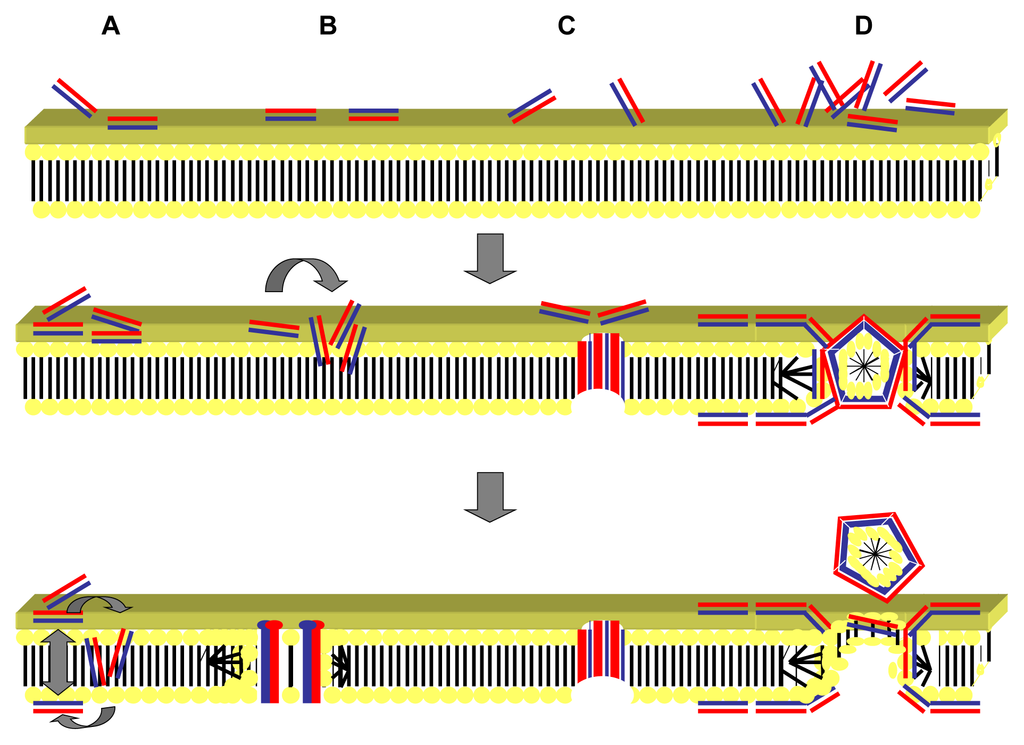
Figure 2.
Proposed mechanisms of action of antimicrobial peptides. Antimicrobial peptides (cylinders) with the charged hydrophilic regions (red) and hydrophobic regions (blue). (A) The “aggregate” model: the antimicrobial peptides reorient to form an aggregate that spans the membrane, composed of peptide and lipid micelle complexes but with no particular orientation adopted; (B) The “toroidal pore” model occurs when the peptides insert perpendicular to the plane of the lipid bilayer, with hydrophilic groups on the peptide interacting with the membranous phospholipid head groups and the hydrophobic regions associating with the lipid core. A lipid bilayer lined pore is created as the membrane curves inwards; (C) The “barrel-stave” model also involves insertion of the peptides at a perpendicular orientation to the plane of the bilayer but staves are formed in a barrel shaped cluster due to hydrophilic portions of the peptide interacting with the lumen of the pore and hydrophobic regions of the peptide associating with the lipid bilayer; (D) The “carpet” model involves the aggregation of peptides at a parallel orientation to the lipid bilayer with localized carpeting of areas of the membrane. Micelles are formed above a critical threshold concentration leading to a detergent-like activity and the formation of pores in the membrane. Adapted from Jenssen, Hamill and Hancock 2006 [147].
The toroidal pore model involves the peptide inserting perpendicular into the cell membrane via electrostatic interactions between the hydrophilic regions of the peptide and the phospholipid head of the bilayer. Hydrophobic regions of the peptide bend the lipid monolayers forming a central water core lined by lipid head groups and inserted peptides [148]. In doing this the membrane bends inwards such that the bilayer lines the channel as well as the peptides. A toroidal pore is thus formed by positive curvature, allowing entry of further antimicrobial peptide [149]. In the aggregate model a similar process occurs to that of the toroidal pore model. However, peptides do not adopt any specific orientation upon insertion into the membrane but cover the membrane as an aggregate of peptide and lipid micelles [150]. Channels that do form vary greatly, so much that partial membrane insertion may lead to the formation of negative curvature and peptide aggregation within the bilayer [151].
Perpendicular insertion of the peptides forming barrel-like clusters or staves occurs in the barrel-stave model [152]. Pores can occur from as little as three peptide molecules and theoretically to allow these pores to form, peptides must have an amphipathic or hydrophobic α-helix, β-sheet structure or both [153]. Usually the barrel-stave model results in a transmembrane pore of unilateral size. Within this pore hydrophilic regions of the peptide oppose the lumen, forming the interior and Van der Waal’s attractions occurring between the hydrophobic peptide regions and the lipid core [154]. The carpet model suggests that peptides accumulate parallel to the membrane with the hydrophobic regions of the peptide associating with the anionic phospholipid head groups on the membrane surface and hydrophilic regions attracted to the polar solvent [155]. Localization of peptides occurs forming a carpet-like coating on the membrane until a threshold concentration is reached. At this threshold a detergent-like process occurs with the eventual formation of micelles and transient pores. Disruption of the membrane structure leads to membrane disintegration [156]. It has been hypothesized that the additional outer lipid membrane present on Gram-negative organisms consisting of lipopolysaccharides allows a self-promoted uptake pathway to occur for cationic peptides [157,158]. As the majority of these peptides have a high affinity for lipopolysaccharides they bind to them, competitively replacing divalent cations such as magnesium and calcium ions from their relative binding sites [159]. Both magnesium and calcium ions are required for cell surface stability via the cross-linking of carboxylated and phosphorylated head groups of lipids [160]. Removal of these divalent cations leads to distortion of the outer membrane forming holes through which further peptide and other small molecules (such as conventional antibiotics) can cross. The self-promoted uptake model provides an explanation as to why in Gram-negative bacteria many cationic antimicrobial peptides act in synergy with conventional antibiotics [161].
Synergy of cationic antimicrobial peptides with standard antimicrobials is not limited to just Gram-negative bacteria but has been proven for both Gram-positive [162] and fungi also [163]. A similar pattern of membrane disruption has been demonstrated for antimicrobial peptides against a range of fungal pathogens. The rabbit α-defensin NP-2, magainin-2 and bovine lactoferrin have been shown to cause membrane permeabilization and cell wall damage in Candida albicans [164–166]. The antimicrobial lipopeptide iturin, obtained from cultures of Bacillus subtilis, is fungicidal through its activity on cell membranes [167]. Lipopeptide aggregates and lipopeptide/phospholipid complexes form at the peptide-membrane interface resulting in the creation of ionic pores that allows the increased influx of potassium ions and fungal cell death [168]. These pores may also provide a means by which ATP is released from damaged cells. Membrane damage results in leakage and localized increases in extracellular ATP as the microbial membrane is damaged but intracellular metabolic processes continue. Vylkova et al. hypothesized that this may contribute to cell death in Candida albicans and other microorganisms by facilitating further peptide uptake or activating the host’s innate immune response with ATP acting as a chemo-attractant at the site of infection [169].
2.2. Mechanism of Action of Antimicrobial Peptides: Intracellular Targeting
Membrane damage is only one of many mechanisms that antimicrobial peptides may possess in exerting microbial cell death. In many cases it may not be the principle mechanism. There is increasing evidence for intracellular targeting of microbes (Figure 3: part E–I) as both alternative and synergistic pathways to membrane rupture and cell lysis. The antimicrobial peptide buforin II, possessing a linear proline hinge and containing an amphipathic α-helical peptide, has been proven to translocate across the cell membrane without loss of the transmembrane potential and with intracellular contents intact even at five times the MIC. Cellular function of Escherichia coli is inhibited by accumulation of buforin II in the cytoplasm and via binding to DNA and RNA [170]. Binding to and inhibition of cellular nucleic acids by cationic antimicrobial peptides is feasible due to the polyanionic charges present in nucleic acids and also some intracellular enzymes [26,171].
