Abstract
The effects of various inhibitors on crude, commercial and partially purified commercial mushroom tyrosinase were examined by comparing IC50 values. Kojic acid, salicylhydroxamic acid, tropolone, methimazole, and ammonium tetrathiomolybdate had relatively similar IC50 values for the crude, commercial and partially purified enzyme. 4-Hexylresorcinol seemed to have a somewhat higher IC50 value using crude extracts, compared to commercial or purified tyrosinase. Some inhibitors (NaCl, esculetin, biphenol, phloridzin) showed variations in IC50 values between the enzyme samples. In contrast, hydroquinone, lysozyme, Zn2+, and anisaldehyde showed little or no inhibition in concentration ranges reported to be effective inhibitors. Organic solvents (DMSO and ethanol) had IC50 values that were similar for some of the tyrosinase samples. Depending of the source of tyrosinase and choice of inhibitor, variations in IC50 values were observed.
1. Introduction
Tyrosinase (E.C. 1.14.18.1) is a ubiquitous enzyme involved in pigmentation. It catalyzes hydroxylation of monophenols (cresolase activity) and oxidation of diphenols (catecholase activity) in the presence of molecular oxygen. Enzymes which catalyze the latter reaction are often referred to as catechol oxidases and/or polyphenol oxidases (E.C. 1.10.3.1). The distinction between polyphenoloxidases and tyrosinases is not always clear because under the right conditions many polyphenol oxidases can hydroxylate monophenols [1]. Products formed as a result of tyrosinase activity can produce precursors for skin pigmentation, defense and protective mechanisms in plants and fungi, and pigments for some flowers [2–6]. On the other hand, products from tyrosinase activity can also lead to deleterious effects such as those associated with browning reactions in fruits and vegetables and black spotting of shrimp and lobsters [7–8].
Commercial mushroom tyrosinase (MT) is often used to screen for anti-browning agents and skin whitening agents isolated from natural sources and made synthetically. As such, it serves as an in vitro model for the human tyrosinase in the search for decreasing skin pigmentation. Commercial MT preparations differ in tyrosinase activity, the presence of carbohydrates, organic material, and other proteins and enzymes [9–10]. All of these contaminants have the potential to affect tyrosinase activity in vitro, especially with regard to screening compounds for tyrosinase inhibition. Several reports have appeared that indicate commercial MT preparations contain laccase [9–12]. Laccase activity can interfere with estimations of tyrosinase activity since the enzyme can use many of the same substrates as tyrosinase. In addition, laccase contamination in commercial MT preparations, and not tyrosinase, was attributed to quinone methide formation [12]. The effects of glycosidase activities in commercial MT preparations are just as problematical. For example, esculin, a phenolic glycoside with no tyrosinase inhibitory activity, can be converted into esculetin, a phenolic tyrosinase inhibitor, by a glucosidase present in commercial MT preparations [9]. Therefore, use of tyrosinase for screening of tyrosinase inhibitors may depend on the purity of the enzyme as well as the assay conditions. It has been pointed out that MT has different enzymatic and physical properties compared to the human enzyme and extrapolation of data using MT may not be transferrable to reactions found in humans [13,14].
Over the last few years, several hundred tyrosinase inhibitors have been reported. In the majority of these examples, commercial MT was used to screen or evaluate these inhibitors. These inhibitors vary from simple compounds like NaCl and DMSO to more complex organic compounds isolated from natural sources and those compounds synthesized chemically. Reviews of MT and its inhibitors have appeared that detail properties of MT and some of the natural and synthetic inhibitors of MT [15–17].
