Anti-Tumor Activity of a Novel Protein Obtained from Tartary Buckwheat
Abstract
:1. Introduction
2. Results and Discussion
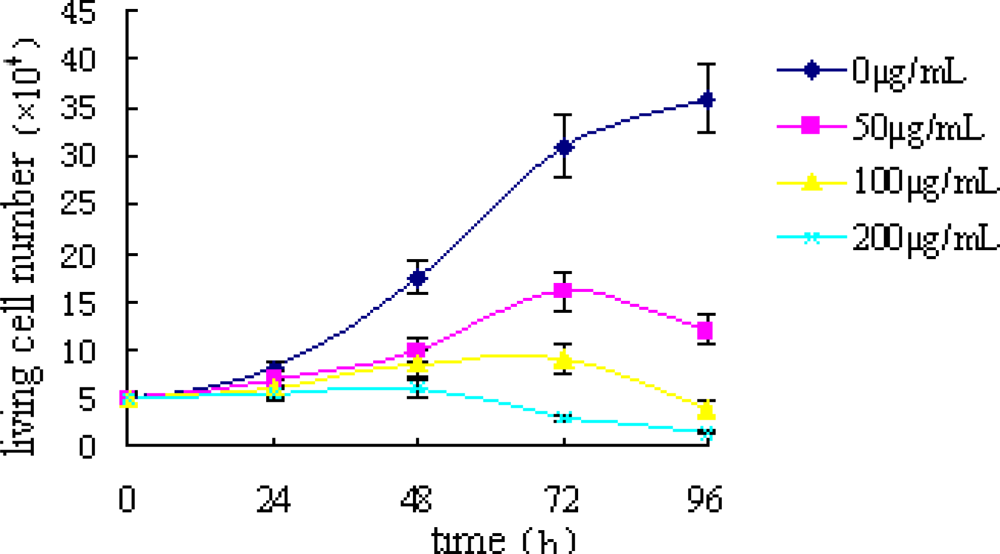
2.1. Trypan Blue Exclusion Test
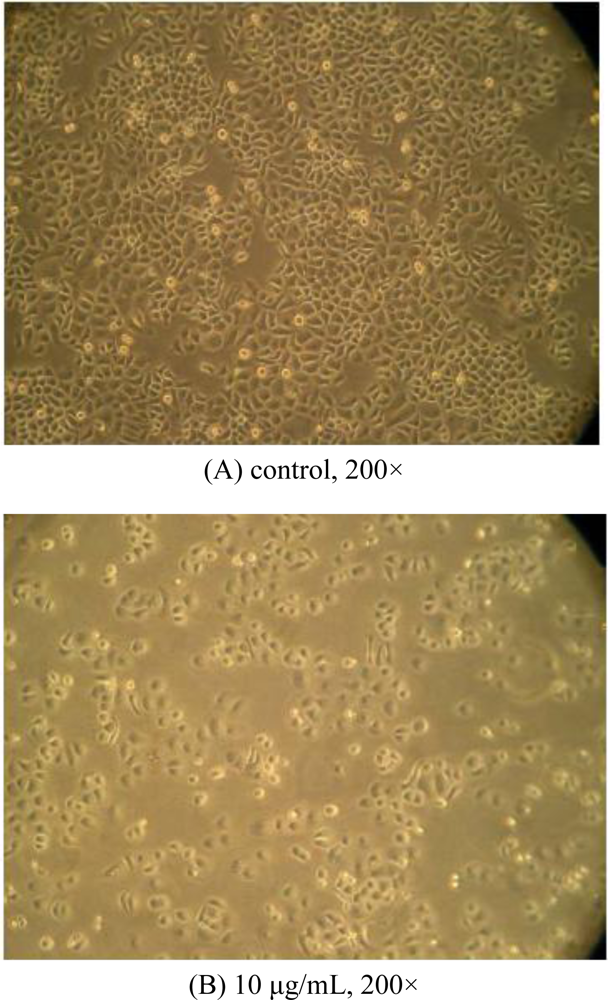
2.2. Morphological Observations by Inverted Microscopy
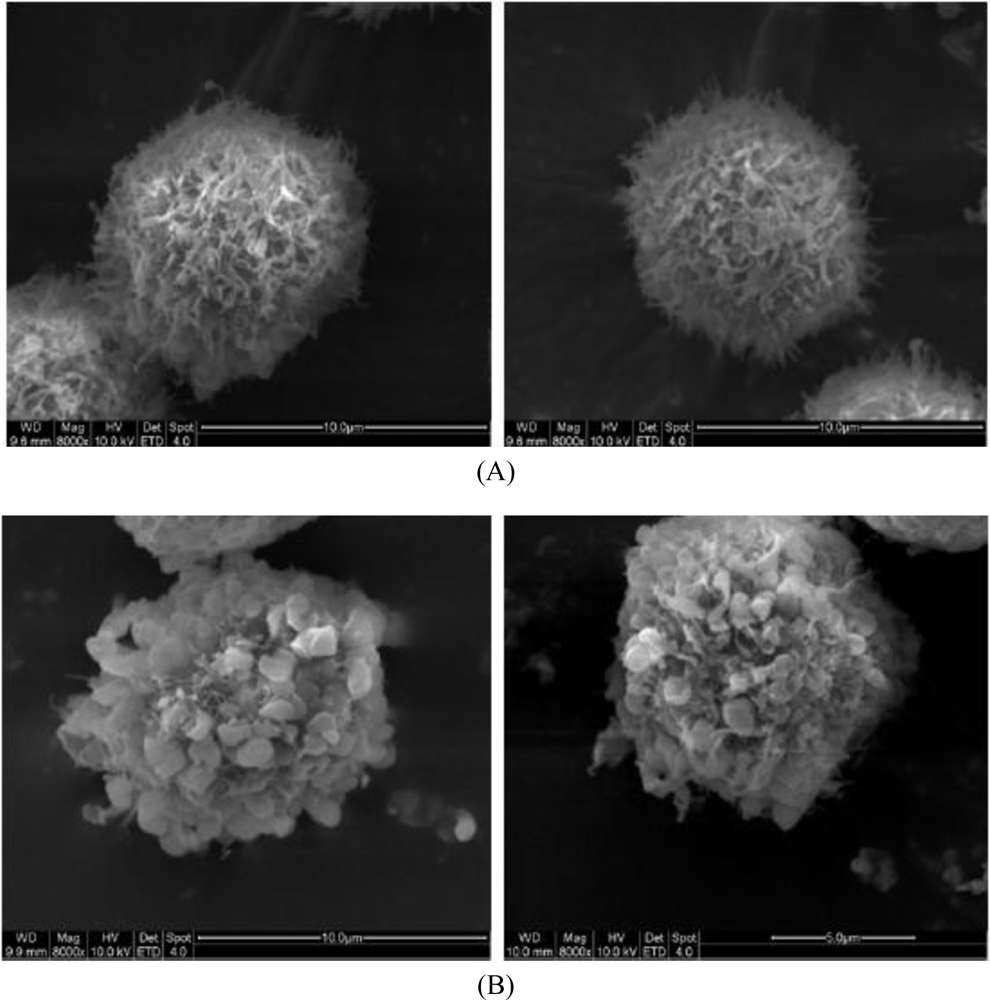
2.3. Morphological Observation by SEM
2.4. Cell Cycle Analysis
2.5. Effect of TBWSP31 on Levels of bcl-2 and Fas Proteins in Bcap37 Cells
3. Experimental Section
3.1. Materials
3.2. Preparation of TBWSP31
3.3. Cell Culture
3.4. Trypan Blue Exclusion Test
3.5. Light Microscopy
3.6. SEM
3.7. Quantitative Detection of Cell DNA with Flow Cytometry (FCM)
3.8. Determination of the Expression of bcl-2 and Fas
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Tahir, I; Farooq, S. Grain composition in some buckwheat cultivars (Fagopyrum spp.) with particular reference to protein fractions. Qual. Plant Food Hum. Nutr 1985, 35, 153–158. [Google Scholar]
- Bonafaccia, G; Gambelli, L; Fabjan, N; Kreft, I. Trace elements in flour and bran from common and tartary buckwheat. Food Chem 2003, 83, 1–5. [Google Scholar]
- Kawakmi, A; Kayahara, H; Ujihara, A. Properties and elimination of bitter components derived from tartary buckwheat (Fagopyrum tataricum) flour. NSKKK 1995, 42, 892–898. [Google Scholar]
- Bonafaccia, G; Marocchini, M; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem 2003, 80, 9–15. [Google Scholar]