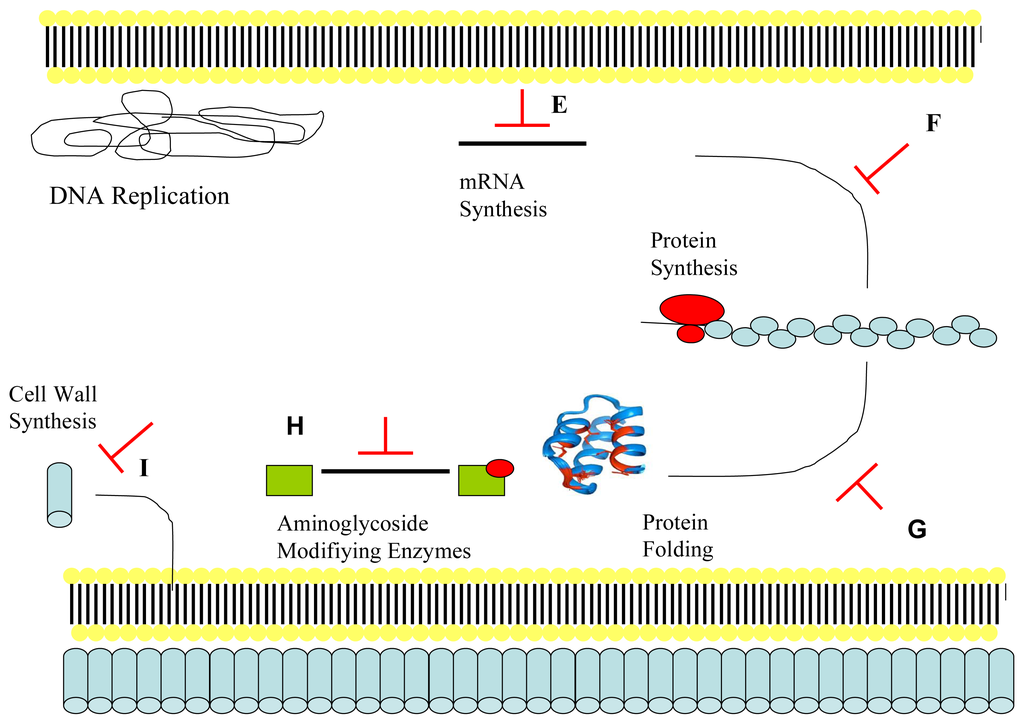
Figure 3.
The intracellular action of some antimicrobial peptides (E−I). (E) Dermaseptin, buforin-II and pleurocidin are antimicrobial peptides that have been shown to inhibit both DNA and RNA synthesis at MIC values [188]. Dermaseptin inhibits RNA synthesis in bacteria at MIC concentration or higher within 5 minutes, with lack of bactericidal action within 30 minutes in Escherichia coli evidence of a mainly intracellular action [189]. Buforin-II contains a single proline residue within its primary structure that allows translocation across cell membranes without membrane destruction, with binding to nucleic acids resulting in cell death [170,190]; (F) PR-39 and indolicidin have been shown to inhibit the rate of protein synthesis and is therefore a plausible target for antimicrobial peptides [13,180,191]; (G) Pyrrhocoricin and drosocin act one step later than the molecules of part E (dermaseptin, buforin-II and pleurocidin) and have been shown to reduce enzymatic activity via inhibition of ATPase activity of the heat-shock protein DnaK, an enzyme involved in chaperone-assisted protein folding [192–194]. Otvos and colleagues formed a chimeric dimer, with newly formed activity against Staphylococcus aureus and increased activity against previously sensitive Escherichia coli, by synthesizing a molecule that possessed pyrrhocoricin’s DnaK binding domain and drosocin’s high membrane permeating ability [195]; (H) Antimicrobial peptides may also inhibit resistance mechanisms linked to bacterial pathogenesis for example enzymes with anionic binding site pockets linked to the modification of aminoglycoside antibiotics [171]; (I) Lantibiotics such as mersacidin and nisin target the formation of structural components of the cell wall, specifically the transglycosylation of lipid II, necessary for the synthesis of peptidoglycan [196,197]. Adapted from Jenssen, Hamill and Hancock 2006 [147].
Some antimicrobial peptides such as indolicidin have been demonstrated to penetrate bacterial cell membranes rendering them relatively undamaged but with antibacterial activity achieved by inhibition of RNA, DNA and protein synthesis [172]. This contrasts to a mainly membrane active targeting of fungal cells by indolicidin via direct interactions with the phospholipid bilayer [173]. Other hypotheses for intracellular action include stimulation of the autolytic enzyme cascade [174]. Cationic antimicrobial peptides, for example lactoferrin and lysozyme, may mimic the action of β-lactam antibiotics and activate autolytic cell wall enzymes such as muramidases causing bacteriolysis [175]. Intracellular targeting of protein synthesis by degradation of proteins required for DNA replication has been shown to be a primary mechanism of action for indolicidin and the pig intestinal peptide PR-39 [176–178]. Indolicidin and lactoferricin B have also been shown to induce filamentation, a process in which bacterial cells continue to elongate but cannot divide as the septum does not develop, and significant cell lysis [179–181]. Filamentation results from inhibition of DNA synthesis [182].
In mammals the antimicrobial cathelicidin peptides LL-37 and PR-39 have multiple membrane and intracellular mechanisms of action but they can also mitigate the immune response to foreign pathogens [183,184]. LL-37 induces the selective movement of neutrophils, monocytes and CD4 T-lymphocyte cells allowing an increase in the adaptive and innate immune response [185,186]. The porcine peptide PR-39 promotes healing via stimulation of angiogenesis mediated by its degradation of hypoxia inducible factor 1a protein [187].
Alternative bactericidal intracellular mechanisms are outlined in Figure 3: parts E-I. By possessing multiple microbial targets, antimicrobial peptides can be a therapeutically valuable tool as the potential for microbial resistance is low (perhaps negligible). The chances of resistance developing is lessened further by many antimicrobial peptides possessing similar MIC and minimum bactericidal concentration (MBC) values (MBC no more than double the value obtained for MIC), owing to mainly bactericidal action [198]. The intracellular mode of action of antimicrobial peptides against fungal pathogens is linked to interactions with the fungal mitochondrion, with targeting and perturbation of the mitochondrial membrane shown by Helmerhorst et al. [199]. The cationic peptides histatins, derived from the saliva of humans, bind to receptors present on the fungal cell membrane and when present in the cytoplasm they target fungal mitochondria [200]. The echinocandins are an antimicrobial peptide family sourced from Aspergillus nidulans that includes the peptides anidulafungin caspofungin, and micafungin [201]. They have been shown to inhibit β-(1,3)-glucan synthase, an enzyme responsible for the production of the essential fungal cell wall component β-(1,3)-glucan [202–204].
3. Development of Resistance to Antimicrobial Peptides
The multiple modes of action utilized by antimicrobial peptides reduces the ability of microorganisms to develop resistance, with cidal activity also shown against bacteria resistant to standard antibiotics [205]. The formation of a highly hydrated extracellular polymeric phenotype or biofilm contributes to antimicrobial resistance by blocking the transport of antimicrobials through the biofilm matrix. Possible mechanisms for this to occur are by binding of the biofilm to them directly, as in the case of positively charged aminoglycoside antibiotics, restricting their permeation and by restricting diffusion of larger antimicrobials [206,207].
Extracellular DNA may also play a role in increased resistance of biofilm forms of Pseudomonas aeruginosa against cationic antimicrobial peptides. Extracellular DNA is a cation chelator and acts to sequester cations from the surrounding environment and also plays a role in the modification of the cationic antimicrobial peptide binding site lipid A by the sugar dehydrogenases enzyme Undecaprenyl phosphate-glucose dehydrogenase and covalent binding to 4-amino-4-deoxy-L-arabinose [208]. Resistance is conferred via covalent modification of the cationic antimicrobial binding site lipid A. Lipid A is a hydrophobic anchor situated on the outer surface of the inner membrane of Gram-negative bacteria and acts as an anionic membrane target for cationic drugs such as polymyxin and also cationic antimicrobial peptides. Covalent binding to 4-amino-4-deoxy-L-arabinose moiety reduces both the anionic charge of lipid A and its affinity for cationic antimicrobials. In Gram-negative bacteria such as Escherichia coli the expression of genes (arn and ugd operons) involved in Undecaprenyl phosphate-4-amino-4-deoxy-L-arabinose production are controlled by the two component quorum sensing systems PhoP/PhoQ and PmrA/PmrB; and/or the RcsA/RcsB/RcsC system as outlined in Figures 4, 5 and 6.