Screening for tyrosinase inhibitors has many problems and pitfalls. If a monophenol (i.e., tyrosine) is used as a substrate, there is often a lag period when monitoring the enzymatic activity. Steady state rates for determining tyrosinase activity may appear after a long time period. Compounds which act as inhibitors may extend this lag period and make determination of steady state rates more difficult and time consuming. Monitoring oxidation of a diphenol (i.e., DOPA) in the presence of inhibitors is also problematical. Steady state rates are often determined from the linear portion of these curves whenever possible. The determination of steady state rates can problematical in the presence of tyrosinase inhibitors because the absorbance vs. time curve shapes can vary with the concentration of inhibitor and the type of inhibitor. This makes estimations of steady state rates more difficult since the linear portion of the curve can change in duration and when it is first observable. This also implies that end point assays, absorbance measurements at two different time points, may not be reliable indicators of steady state rates with regard to MT.
Because the majority of reports use commercial MT as a source of tyrosinase, we examined whether the purity of the enzyme could affect estimations of IC50 values, a parameter often used to indicate the potency of a tyrosinase inhibitor. We chose 18 reported tyrosinase inhibitors to test their effect on crude, commercial, and purified MT. These inhibitors were chosen based on their availability from commercial sources and our own interest in them.
2. Results and Discussion
IC50 values for 18 inhibitors of MT were determined using a crude MT extract, commercial MT, or a purified MT sample. Commercial and purified MT isolated from commercial preparations contained no latent tyrosinase. Crude extracts of MT appeared to contain latent tyrosinase and assays were conducted in the presence of 0.1% SDS to account for latent and active enzyme present (data not shown). We arranged these inhibitors into groups based on IC50 value similarities between the different tyrosinase samples and to IC50 values for commercial and/or purified MT reported in the literature (Table 1 and references therein [18–31]).

Table 1.
IC50 values for mushroom tyrosinase inhibitors.
The first group of inhibitors (kojic acid, SHAM, 4 HR, tropolone, methimazole, ATTM) appeared to have similar IC50 values for both the commercial and purified MT (Table 1). These IC50 values closely resembled or were within ranges to those IC50 values reported in the literature. Using crude MT, IC50 values for these inhibitors, with perhaps the exception of 4 HR, were also similar to those obtained using commercial or purified MT. 4-Substituted resorcinols, along with tropolone, kojic acid, and mimosine referenced therein [32], were reported to be slow binding competitive inhibitors of tyrosinase and showed biphasic absorbance vs. time curves. We also observed biphasic absorbance vs. time curves for these inhibitors and only used initial rates for determination of their IC50 values. Some investigators use end point assays, measuring absorbencies at two different time points for rate estimations, to determine IC50 values for tyrosinase inhibitors. For slow binding inhibitors, and other compounds not yet identified as slow binding inhibitors, use of end point assays may lead to IC50 values that are not similar to that those measured under initial rate conditions or constant rate conditions. Park et al. [23] reported inhibition of tyrosinase by ATTM. Their absorbance vs. time curves also showed a biphasic response, which suggests this compound may also be a slow binding inhibitor. We also observed these biphasic curves and inhibition by ATTM below 10–20 μM. However, at higher concentrations of ATTM we observed absorbance vs. time curves that showed an initial decrease in absorbance that eventually “leveled out” and later began a gradual increase in absorbance depending on the concentration of ATTM. Thus, it was difficult to determine rates at greater than 10 μM ATTM.
Many investigators use kojic acid as a reference inhibitor, and it is surprising that IC50 values for kojic acid vary over such a wide range (Table 1, [18]). Our IC50 values are within the lower end of these ranges. Figure 1 shows a plot of MT activity vs. kojic acid concentration for commercial and purified MT used to determine an IC50 value. We show these two curves because most IC50 values are reported using the commercial MT and sometimes using a purified MT. Even though the commercial and purified tyrosinase showed different enzyme activity in the absence of kojic acid, the IC50 values and inhibition curves were similar. In general, for inhibitors in group 1 it appears that the purity of tyrosinase does not have a significant effect on IC50 values.