- Fabjan, N; Rode, J; Kosir, IJ; Wang, ZH; Zhang, Z; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem 2003, 51, 6452–6455. [Google Scholar]
- Li, SQ; Zhang, HQ. Advances in the development of functional foods from buckwheat. Crit. Rev. Food Sci. Nutr 2001, 41, 451–464. [Google Scholar]
- Kayashita, J; Shimaoka, I; Nakajoh, M; Yamazaki, M. Hypocholesterolemic Effect of Buckwheat Protein Extract in Rats Fed Cholesterol Enriched Diets. Nutr. Res 1995, 15, 691–698. [Google Scholar]
- Kayashita, J; Shimaoka, I; Nakajoh, M; Yamazaki, M; Kato, N. Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-fed rats because of its low digestibility. J. Nutr 1997, 127, 1395–1400. [Google Scholar]
- Tomotake, H; Shimaoka, I; Kayashita, J; Yokoyama, F; Nakajoh, M; Kato, N. Stronger suppression of plasma cholesterol and enhancement of the fecal excretion of steroids by a buckwheat protein product than by a soy protein isolate in rats fed on a cholesterol-free diet. Biosci. Biotechnol. Biochem 2001, 65, 1412–1414. [Google Scholar]
- Tomotake, H; Yamamoto, N; Yanaka, N; Ohinata, H; Yamazaki, R; Kayashita, J; Kato, N. High protein buckwheat flour suppresses hypercholesterolemia in rats and gallstone formation in mice by hypercholesterolemic diet and body fat in rats because of its low protein digestibility. Nutrition 2006, 22, 166–173. [Google Scholar]
- Kayashita, J; Shimaoka, I; Yamazaki, M; Kato, N. Buckwheat protein extract ameliorates atropine-induced constipation in rats. Curr. Adv. Buckwheat Res 1995, 2, 941–946. [Google Scholar]