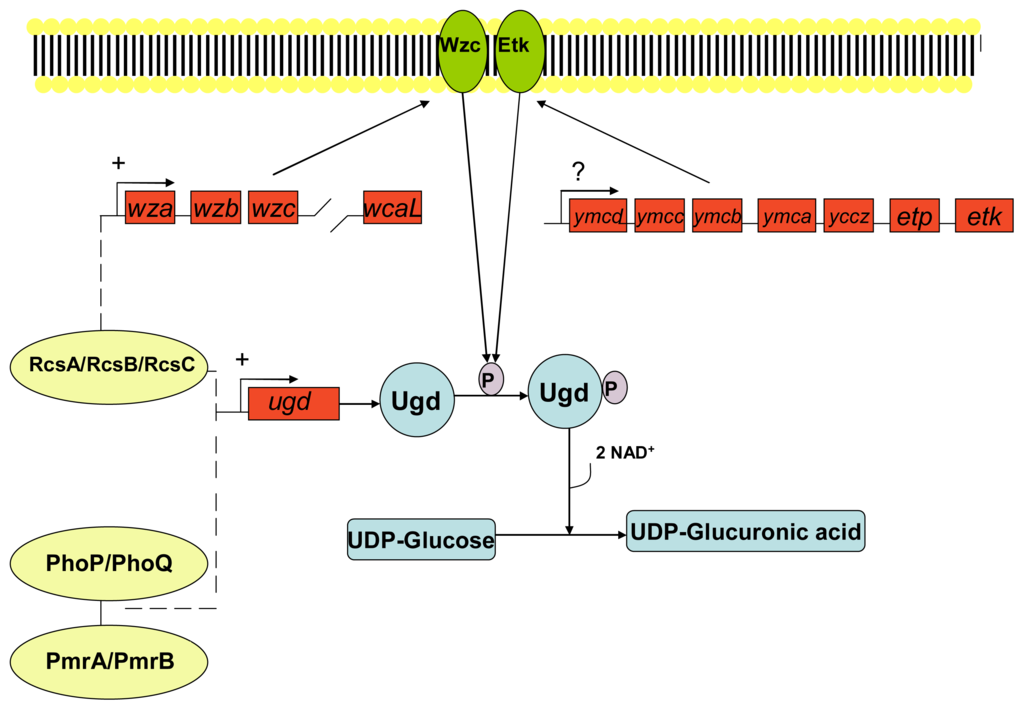
Figure 4.
The synthesis of Undecaprenyl phosphate-glucuronic acid via quorum sensing systems. The two component quorum sensing systems PhoP/PhoQ and PmrA/PmrB; or the RcsA/RcsB/RcsC system alone allow the expression of genes (ugd) involved in the intracellular synthesis of the 4-amino-4-deoxy-L-arabinose precursor: Undecaprenyl phosphate-glucuronic acid. Both scenarios involve the phosphorylation of the protein Ugd by the membrane bound autophosphorylated protein-tyrosine kinase Wzc and/or Etk. Etk and Wzc are a BY-kinase (a newly defined group of enzymes involved in protein-tyrosine phosphorylation) of Escherichia coli and are involved in the production of the group IV capsule surrounding the cell membrane [209]. The wca operon is upregulated by the RcsA/RcsB/RcsC system and consists of 19 genes with the third gene in order of transcription being the wzc gene that has been shown to encode a membrane bound autophosphorylated protein-tyrosine kinase Wzc [210]. Etk is coded for by the etk gene present on the ymc operon of some pathogenic strains of Escherichia coli [211]. The mechanism of the ymc operon is itself unknown, although it could possibly be a promoter of etk expression. Key: UDP: Undecaprenyl phosphate. (Adapted from Lacour, 2008 [212])
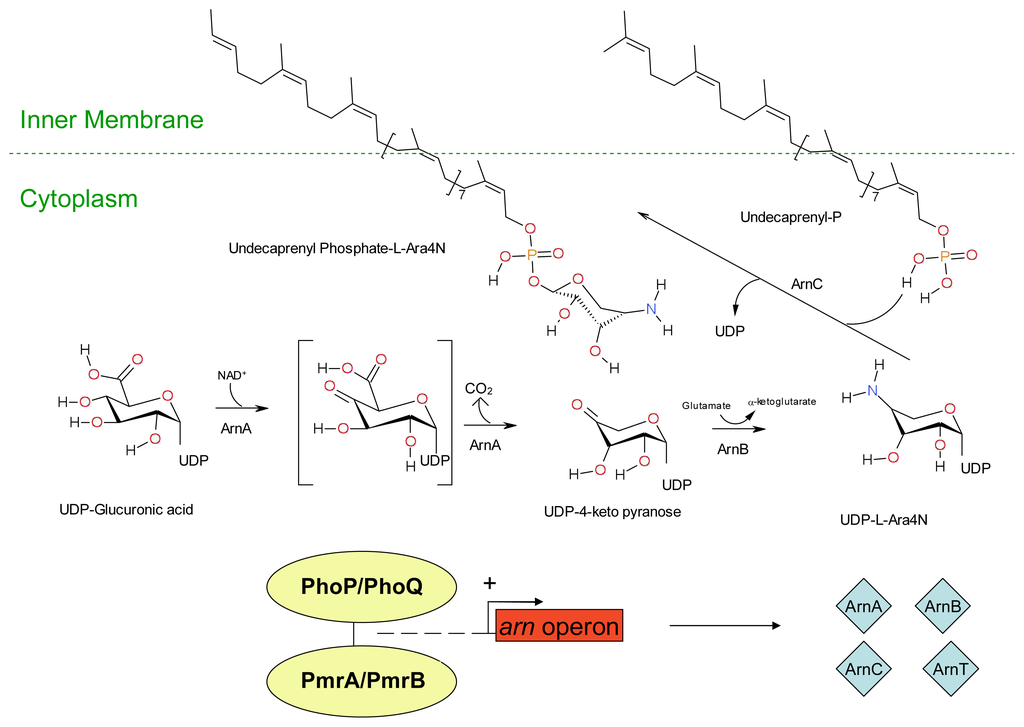
Figure 5.
The synthesis of Undecaprenyl Phosphate-α-4-amino-4-deoxy-L-arabinose from Undecaprenyl-Glucuronic acid. The enzymes ArnA, ArnB, ArnC and ArnT are produced by transcription of the arn operon which is upregulated by the RcsA/RcsB/RcsC and PmrA/PmrB quorum sensing systems. The enzyme ArnA catalyses the oxidative decarboxylation of Undecaprenyl -glucuronic acid, thus forming an Undecaprenyl -4-keto-pyranose intermediate. ArnC catalyzes the transfer of the 4-amino-4-deoxy-L-arabinose moiety to Undecaprenyl phosphate in the inner membrane, forming the Undecaprenyl phosphate-α-4-amino-4-deoxy-L-arabinose. Key: UDP: Undecaprenyl phosphate. (Adapted from Lacour, S. 2008 [212]).
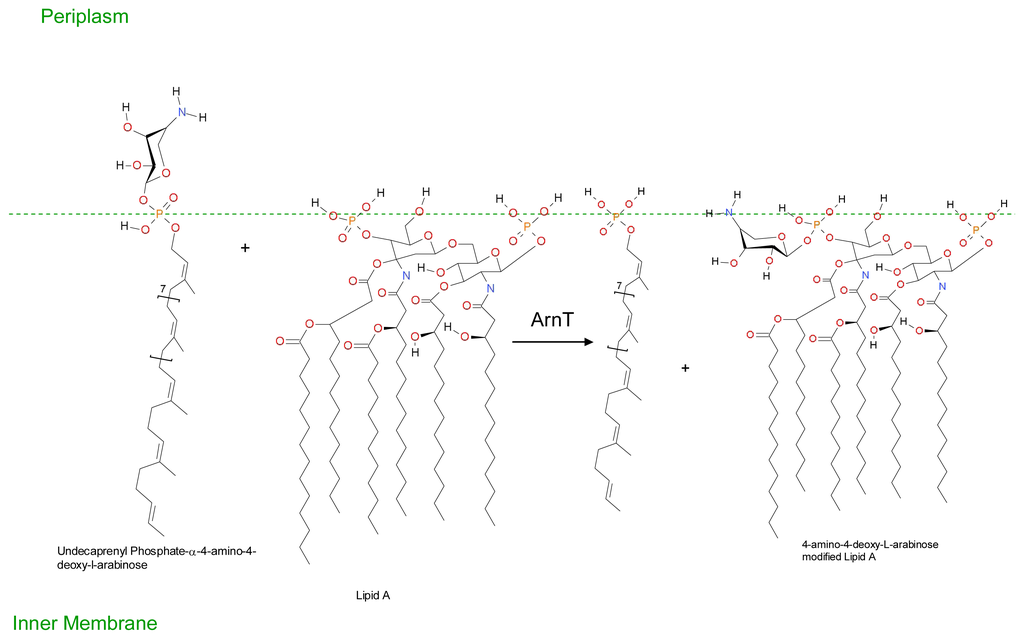
Figure 6.
The transfer of 4-amino-4-deoxy-L-arabinose moiety to Lipid A. Translocation to the outer surface of the inner membrane occurs via an unknown mechanism. ArnT transfer the 4-amino-4-deoxy-L-arabinose moiety from Undecaprenyl phosphate-α-4-amino-4-deoxy-L-arabinose to Lipid A, thereby reducing the affinity of Lipid A for polymyxin and other cationic antimicrobial peptides. (Adapted from Lacour, S. 2008 [212]).