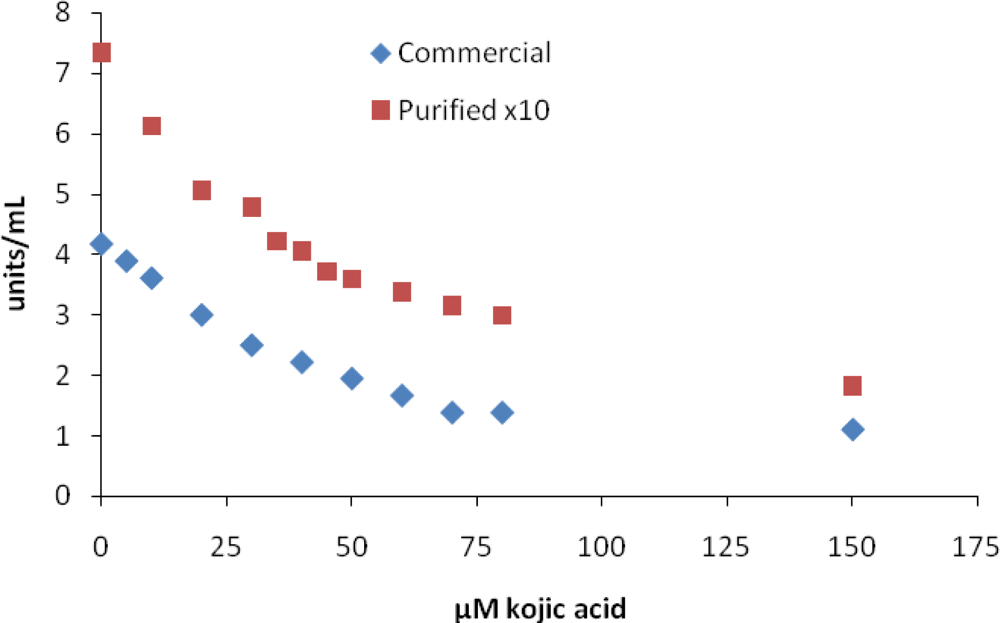
Figure 1.
Inhibition of mushroom tyrosinase by kojic acid. Assays were carried out as described in the Experimental section.
The second group of inhibitors (NaCl, esculetin, biphenol, phloridzin) seemed to show a few differences in IC50 values between the crude, commercial and purified enzyme (Table 1). For the most part, this group of inhibitors also appeared to have much larger IC50 values than those reported in Table 1. For example, NaCl and esculetin seemed to show an increase in IC50 values from crude to commercial to purified MT. In contrast, biphenol did not show quite as large a variation in IC50 values among the tyrosinase samples, although the IC50 for biphenol was much smaller for commercial MT. Except for phloridzin, the IC50 values for NaCl, esculetin and biphenol seemed to be larger for the purified enzyme compared to the commercial or crude enzyme in this group of inhibitors.
Figure 2 shows the plot of MT activity vs. NaCl concentration using the commercial and purified MT. These IC50 values for NaCl were much higher than that reported by Park et al. [24], who suggested that Cl− was responsible for inhibition and not Na+. They also used DOPA as a substrate for tyrosinase. We have no reasonable explanation for the difference in our results vs. those by Park et al. [24] except for the source of commercial tyrosinase used in the two studies. However, even our purified MT showed a much higher IC50 value than the commercial MT.