- Kayashita, J; Shimaoka, I; Nakajoh, M; Kato, N. Feeding of buckwheat protein extract reduces hepatic triglyceride concentration, adipose tissue weight, and hepatic lipogenesis in rats. J. Nutr. Biochem 1996, 7, 555–559. [Google Scholar]
- Liu, Z; Ishikawa, W; Huang, X; Tomotake, H; Kayashita, J; Watanabe, H; Nakajoh, M; Kato, N. A buckwheat protein product suppresses 1,2-dimethyhydrazine-induced colon carcinogenesis in rats by reducing cell proliferation. J. Nutr 2001, 131, 1850–1853. [Google Scholar]
- Kayashita, J; Shimaoka, I; Nakajoh, M; Kishida, N; Kato, N. Consumption of a buckwheat protein extract retards 7,12-dimetylbenz(α)anthracene-induced mammary carcinogenesis in rats. Biosci. Biotechnol. Biochem 1999, 63, 183–1839. [Google Scholar]
- Shen, SC; Ko, CH; Tseng, SW; Tsai, SH; Chen, YC. Structurally related antitumor effects of flavanones in vitro and in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol 2004, 197, 84–95. [Google Scholar]
- Reed, JC. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol. Med 2001, 7, 314–319. [Google Scholar]
- Frankfurt, OS; Krishan, A. Apoptosis-based drug screening and detection of selective toxicity to cancer cells. Anticancer Drugs 2003, 14, 555–561. [Google Scholar]
- Fabris, C; Valduga, G; Miotto, G; Borsetto, L; Jori, G; Garbisa, S; Reddi, E. Photosensitization with zinc (II) phthalocyanine as a switch in the decision between apoptosis and necrosis. Cancer Res 2001, 61, 7495–7500. [Google Scholar]
- MacFarlane, M. TRAIL-induced signaling and apoptosis. Toxicol. Lett 2003, 139, 89–97. [Google Scholar]