4. Future Perspectives
As biocides antimicrobial peptides have the potential to eradicate the most resistant forms of clinically relevant biofilm forming pathogens. Lipopeptides such as polymyxin B and daptomycin are already utilized in topical formulations [213], therefore the potential exists for the ornithine and tryptophan containing peptides to be exploited similarly. More advanced forms of topical treatment would mimic the use of the polycationic lipopeptide colistin and the polycationic trisaccharides tobramycin and gentamicin as aerosol therapy for the treatment of persistent lung infections in cystic fibrosis patients [214,215]. The potential for natural and synthetic peptides as therapeutic molecules go beyond the boundaries of microbial biofilm infection. Research has extended to the use of these peptides from areas as diverse as cancer treatment [216], to the eradication of sexually transmitted diseases [217], such as HIV [218], with a dual role as an effective contraceptive spermicide [219,220]. A group of cationic peptides, referred to as cell-penetrating peptides can translocate into the cell cytoplasm without disruption of the cell membrane. Peptides such as Apidaecins have the potential to act as precursors for the transport of alternative drugs to mammalian cells [221]. Despite the fact there are thousands of naturally sourced antimicrobial peptides and millions of potential synthetic possibilities there have been limited clinical trials based on antimicrobial peptides [222]. Only a relative few, for example daptomycin, have entered into clinical trials and therapeutic use based on in vitro results and animal studies [106,223,224]. Issues still remain with regard to the stability of peptide based formulations in vivo and the large scale production costs of these peptides. It is expected that future research will allow the area of antimicrobial peptides to be harnessed therapeutically with the same degree of evolutionary success as they are utilized in nature as components of innate immunity. Antimicrobial peptides fulfill a number of the criteria expected from an ‘ideal’ biocide, namely performance (high cidal activity and a rapid rate of kill across a range of microorganisms), environmental fate (facile bioremediation), safety and cost.
Acknowledgements
The authors gratefully acknowledge support from the Intertrade Ireland FUSION scheme for GL.
References
- Falagas, ME; Fragoulis, KN; Karydis, I. A comparative study on the cost of new antibiotics and drugs of other therapeutic categories. PLoS ONE 2006, 1, 1–4. [Google Scholar]
- Chambers, HF; Deleo, FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol 2009, 7, 629–641. [Google Scholar]
- Wenzel, RP. The antibiotic pipeline-challenges, costs and values. N Engl J Med 2001, 523–526. [Google Scholar]
- Boucher, HW; Talbot, GH; Bradley, JS; Edwards, JE; Gilbert, D; Rice, LB; Scheld, M; Spellberg, B; Bartlett, J. Bad bugs no drugs: No ESKAPE! An update from the infectious diseases society of America. Clin. Infect. Dis 2009, 48, 1–12. [Google Scholar]
- Rice, LB. Do we really need new anti-infective drugs? Curr. Opin. Pharmacol 2003, 3, 459–463. [Google Scholar]
- Andreu, D; Rivas, L. Animal antimicrobial peptides: An overview. Biopolymers 1998, 47, 415–433. [Google Scholar]
- Joerger, RD. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci 2003, 82, 640–647. [Google Scholar]
- Brown, KL; Hancock, RE. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol 2006, 18, 24–30. [Google Scholar]
- Bowdish, DM; Davidson, DJ; Lau, YE; Lee, K; Scott, MG; Hancock, RE. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol 2005, 77, 451–459. [Google Scholar]
- Rosenfeld, Y; Sahl, HG; Shai, Y. Parameters involved in antimicrobial and endotoxin detoxification activities of antimicrobial peptides. Biochemistry 2008, 47, 6468–6478. [Google Scholar]
- Elsbach, P. What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J. Clin. Invest 2003, 111, 1643–1645. [Google Scholar]
- Yeaman, MR; Yount, NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev 2003, 55, 27–55. [Google Scholar]
- Brogden, KA. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol 2005, 3, 238–250. [Google Scholar]
- Ganz, T; Lehrer, RI. Antibiotic peptides from higher eukaryotes: Biology and applications. Mol. Med. Today 1999, 5, 292–297. [Google Scholar]
- Van’t Hof, W; Veerman, EC; Helmerhorst, EJ; Amerongen, AV. Antimicrobial peptides: Properties and applicability. Biol. Chem 2001, 382, 597–619. [Google Scholar]
- Hancock, RE; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 2000, 8, 402–410. [Google Scholar]
- Brahmachary, M; Krishnan, SP; Koh, JL; Khan, AM; Seah, SH; Tan, TW; Brusic, V; Bajic, VB. ANTIMIC: A database of antimicrobial sequences. Nucleic Acids Res 2004, 32, D586–D589. [Google Scholar]
- Hwang, PM; Vogel, HJ. Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol 1998, 76, 235–246. [Google Scholar]
- Powers, JP; Hancock, RE. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar]
- Hilpert, K; Elliott, MR; Volkmer-Engert, R; Henklein, P; Donini, O; Zhou, Q; Winkler, DF; Hancock, RE. Sequence requirements and an optimization strategy for short antimicrobial peptides. Chem. Biol 2006, 13, 1101–1107. [Google Scholar]
- Bisht, GS; Rawat, DS; Kumar, A; Kumar, R; Pasha, S. Antimicrobial activity of rationally designed amino terminal modified peptides. Bioorg. Med. Chem. Lett 2007, 17, 4343–4346. [Google Scholar]
- Hicks, RP; Bhonsle, JB; Venugopal, D; Koser, BW; Magill, AJ. De novo design of selective antibiotic peptides by incorporation of unnatural amino acids. J. Med. Chem 2007, 50, 3026–3036. [Google Scholar]
- Laverty, G; McLaughlin, M; Shaw, C; Gorman, SP; Gilmore, BF. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem. Biol. Drug Des 2010, 75, 563–569. [Google Scholar]
- Marshall, SH; Arenas, G. Antimicrobial Peptides: A natural alternative to chemical antibiotics and a potential for applied biotechnology. Electron. J. Biomed 2003, 6, 272–284. [Google Scholar]
- Hancock, RE; Brown, KL; Mookherjee, N. Host defence peptides from invertebrates—Emerging antimicrobial strategies. Immunobiology 2006, 211, 315–322. [Google Scholar]
- McPhee, JB; Hancock, RE. Function and therapeutic potential of host defence peptides. J. Pept. Sci 2005, 11, 677–687. [Google Scholar]
- Guskey, MT; Tsuji, BT. A comparative review of the lipoglycopeptides: Oritavancin, dalbavancin, and telavancin. Pharmacotherapy 2010, 30, 80–94. [Google Scholar]
- Zelezetsky, I; Pag, U; Sahl, HG; Tossi, A. Tuning the biological properties of amphipathic alpha-helical antimicrobial peptides: Rational use of minimal amino acid substitutions. Peptides 2005, 26, 2368–2376. [Google Scholar]
- Thomas, S; Karnik, S; Barai, RS; Jayaraman, VK; Idicula-Thomas, S. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Res 2010, 38, D774–D780. [Google Scholar]
- Maloy, WL; Kari, UP. Structure-activity studies on magainins and other host defense peptides. Biopolymers 1995, 37, 105–122. [Google Scholar]
- Saberwal, G; Nagaraj, R. Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: Facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim. Biophys. Acta 1994, 1197, 109–131. [Google Scholar]
- Ganz, T; Lehrer, RI. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol 1998, 10, 41–44. [Google Scholar]
- Hancock, RE; Chapple, DS. Peptide antibiotics. Antimicrob. Agents Chemother 1999, 43, 1317–1323. [Google Scholar]
- Shai, Y. From innate immunity to de-novo designed antimicrobial peptides. Curr. Pharm. Des 2002, 8, 715–725. [Google Scholar]
- Bhunia, A; Domadia, PN; Bhattacharjya, S. Structural and thermodynamic analyses of the interaction between melittin and lipopolysaccharide. Biochim. Biophys. Acta 2007, 1768, 3282–3291. [Google Scholar]
- Zasloff, M. Magainins, a class of antimicrobial peptides from xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar]