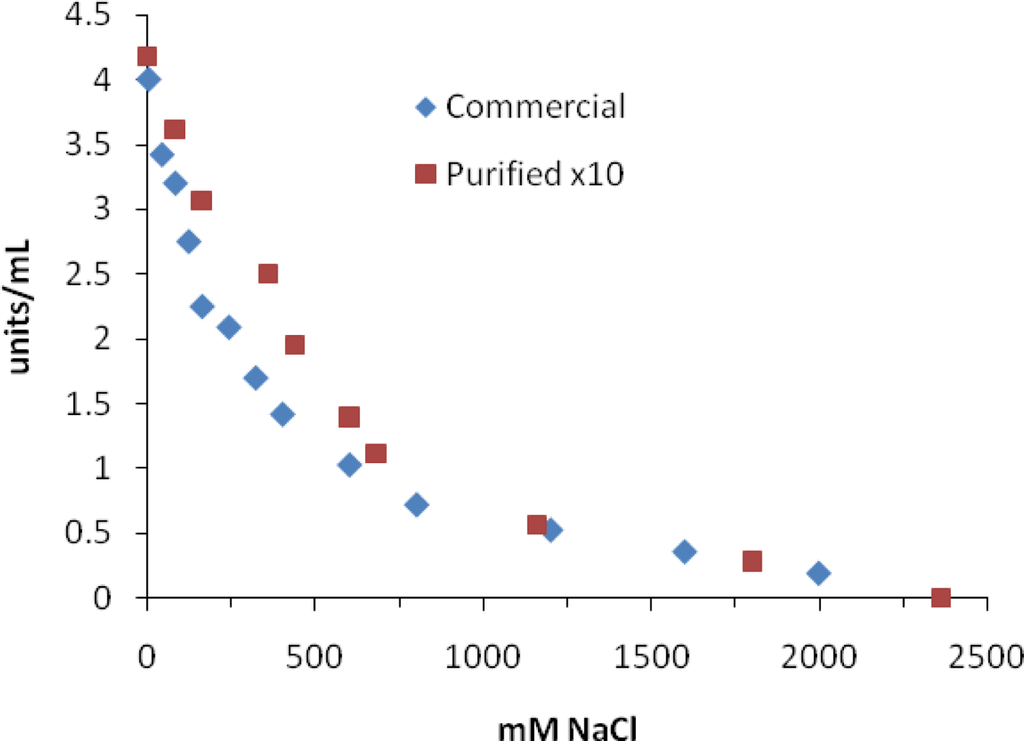
Figure 2.
Inhibition of mushroom tyrosinase by NaCl. Assays were carried out as described in the Experimental section.
Figure 3 shows a plot of activity of MT vs. esculetin concentration for commercial and purified MT. IC50 values for esculetin were much higher than that reported by Masamoto [25], although Flurkey et al. [10] did report a comparable IC50 value for esculetin using commercial MT. Even though esculetin has been reported to be an inhibitor, Munoz-Munoz et al. [33] have shown that esculetin can be used as an alternative substrate for MT and peroxidase using a chronometric assay. Sollai et al. [34] has also shown that esculetin can be oxidized by MT, plant polyphenol oxidases, and laccase although at slow rates. Thus, apparent inhibition by esculetin could be explained by competition for two different substrates. However, it is still hard to reconcile the differences in IC50 values between the MT samples we tested, especially the 685 μM IC50 value for purified MT.
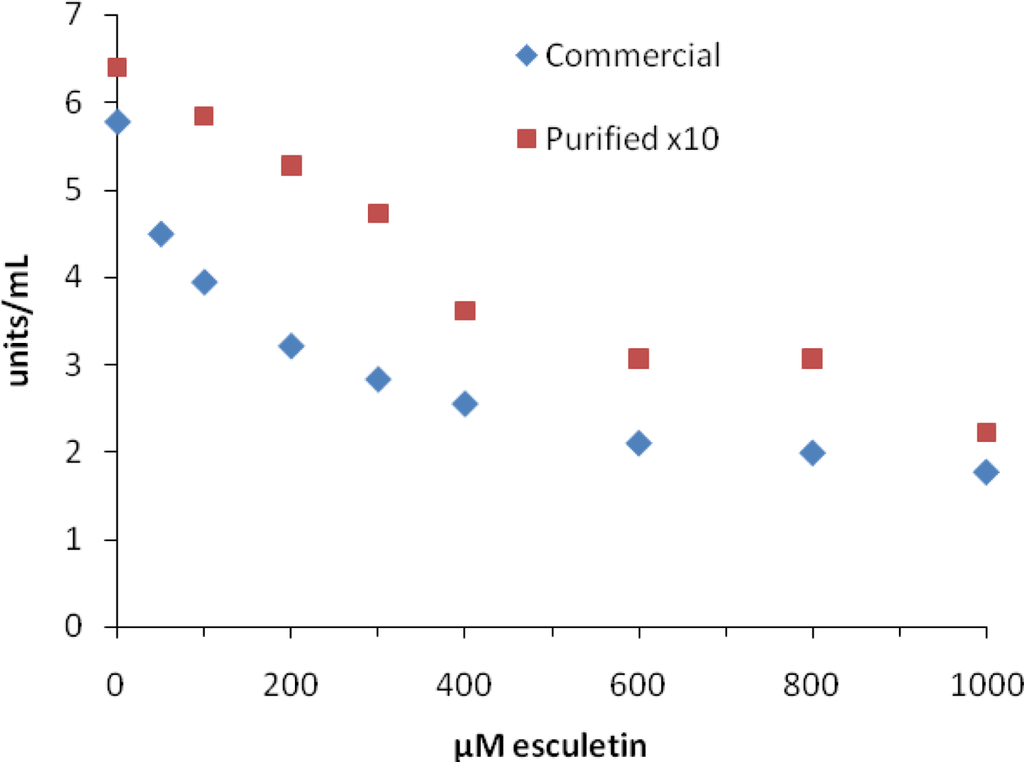
Figure 3.
Inhibition of mushroom tyrosinase by esculetin. Assays were carried out as described in the Experimental section.
Kim et al. [26] obtained an IC50 value for biphenol of approximately 2 μM using tyrosine as a substrate instead of DOPA, although they monitored dopachrome formation from tyrosine. Using DOPA as a substrate, we observed a large variation in IC50 values for biphenol between crude and purified MT vs. commercial MT. In addition, we observed increases in tyrosinase activity at concentrations above 20 μM for commercial MT and 100 μM biphenol for purified MT (data not shown). Using crude tyrosinase extracts, there was a 50% decrease in enzyme activity from 0 to 50 μM biphenol but no further inhibition was observed up to 500 μM biphenol (data not shown).