- McAree, B; O’Donnell, ME; Spence, A; Lioe, TF; McManus, DT; Spence, RAJ. Breast cancer in women under 40 years of age: A series of 57 cases from Northern Ireland. Breast 2010, 19, 2. 97–104. [Google Scholar]
- Guo, XN; Zhu, KX; Zhang, H; Yao, HY. Purification and characterization of the antitumor protein from Chinese tartary buckwheat water-soluble extracts. J. Agric. Food Chem 2007, 55, 6958–6961. [Google Scholar]
- Desjardins, LM; Macmanus, JP. An adherent cell model to study different stages of apoptosis. Exp. Cell Res 1995, 216, 380–387. [Google Scholar]
- Rao, PVL; Jayaraj, R; Bhaskar, ASB; Kumar, O; Bhattacharya, R; Saxena, P; Dash, PK; Vijayaraghavan. Mechanism of ricin-induced apoptosis in human cervical cancer cells. Biochem. Pharmacol 2005, 69, 865–885. [Google Scholar]
- Coleman, ML; Sahai, EA; Yeo, M; Bosch, M; Dewar, A; Olson, MF. Membrane blebbing during apoptosisresults from caspase-mediated activation of ROCK I. Nat. Cell Biol 2001, 3, 339–345. [Google Scholar]
- Sebbagh, M; Renvoizé, C; Hamelin, J; Riché, N; Bertoglio, J; Breard, J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol 2001, 3, 346–352. [Google Scholar]
- Krajewski, S; Tanaka, S; Takayama, S; Schibler, MJ; Fenton, W; Reed, JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res 1993, 53, 4701–4714. [Google Scholar]
- Hug, H. Fas-mediated apoptosis in tumor formation and defense. Biol. Chem 1997, 378, 1405–1412. [Google Scholar]