- Bulet, P; Hetru, C; Dimarcq, JL; Hoffmann, D. Antimicrobial peptides in insects; Structure and function. Dev. Comp. Immunol 1999, 23, 329–344. [Google Scholar]
- Powers, JP; Rozek, A; Hancock, RE. Structure-activity relationships for the beta-hairpin cationic antimicrobial peptide polyphemusin I. Biochim. Biophys. Acta 2004, 1698, 239–250. [Google Scholar]
- Boman, HG. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol 1995, 13, 61–92. [Google Scholar]
- Yang, ST; Yub Shin, SY; Kim, YC; Kim, Y; Hahm, KS; Kim, JI. Conformation-dependent antibiotic activity of tritrpticin, a cathelicidin-derived antimicrobial peptide. Biochem. Biophys. Res. Commun 2002, 296, 1044–1050. [Google Scholar]
- Selsted, ME; Novotny, MJ; Morris, WL; Tang, YQ; Smith, W; Cullor, JS. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem 1992, 267, 4292–4295. [Google Scholar]
- Kim, SM; Kim, JM; Joshi, BP; Cho, H; Lee, KH. Indolicidin-derived antimicrobial peptide analogs with greater bacterial selectivity and requirements for antibacterial and hemolytic activities. Biochim. Biophys. Acta 2009, 1794, 185–192. [Google Scholar]
- Halevy, R; Rozek, A; Kolusheva, S; Hancock, RE; Jelinek, R. Membrane binding and permeation by indolicidin analogs studied by a biomimetic lipid/polydiacetylene vesicle assay. Peptides 2003, 24, 1753–1761. [Google Scholar]
- Foster, JW; Woodruff, HB. Antibiotic substances produced by bacteria. Ann. N. Y. Acad. Sci 2010, 1213, 125–136. [Google Scholar]
- Greenhalgh, DG. Topical antimicrobial agents for burn wounds. Clin. Plast. Surg 2009, 36, 597–606. [Google Scholar]
- Laforce, FM; Boose, DS. Sublethal damage of Escherichia coli by lung lavage. Am. Rev. Respir. Dis 1981, 124, 733–737. [Google Scholar]
- LaForce, FM; Boose, DS. Effect of zinc and phosphate on an antibacterial peptide isolated from lung lavage. Infect. Immun 1984, 45, 692–696. [Google Scholar]
- Harris, F; Dennison, SR; Phoenix, DA. Anionic antimicrobial peptides from eukaryotic organisms. Curr. Protein Pept. Sci 2009, 10, 585–606. [Google Scholar]
- Lai, R; Liu, H; Hui Lee, W; Zhang, Y. An anionic antimicrobial peptide from toad. Bombina Maxima. Biochem. Biophys. Res. Commun 2002, 295, 796–799. [Google Scholar]
- Steffen, H; Rieg, S; Wiedemann, I; Kalbacher, H; Deeg, M; Sahl, HG; Peschel, A; Gotz, F; Garbe, C; Schittek, B. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob. Agents Chemother 2006, 50, 2608–2620. [Google Scholar]
- Goumon, Y; Lugardon, K; Gadroy, P; Strub, JM; Welters, ID; Stefano, GB; Aunis, D; Metz-Boutigue, MH. Processing of proenkephalin-a in bovine chromaffin cells. Identification of natural derived fragments by N-terminal sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Biol. Chem 2000, 275, 38355–38362. [Google Scholar]
- Diego-Garcia, E; Batista, CV; Garcia-Gomez, BI; Lucas, S; Candido, DM; Gomez-Lagunas, F; Possani, LD. The Brazilian scorpion Tityus costatus karsch: Genes, peptides and function. Toxicon 2005, 45, 273–283. [Google Scholar]
- Bruhn, H; Winkelmann, J; Andersen, C; Andra, J; Leippe, M. Dissection of the mechanisms of cytolytic and antibacterial activity of lysenin, a defence protein of the annelid Eisenia Fetida. Dev. Comp. Immunol 2006, 30, 597–606. [Google Scholar]
- Prochazkova, P; Silerova, M; Felsberg, J; Joskova, R; Beschin, A; de Baetselier, P; Bilej, M. Relationship between hemolytic molecules in Eisenia Fetida earthworms. Dev. Comp. Immunol 2006, 30, 381–392. [Google Scholar]
- Brogden, KA; de Lucca, AJ; Bland, J; Elliott, S. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc. Natl. Acad. Sci. USA 1996, 93, 412–416. [Google Scholar]
- Grubor, B; Meyerholz, DK; Ackermann, MR. Collectins and cationic antimicrobial peptides of the respiratory epithelia. Vet. Pathol 2006, 43, 595–612. [Google Scholar]
- Schutte, BC; McCray, PB, Jr. [Beta]-defensins in lung host defense. Annu. Rev. Physiol 2002, 64, 709–748. [Google Scholar]
- Brogden, KA; Ackermann, M; McCray, PB, Jr; Tack, BF. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob Agents 2003, 22, 465–478. [Google Scholar]
- Vandendriessche, L. Inhibitors of ribonuclease activity. Arch. Biochem. Biophys 1956, 65, 347–353. [Google Scholar]
- Sela, M. Inhibition of ribonuclease by copolymers of glutamic acid and aromatic amino acids. J. Biol. Chem 1962, 237, 418–421. [Google Scholar]
- Conlon, JM; Al-Ghaferi, N; Abraham, B; Leprince, J. Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 2007, 42, 349–357. [Google Scholar]
- Conlon, JM; Raza, H; Coquet, L; Jouenne, T; Leprince, J; Vaudry, H; King, JD. Purification of peptides with differential cytolytic activities from the skin secretions of the central American frog, Lithobates Vaillanti (Ranidae). Comp. Biochem. Physiol. C. Toxicol. Pharmacol 2009, 150, 150–154. [Google Scholar]
- VanCompernolle, SE; Taylor, RJ; Oswald-Richter, K; Jiang, J; Youree, BE; Bowie, JH; Tyler, MJ; Conlon, JM; Wade, D; Aiken, C; et al. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J. Virol 2005, 79, 11598–11606. [Google Scholar]
- Nicolas, P; Mor, A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol 1995, 49, 277–304. [Google Scholar]
- Simmaco, M; Mignogna, G; Barra, D. Antimicrobial peptides from amphibian skin: What do they tell us? Biopolymers 1998, 47, 435–450. [Google Scholar]
- Tyler, MJ; Stone, DJ; Bowie, JH. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar]
- Nutkins, JC; Williams, DH. Identification of highly acidic peptides from processing of the skin prepropeptides of Xenopus laevis. Eur. J. Biochem 1989, 181, 97–102. [Google Scholar]
- Mor, A; Hani, K; Nicolas, P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J. Biol. Chem 1994, 269, 31635–31641. [Google Scholar]
- Conlon, JM; Kolodziejek, J; Nowotny, N. Antimicrobial peptides from ranid frogs: Taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim. Biophys. Acta 2004, 1696, 1–14. [Google Scholar]
- Tossi, A; Sandri, L; Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar]
- Mignogna, G; Simmaco, M; Kreil, G; Barra, D. Antibacterial and haemolytic peptides containing d-alloisoleucine from the skin of Bombina variegata. EMBO J 1993, 12, 4829–4832. [Google Scholar]
- Clark, DP; Durell, S; Maloy, WL; Zasloff, M. Ranalexin. A novel antimicrobial peptide from bullfrog (Rana Catesbeiana) skin, structurally related to the bacterial antibiotic, polymyxin. J. Biol. Chem 1994, 269, 10849–10855. [Google Scholar]
- Mor, A; Nguyen, VH; Delfour, A; Migliore-Samour, D; Nicolas, P. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 1991, 30, 8824–8830. [Google Scholar]
- Raftery, MJ; Waugh, RJ; Bowie, JH; Wallace, JC; Tyler, MJ. The structures of the frenatin peptides from the skin secretion of the giant tree frog Litoria infrafrenata. J. Pept. Sci 1996, 2, 117–124. [Google Scholar]
- Lee, WH; Li, Y; Lai, R; Li, S; Zhang, Y; Wang, W. Variety of antimicrobial peptides in the bombina maxima toad and evidence of their rapid diversification. Eur. J. Immunol 2005, 35, 1220–1229. [Google Scholar]
- Lai, R; Zheng, YT; Shen, JH; Liu, GJ; Liu, H; Lee, WH; Tang, SZ; Zhang, Y. Antimicrobial peptides from skin secretions of Chinese red belly toad Bombina maxima. Peptides 2002, 23, 427–435. [Google Scholar]
- Hernandez, C; Mor, A; Dagger, F; Nicolas, P; Hernandez, A; Benedetti, EL; Dunia, I. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur. J. Cell Biol 1992, 59, 414–424. [Google Scholar]
- Strom, MB; Haug, BE; Skar, ML; Stensen, W; Stiberg, T; Svendsen, JS. The pharmacophore of short cationic antibacterial peptides. J. Med. Chem 2003, 46, 1567–1570. [Google Scholar]
- Haug, BE; Stensen, W; Stiberg, T; Svendsen, JS. Bulky nonproteinogenic amino acids permit the design of very small and effective cationic antibacterial peptides. J. Med. Chem 2004, 47, 4159–4162. [Google Scholar]
- Sieber, P. Modification of tryptophan residues during acidolysis of 4-methoxy-2,3,6-trimethylbenzenesulfonyl groups. Effects of scavengers. Tetrahedron Lett 1987, 28, 1637–1640. [Google Scholar]