In general, it was difficult to discern a consistent pattern in IC50 values in group 2 with regard to the purity of tyrosinase. It is possible that some of these inhibitors could be affected by material in crude extracts and in commercial samples of MT differently or behaved differently with a more purified source of enzyme.
The third group of inhibitors (hydroquinone, lysozyme, ZnSO4, anisaldehyde) tested seemed to show little or no inhibition of tyrosinase or required concentrations to reach 50% inhibition that were much different than those reported by other investigators (Table 1). This was true for all of the different tyrosinase samples tested. For example, using DOPA as a substrate we found that hydroquinone increased reactions rates at lower concentrations of hydroquinone and caused inhibition at higher concentrations for some of the enzyme samples (Figure 4). Although hydroquinone has been reported to be an inhibitor of MT, Kasraee [35] and Passi and Nazzaro-Porro [36] have reported that hydroquinone can act as a substrate for MT in which the oxidation product of hydroquinone, parabenzoquinone, can oxidize DOPA. Enhancement in our observed rates at lower concentrations of hydroquinone could be a result of hydroquinone coupled oxidation of DOPA as well as enzyme catalyzed oxidation of DOPA. This apparent activation varied between 20–500 μM hydroquinone for the different MT samples (Figure 4). The crude tyrosinase showed a decrease in activity beyond 100 μM hydroquinone, while the commercial enzyme showed increased activity up to 400 μM hydroquinone. In contrast, the purified enzyme showed an increase in activity with increasing hydroquinone up to 200 μM and then activity seemed to remain relatively constant up to 500 μM hydroquinone.