- Li, J; Huang, CY; Zheng, RL; Cui, KR; Li, JF. Hydrogen peroxide induces apoptosis in human hepatoma cells and alters cell redox status. Cell Biol. Int 2000, 24, 9–23. [Google Scholar]
- Rello, S; Stockert, JC; Moreno, V; Gamez, A; Pacheco, M; Juarranz, A; Canete, M; Villanueva, A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005, 10, 201–208. [Google Scholar]
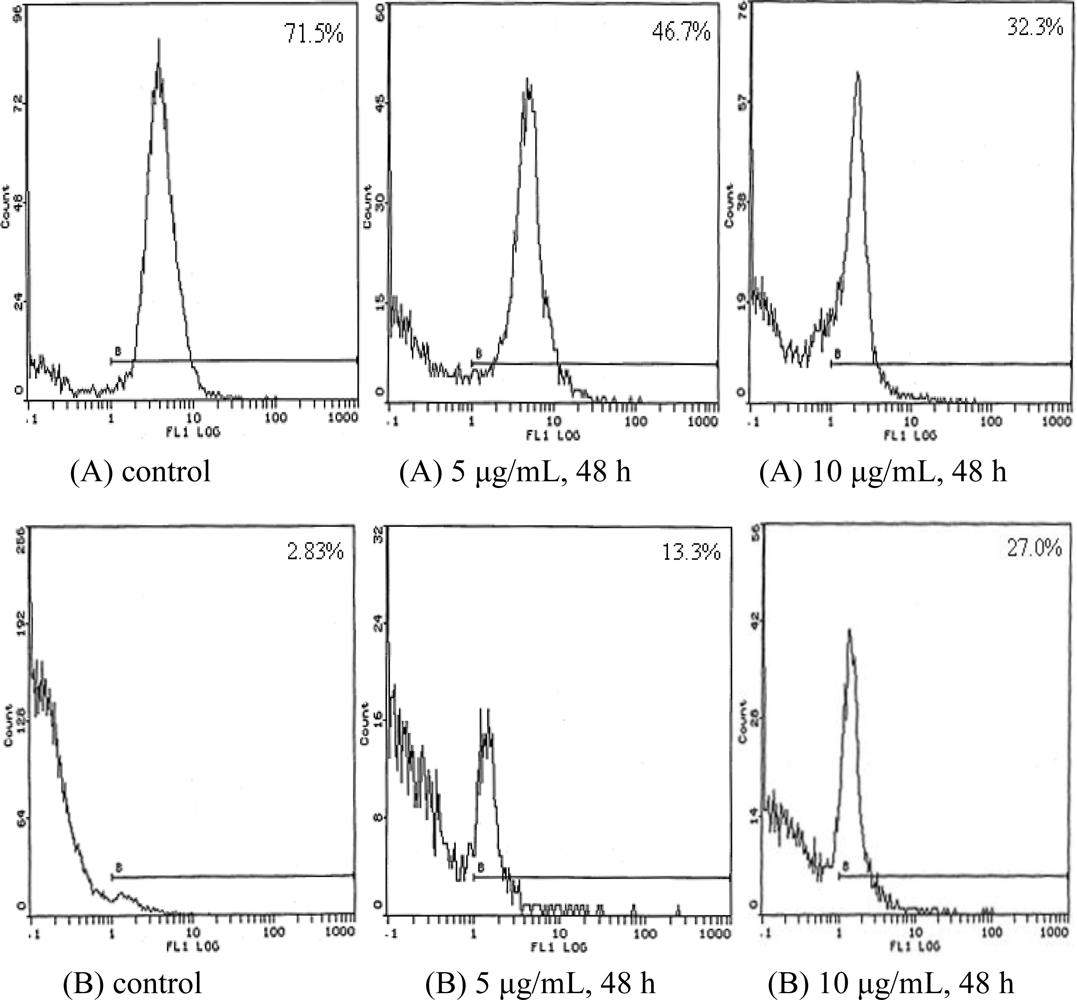
| Time (h) | Concentration (μg/mL) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| 24 | control | 53.6 | 33.5 | 12.9 |
| 5 | 57.3 | 31.4 | 11.3 | |
| 10 | 68.4 | 28.9 | 2.7 | |
| 48 | control | 54.9 | 32.7 | 12.4 |
| 5 | 70.9 | 28.5 | 0.6 | |
| 10 | 77.6 | 21.8 | 0.7 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, X.; Zhu, K.; Zhang, H.; Yao, H. Anti-Tumor Activity of a Novel Protein Obtained from Tartary Buckwheat. Int. J. Mol. Sci. 2010, 11, 5201-5211. https://doi.org/10.3390/ijms11125201
Guo X, Zhu K, Zhang H, Yao H. Anti-Tumor Activity of a Novel Protein Obtained from Tartary Buckwheat. International Journal of Molecular Sciences. 2010; 11(12):5201-5211. https://doi.org/10.3390/ijms11125201
Chicago/Turabian StyleGuo, Xiaona, Kexue Zhu, Hui Zhang, and Huiyuan Yao. 2010. "Anti-Tumor Activity of a Novel Protein Obtained from Tartary Buckwheat" International Journal of Molecular Sciences 11, no. 12: 5201-5211. https://doi.org/10.3390/ijms11125201
APA StyleGuo, X., Zhu, K., Zhang, H., & Yao, H. (2010). Anti-Tumor Activity of a Novel Protein Obtained from Tartary Buckwheat. International Journal of Molecular Sciences, 11(12), 5201-5211. https://doi.org/10.3390/ijms11125201