- Choi, H; Aldrich, JV. Comparison of methods for the fmoc solid-phase synthesis and cleavage of a peptide containing both tryptophan and arginine. Int. J. Pept. Protein Res 1993, 42, 58–63. [Google Scholar]
- Makovitzki, A; Baram, J; Shai, Y. Antimicrobial lipopolypeptides composed of palmitoyl di- and tricationic peptides: In vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 2008, 47, 10630–10636. [Google Scholar]
- Vallon-Eberhard, A; Makovitzki, A; Beauvais, A; Latge, JP; Jung, S; Shai, Y. Efficient clearance of aspergillus fumigatus in murine lungs by an ultrashort antimicrobial lipopeptide, palmitoyl-Lys-Ala-DAla-Lys. Antimicrob. Agents Chemother 2008, 52, 3118–3126. [Google Scholar]
- Makovitzki, A; Avrahami, D; Shai, Y. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. USA 2006, 103, 15997–16002. [Google Scholar]
- Hancock, RE; Sahl, HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol 2006, 24, 1551–1557. [Google Scholar]
- Schmidtchen, A; Frick, IM; Andersson, E; Tapper, H; Bjorck, L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol 2002, 46, 157–168. [Google Scholar]
- Chen, Y; Vasil, AI; Rehaume, L; Mant, CT; Burns, JL; Vasil, ML; Hancock, RE; Hodges, RS. Comparison of biophysical and biologic properties of alpha-helical enantiomeric antimicrobial peptides. Chem. Biol. Drug Des 2006, 67, 162–173. [Google Scholar]
- Papo, N; Oren, Z; Pag, U; Sahl, HG; Shai, Y. The consequence of sequence alteration of an amphipathic alpha-helical antimicrobial peptide and its diastereomers. J. Biol. Chem 2002, 277, 33913–33921. [Google Scholar]
- Thennarasu, S; Lee, DK; Tan, A; Prasad Kari, U; Ramamoorthy, A. Antimicrobial activity and membrane selective interactions of a synthetic lipopeptide MSI-843. Biochim. Biophys. Acta 2005, 1711, 49–58. [Google Scholar]
- Zhang, L; Falla, TJ. Antimicrobial peptides: Therapeutic potential. Expert Opin. Pharmacother 2006, 7, 653–663. [Google Scholar]
- Hilpert, K; Volkmer-Engert, R; Walter, T; Hancock, RE. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol 2005, 23, 1008–1012. [Google Scholar]
- Haug, BE; Svendsen, JS. The role of tryptophan in the antibacterial activity of a 15-residue bovine lactoferricin peptide. J. Pept. Sci 2001, 7, 190–196. [Google Scholar]
- Lejon, T; Svendsen, JS; Haug, BE. Simple parameterization of non-proteinogenic amino acids for QSAR of antibacterial peptides. J. Pept. Sci 2002, 8, 302–306. [Google Scholar]
- Strom, MB; Rekdal, O; Svendsen, JS. Antibacterial activity of 15-residue lactoferricin derivatives. J. Pept. Res 2000, 56, 265–274. [Google Scholar]
- Strom, MB; Haug, BE; Rekdal, O; Skar, ML; Stensen, W; Svendsen, JS. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochem. Cell Biol 2002, 80, 65–74. [Google Scholar]
- Strom, MB; Rekdal, O; Svendsen, JS. The effects of charge and lipophilicity on the antibacterial activity of undecapeptides derived from bovine lactoferricin. J. Pept. Sci 2002, 8, 36–43. [Google Scholar]
- Strom, MB; Rekdal, O; Svendsen, JS. Antimicrobial activity of short arginine- and tryptophan-rich peptides. J. Pept. Sci 2002, 8, 431–437. [Google Scholar]
- Japelj, B; Zorko, M; Majerle, A; Pristovsek, P; Sanchez-Gomez, S; Martinez de Tejada, G; Moriyon, I; Blondelle, SE; Brandenburg, K; Andra, J; et al. The acyl group as the central element of the structural organization of antimicrobial lipopeptide. J. Am. Chem. Soc 2007, 129, 1022–1023. [Google Scholar]
- Jerala, R. Synthetic lipopeptides: A novel class of anti-infectives. Expert Opin. Investig. Drugs 2007, 16, 1159–1169. [Google Scholar]
- Avrahami, D; Shai, Y. A new group of antifungal and antibacterial lipopeptides derived from non-membrane active peptides conjugated to palmitic acid. J. Biol. Chem 2004, 279, 12277–12285. [Google Scholar]
- Kabara, JJ; Swieczkowski, DM; Conley, AJ; Truant, JP. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother 1972, 2, 23–28. [Google Scholar]
- Kitahara, T; Koyama, N; Matsuda, J; Aoyama, Y; Hirakata, Y; Kamihira, S; Kohno, S; Nakashima, M; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull 2004, 27, 1321–1326. [Google Scholar]
- Shalev, DE; Rotem, S; Fish, A; Mor, A. Consequences of N-acylation on structure and membrane binding properties of dermaseptin derivative K4-S4-(1–13). J. Biol. Chem 2006, 281, 9432–9438. [Google Scholar]
- Tsubery, H; Ofek, I; Cohen, S; Fridkin, M. N-terminal modifications of polymyxin B nonapeptide and their effect on antibacterial activity. Peptides 2001, 22, 1675–1681. [Google Scholar]
- Malina, A; Shai, Y. Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem. J 2005, 390, 695–702. [Google Scholar]
- Arbeit, RD; Maki, D; Tally, FP; Campanaro, E; Eisenstein, BI. Daptomycin 98–01 and 99–01 Investigators. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis 2004, 38, 1673–1681. [Google Scholar]
- Livermore, DM. Future directions with daptomycin. J Antimicrob Chemother 2008, 62, iii41–iii49. [Google Scholar]
- Mohr, JF; Friedrich, LV; Yankelev, S; Lamp, KC. Daptomycin for the treatment of Enterococcal bacteraemia: Results from the Cubicin Outcomes Registry and Experience (CORE). Int. J. Antimicrob. Agents 2009, 33, 543–548. [Google Scholar]
- Andrew, JH; Wale, MC; Wale, LJ; Greenwood, D. The effect of cultural conditions on the activity of LY146032 against Staphylococci and Streptococci. J. Antimicrob. Chemother 1987, 20, 213–221. [Google Scholar]
- Chow, AW; Cheng, N. In vitro activities of daptomycin (LY146032) and paldimycin (U-70,138F) against anaerobic gram-positive bacteria. Antimicrob. Agents Chemother 1988, 32, 788–790. [Google Scholar]
- Alder, JD. Daptomycin: A new drug class for the treatment of gram-positive infections. Drugs Today (Barc. ) 2005, 41, 81–90. [Google Scholar]
- Silverman, JA; Mortin, LI; Vanpraagh, AD; Li, T; Alder, J. Inhibition of daptomycin by pulmonary surfactant: In vitro modeling and clinical impact. J. Infect. Dis 2005, 191, 2149–2152. [Google Scholar]
- Steenbergen, JN; Alder, J; Thorne, GM; Tally, FP. Daptomycin: A lipopeptide antibiotic for the treatment of serious gram-positive infections. J. Antimicrob. Chemother 2005, 55, 283–288. [Google Scholar]
- Jung, D; Rozek, A; Okon, M; Hancock, RE. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem. Biol 2004, 11, 949–957. [Google Scholar]
- Silverman, JA; Perlmutter, NG; Shapiro, HM. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother 2003, 47, 2538–2544. [Google Scholar]
- Shoemaker, DM; Simou, J; Roland, WE. A review of daptomycin for injection (cubicin) in the treatment of complicated skin and skin structure infections. Ther. Clin. Risk Manag 2006, 2, 169–174. [Google Scholar]
- Ainsworth, GC; Brown, AM; Brownlee, G. Aerosporin, an antibiotic produced by Bacillus aerosporus Greer. Nature 1947, 160, 263. [Google Scholar]
- Stansly, PG; Shepherd, RG; White, HL. Polymyxin: A new chemotherapeutic agent. Johns. Hopk. Hosp. Bull 1947, 81, 43–54. [Google Scholar]
- Benedict, RG; Langlykke, AF. Antibiotic activity of Bacillus polymyxa. J. Bacteriol 1947, 54, 24–25. [Google Scholar]
- Koyama, Y; Kurosasa, A; Tsuchiya, A; Takakuta, K. A new antibiotic ‘colistin’ produced by spore-forming soil bacteria. J. Antibiot 1950, 3, 457–458. [Google Scholar]
- Kanazawa, K; Sato, Y; Ohki, K; Okimura, K; Uchida, Y; Shindo, M; Sakura, N. Contribution of each amino acid residue in polymyxin B(3) to antimicrobial and lipopolysaccharide binding activity. Chem. Pharm. Bull. (Tokyo) 2009, 57, 240–244. [Google Scholar]
- Kwa, A; Kasiakou, SK; Tam, VH; Falagas, ME. Polymyxin B: Similarities to and differences from colistin (polymyxin E). Expert Rev. Anti Infect. Ther 2007, 5, 811–821. [Google Scholar]
- Falagas, ME; Kasiakou, SK. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit Care 2006, 10. [Google Scholar] [CrossRef]
- Falagas, ME; Kasiakou, SK. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis 2005, 40, 1333–1341. [Google Scholar]
- British Medical Association; Royal Pharmaceutical Society of Great Britain, British National Formulary; Pharmaceutical Press: London, UK, 2009.
- Li, J; Nation, RL; Milne, RW; Turnidge, JD; Coulthard, K. Evaluation of colistin as an agent against multi-resistant gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar]
- Catchpole, CR; Andrews, JM; Brenwald, N; Wise, R. A reassessment of the in-vitro activity of colistin sulphomethate sodium. J. Antimicrob. Chemother 1997, 39, 255–260. [Google Scholar]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev 1992, 56, 395–411. [Google Scholar]
- Vaara, M; Porro, M. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob. Agents Chemother 1996, 40, 1801–1805. [Google Scholar]
- Clausell, A; Garcia-Subirats, M; Pujol, M; Busquets, MA; Rabanal, F; Cajal, Y. Gram-negative outer and inner membrane models: Insertion of cyclic cationic lipopeptides. J. Phys. Chem. B 2007, 111, 551–563. [Google Scholar]
- Groisman, EA; Kayser, J; Soncini, FC. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J. Bacteriol 1997, 179, 7040–7045. [Google Scholar]
- Conrad, RS; Galanos, C. Fatty acid alterations and polymyxin B binding by lipopolysaccharides from Pseudomonas aeruginosa adapted to polymyxin B resistance. Antimicrob. Agents Chemother 1989, 33, 1724–1728. [Google Scholar]
- Gilleland, HE, Jr; Lyle, RD. Chemical alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J. Bacteriol 1979, 138, 839–845. [Google Scholar]
- Shand, GH; Anwar, H; Brown, MR. Outer membrane proteins of polymyxin resistant Pseudomonas aeruginosa: Effect of magnesium depletion. J. Antimicrob. Chemother 1988, 22, 811–821. [Google Scholar]
- Warren, HS; Kania, SA; Siber, GR. Binding and neutralization of bacterial lipopolysaccharide by colistin nonapeptide. Antimicrob. Agents Chemother 1985, 28, 107–112. [Google Scholar]
- Cardoso, LS; Araujo, MI; Goes, AM; Pacifico, LG; Oliveira, RR; Oliveira, SC. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb. Cell. Fact 2007, 6, 1–6. [Google Scholar]
- Koch-Weser, J; Sidel, VW; Federman, EB; Kanarek, P; Finer, DC; Eaton, AE. Adverse effects of sodium colistimethate. manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med 1970, 72, 857–868. [Google Scholar]
- Brown, JM; Dorman, DC; Roy, LP. Acute renal failure due to overdosage of colistin. Med. J. Aust 1970, 2, 923–924. [Google Scholar]
- Falagas, ME; Rizos, M; Bliziotis, IA; Rellos, K; Kasiakou, SK; Michalopoulos, A. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect Dis 2005, 5. [Google Scholar] [CrossRef] [Green Version]
- Bergen, PJ; Li, J; Rayner, CR; Nation, RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother 2006, 50, 1953–1958. [Google Scholar]
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta 1999, 1462, 1–10. [Google Scholar]
- Neuhaus, FC; Baddiley, J. A continuum of anionic charge: Structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev 2003, 67, 686–723. [Google Scholar]
- Muhle, SA; Tam, JP. Design of gram-negative selective antimicrobial peptides. Biochemistry 2001, 40, 5777–5785. [Google Scholar]
- Avrahami, D; Shai, Y. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to d,l-amino acid-containing antimicrobial peptides: A plausible mode of action. Biochemistry 2003, 42, 14946–14956. [Google Scholar]
- Sung, WS; Lee, DG. Pleurocidin-derived antifungal peptides with selective membrane-disruption effect. Biochem. Biophys. Res. Commun 2008, 369, 858–861. [Google Scholar]
- Lopez-Garcia, B; Marcos, JF; Abad, C; Perez-Paya, E. Stabilisation of mixed peptide/lipid complexes in selective antifungal hexapeptides. Biochim. Biophys. Acta 2004, 1660, 131–137. [Google Scholar]
- Jenssen, H; Hamill, P; Hancock, RE. Peptide antimicrobial agents. Clin. Microbiol. Rev 2006, 19, 491–511. [Google Scholar]
- Hallock, KJ; Lee, DK; Ramamoorthy, A. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys. J 2003, 84, 3052–3060. [Google Scholar]
- Cirac, AD; Moiset, G; Mika, JT; Kocer, A; Salvador, P; Poolman, B; Marrink, SJ; Sengupta, D. The molecular basis for antimicrobial activity of pore-forming cyclic peptides. Biophys. J 2011, 100, 2422–2431. [Google Scholar]
- Matsuzaki, K. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim. Biophys. Acta 1998, 1376, 391–400. [Google Scholar]
- Powers, JP; Tan, A; Ramamoorthy, A; Hancock, RE. Solution structure and interaction of the antimicrobial polyphemusins with lipid membranes. Biochemistry 2005, 44, 15504–15513. [Google Scholar]
- Ehrenstein, G; Lecar, H. Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys 1977, 10, 1–34. [Google Scholar]
- Shai, Y; Oren, Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides 2001, 22, 1629–1641. [Google Scholar]
- He, K; Ludtke, SJ; Worcester, DL; Huang, HW. Neutron scattering in the plane of membranes: Structure of alamethicin pores. Biophys. J 1996, 70, 2659–2666. [Google Scholar]
- Oren, Z; Shai, Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 1998, 47, 451–463. [Google Scholar]
- Bechinger, B; Lohner, K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1529–1539. [Google Scholar]
- Sato, H; Feix, JB. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic alpha-helical antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1245–1256. [Google Scholar]
- Sawyer, JG; Martin, NL; Hancock, RE. Interaction of macrophage cationic proteins with the outer membrane of Pseudomonas aeruginosa. Infect. Immun 1988, 56, 693–698. [Google Scholar]
- Black, CB; Huang, H-W; Cowan, JA. Biological Coordination chemistry of magnesium, sodium, and potassium ions. Protein and nucleotide binding sites. Coord Chem Rev 1994, 135–136, 165–202. [Google Scholar]
- Chen, CZ; Cooper, SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar]
- Giacometti, A; Cirioni, O; Barchiesi, F; Fortuna, M; Scalise, G. In-vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother 1999, 44, 641–645. [Google Scholar]
- Giacometti, A; Cirioni, O; Del Prete, MS; Paggi, AM; D’Errico, MM; Scalise, G. Combination studies between polycationic peptides and clinically used antibiotics against gram-positive and gram-negative bacteria. Peptides 2000, 21, 1155–1160. [Google Scholar]
- Kumar, M; Chaturvedi, AK; Kavishwar, A; Shukla, PK; Kesarwani, AP; Kundu, B. Identification of a novel antifungal nonapeptide generated by combinatorial approach. Int. J. Antimicrob. Agents 2005, 25, 313–320. [Google Scholar]
- Lehrer, RI; Szklarek, D; Ganz, T; Selsted, ME. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect. Immun 1985, 49, 207–211. [Google Scholar]
- Park, Y; Lee, DG; Hahm, KS. HP(2–9)-Magainin 2(1–12), a synthetic hybrid peptide, exerts its antifungal effect on candida albicans by damaging the plasma membrane. J. Pept. Sci 2004, 10, 204–209. [Google Scholar]
- Bellamy, W; Wakabayashi, H; Takase, M; Kawase, K; Shimamura, S; Tomita, M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol 1993, 182, 97–105. [Google Scholar]
- Ajesh, K; Sreejith, K. Peptide antibiotics: An alternative and effective antimicrobial strategy to circumvent fungal infections. Peptides 2009, 30, 999–1006. [Google Scholar]
- Maget-Dana, R; Peypoux, F. Iturins, a special class of pore-forming lipopeptides: Biological and physicochemical properties. Toxicology 1994, 87, 151–174. [Google Scholar]
- Vylkova, S; Sun, JN; Edgerton, M. The role of released ATP in killing Candida albicans and other extracellular microbial pathogens by cationic peptides. Purinergic Signal 2007, 3, 91–97. [Google Scholar]
- Park, CB; Kim, HS; Kim, SC. Mechanism of action of the antimicrobial peptide buforin II: Buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun 1998, 244, 253–257. [Google Scholar]
- Boehr, DD; Draker, KA; Koteva, K; Bains, M; Hancock, RE; Wright, GD. Broad-spectrum peptide inhibitors of aminoglycoside antibiotic resistance enzymes. Chem. Biol 2003, 10, 189–196. [Google Scholar]
- Falla, TJ; Karunaratne, DN; Hancock, RE. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem 1996, 271, 19298–19303. [Google Scholar]
- Lee, DG; Kim, HK; Kim, SA; Park, Y; Park, SC; Jang, SH; Hahm, KS. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun 2003, 305, 305–310. [Google Scholar]
- Ginsburg, I; Koren, E. Are cationic antimicrobial peptides also ‘double-edged swords’? Expert Rev. Anti Infect. Ther 2008, 6, 453–462. [Google Scholar]
- Ginsburg, I. Bactericidal cationic peptides can also function as bacteriolysis-inducing agents mimicking beta-lactam antibiotics? It is enigmatic why this concept is consistently disregarded. Med. Hypotheses 2004, 62, 367–374. [Google Scholar]
- Boman, HG; Agerberth, B; Boman, A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun 1993, 61, 2978–2984. [Google Scholar]
- Holzl, MA; Hofer, J; Steinberger, P; Pfistershammer, K; Zlabinger, GJ. Host antimicrobial proteins as endogenous immunomodulators. Immunol. Lett 2008, 119, 4–11. [Google Scholar]
- Xiong, YQ; Yeaman, MR; Bayer, AS. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob. Agents Chemother 1999, 43, 1111–1117. [Google Scholar]
- Ulvatne, H; Samuelsen, O; Haukland, HH; Kramer, M; Vorland, LH. Lactoferricin B inhibits bacterial macromolecular synthesis in Escherichia coli and Bacillus subtilis. FEMS Microbiol. Lett 2004, 237, 377–384. [Google Scholar]
- Subbalakshmi, C; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett 1998, 160, 91–96. [Google Scholar]
- Healy, DP; Gardner, JC; Puthoff, BK; Kagan, RJ; Neely, AN. Antibiotic-mediated bacterial filamentation: A potentially important laboratory phenomenon. Clin. Microbiol. Newsl 2007, 29, 22–24. [Google Scholar]
- Hsu, CH; Chen, C; Jou, ML; Lee, AY; Lin, YC; Yu, YP; Huang, WT; Wu, SH. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: Evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res 2005, 33, 4053–4064. [Google Scholar]
- Iimura, M; Gallo, RL; Hase, K; Miyamoto, Y; Eckmann, L; Kagnoff, MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol 2005, 174, 4901–4907. [Google Scholar]
- Eckmann, L. Defence molecules in intestinal innate immunity against bacterial infections. Curr. Opin. Gastroenterol 2005, 21, 147–151. [Google Scholar]
- Agerberth, B; Charo, J; Werr, J; Olsson, B; Idali, F; Lindbom, L; Kiessling, R; Jornvall, H; Wigzell, H; Gudmundsson, GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol 2004, 75, 39–48. [Google Scholar]
- Li, J; Post, M; Volk, R; Gao, Y; Li, M; Metais, C; Sato, K; Tsai, J; Aird, W; Rosenberg, RD; et al. PR39, a peptide regulator of angiogenesis. Nat. Med 2000, 6, 49–55. [Google Scholar]
- Rotem, S; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta 2009, 1788, 1582–1592. [Google Scholar]
- Patrzykat, A; Friedrich, CL; Zhang, L; Mendoza, V; Hancock, RE. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother 2002, 46, 605–614. [Google Scholar]
- Park, CB; Kim, MS; Kim, SC. A novel antimicrobial peptide from Bufo Bufo gargarizans. Biochem. Biophys. Res. Commun 1996, 218, 408–413. [Google Scholar]
- Piddock, LJ. Antibacterials-mechanisms of action. Curr. Opin. Microbiol 1998, 1, 502–508. [Google Scholar]
- Hale, JD; Hancock, RE. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti Infect. Ther 2007, 5, 951–959. [Google Scholar]
- Otvos, L, Jr; Rogers, ME; Consolvo, PJ; Condie, BA; Lovas, S; Bulet, P; Blaszczyk-Thurin, M. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry 2000, 39, 14150–14159. [Google Scholar]
- Kragol, G; Lovas, S; Varadi, G; Condie, BA; Hoffmann, R; Otvos, L, Jr. The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 2001, 40, 3016–3026. [Google Scholar]
- Otvos, L, Jr; Snyder, C; Condie, B; Bulet, P; Wade, JD. Chimeric antimicrobial peptides exhibit multiple modes of action. Int. J. Pept. Res. Ther 2005, 11, 29–42. [Google Scholar]
- Brotz, H; Bierbaum, G; Leopold, K; Reynolds, PE; Sahl, HG. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother 1998, 42, 154–160. [Google Scholar]
- Brotz, H; Sahl, HG. New insights into the mechanism of action of lantibiotics—Diverse biological effects by binding to the same molecular target. J. Antimicrob. Chemother 2000, 46, 1–6. [Google Scholar]
- Marr, AK; Gooderham, WJ; Hancock, RE. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol 2006, 6, 468–472. [Google Scholar]
- Helmerhorst, EJ; Breeuwer, P; van’t Hof, W; Walgreen-Weterings, E; Oomen, LC; Veerman, EC; Amerongen, AV; Abee, T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem 1999, 274, 7286–7291. [Google Scholar]
- Kavanagh, K; Dowd, S. Histatins: Antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol 2004, 56, 285–289. [Google Scholar]
- Hodges, RL; Hodges, DW; Goggans, K; Xuei, X; Skatrud, P; McGilvray, D. Genetic modification of an Echinocandin B-producing strain of Aspergillus nidulans to produce mutants blocked in sterigmatocystin biosynthesis. J. Ind. Microbiol 1994, 13, 372–381. [Google Scholar]
- Lorand, T; Kocsis, B. Recent advances in antifungal agents. Mini Rev. Med. Chem 2007, 7, 900–911. [Google Scholar]
- Gonzalez, GM; Tijerina, R; Najvar, LK; Bocanegra, R; Luther, M; Rinaldi, MG; Graybill, JR. Correlation between antifungal susceptibilities of Coccidioides immitis in vitro and antifungal treatment with caspofungin in a mouse model. Antimicrob. Agents Chemother 2001, 45, 1854–1859. [Google Scholar]
- Douglas, CM. Fungal beta(1,3)-d-glucan synthesis. Med. Mycol 2001, 39, 55–66. [Google Scholar]
- Giuliani, A; Pirri, G; Nicoletto, SF. Antimicrobial peptides: An overview of a promising class of therapeutics. Eur. J. Biol 2007, 2, 1–33. [Google Scholar]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother 2001, 45, 999–1007. [Google Scholar]
- Donlan, RM. Role of biofilms in antimicrobial resistance. ASAIO J 2000, 46, S47–S52. [Google Scholar]
- Mulcahy, H; Charron-Mazenod, L; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog 2008, 4, 1–9. [Google Scholar]
- Grangeasse, C; Cozzone, AJ; Deutscher, J; Mijakovic, I. Tyrosine phosphorylation: An emerging regulatory device of bacterial physiology. Trends Biochem. Sci 2007, 32, 86–94. [Google Scholar]
- Vincent, C; Doublet, P; Grangeasse, C; Vaganay, E; Cozzone, AJ; Duclos, B. Cells of Escherichia coli contain a protein-tyrosine kinase, wzc, and a phosphotyrosine-protein phosphatase, wzb. J. Bacteriol 1999, 181, 3472–3477. [Google Scholar]
- Peleg, A; Shifrin, Y; Ilan, O; Nadler-Yona, C; Nov, S; Koby, S; Baruch, K; Altuvia, S; Elgrably-Weiss, M; Abe, CM; et al. Identification of an Escherichia coli operon required for formation of the O-antigen capsule. J. Bacteriol 2005, 187, 5259–5266. [Google Scholar]
- Lacour, S; Bechet, E; Cozzone, AJ; Mijakovic, I; Grangeasse, C. Tyrosine phosphorylation of the UDP-glucose dehydrogenase of Escherichia coli is at the crossroads of colanic acid synthesis and polymyxin resistance. PLoS One 2008, 3, 1–9. [Google Scholar]
- Pirri, G; Giuliani, A; Nicoletto, SF; Pizzuto, L; Rinaldi, AC. Lipopeptides as anti-infectives: A practical perspective. Cent. Eur. J. Biol 2009, 4, 258–273. [Google Scholar]
- Jensen, T; Pedersen, SS; Garne, S; Heilmann, C; Hoiby, N; Koch, C. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J. Antimicrob. Chemother 1987, 19, 831–838. [Google Scholar]
- Kun, P; Landau, LI; Phelan, PD. Nebulized gentamicin in children and adolescents with cystic fibrosis. Aust. Paediatr. J 1984, 20, 43–45. [Google Scholar]
- Hoskin, DW; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar]
- Sambri, V; Marangoni, A; Giacani, L; Gennaro, R; Murgia, R; Cevenini, R; Cinco, M. Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema Pallidum. J. Antimicrob. Chemother 2002, 50, 895–902. [Google Scholar]
- Robinson, WE, Jr; McDougall, B; Tran, D; Selsted, ME. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol 1998, 63, 94–100. [Google Scholar]
- Reddy, KV; Shahani, SK; Meherji, PK. Spermicidal activity of magainins: In vitro and in vivo studies. Contraception 1996, 53, 205–210. [Google Scholar]
- Yedery, RD; Reddy, KV. Antimicrobial peptides as microbicidal contraceptives: Prophecies for prophylactics—A mini review. Eur. J. Contracept. Reprod. Health Care 2005, 10, 32–42. [Google Scholar]
- Henriques, ST; Melo, MN; Castanho, MA. Cell-penetrating peptides and antimicrobial peptides: How different are they. Biochem J 2006, 399, 1–7. [Google Scholar]
- Reddy, KV; Yedery, RD; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar]
- Carpenter, CF; Chambers, HF. Daptomycin: Another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis 2004, 38, 994–1000. [Google Scholar]
- Oleson, FB, Jr; Berman, CL; Kirkpatrick, JB; Regan, KS; Lai, JJ; Tally, FP. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob. Agents Chemother 2000, 44, 2948–2953. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).