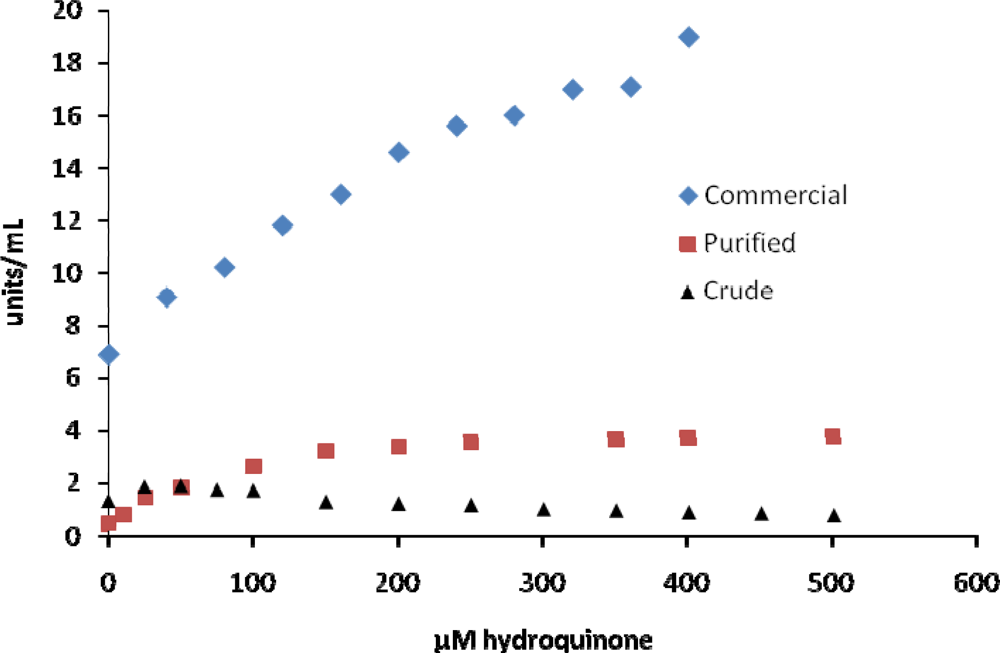
Figure 4.
Inhibition of mushroom tyrosinase by hydroquinone. Assays were carried out as described in the Experimental section.
For lysozyme, we could not add enough inhibitor into the assay to determine an IC50 value and observed less than 50% inhibition, even with 10–20 mg/mL (700–1,400 μM) lysozyme in the assay. This is in contrast to Li et al. [28], who found that lysozyme inhibited MT with an IC50 value of 0.32 μM (4.6 μg/mL). They also suggested that inhibition was dependent upon the time of pre-incubation of lysozyme with tyrosinase. For example, Li et al. [28] observed 8% inhibition on mixing MT with lysozyme just before assaying but found 30% inhibition if MT and lysozyme were incubated for one hour before assaying. We found 8–25% inhibition when large amounts of lysozyme (10–20 mg/mL) were mixed with MT just before assaying, but did not attempt to incubate MT and lysozyme for various times to increase the percent inhibition. We also tried three different sources of lysozyme with the similar results (data not shown).
Effects of Zn2+ and anisaldehyde on mushroom tyrosinase were examined. In monitoring the oxidation of DOPA to dopachrome, we found no inhibition of MT by Zn2+, even at concentrations up to 1 mM (Table 1). This is in contrast to that reported by Han et al. [29]. However, they used a much different assay for tyrosinase that coupled DOPA oxidation to a change in the coloration of thymol blue as a result of protonation. Since our assays monitor DOPA oxidation directly, we can only surmise that Zn2+ may cause some sort of interference in the coupled assay reported by Han et al. [29]. We were able to determine IC50 values for anisaldehyde using the crude, commercial and purified MT (Table 1). These values ranged from 1 to 1.8 mM between the mushroom samples but were much larger that that reported by Ha et al. [30]. Although we did not check for the presence of anisic acid in the anisaldehyde, anisic acid has been shown to be a MT inhibitor with an IC50 value of 680 μM [37]. In general, the IC50 values in this third group of inhibitors were much different than that reported by citations listed in Table 1 even though the crude, commercial and purified MT appeared to behave in a similar manner with these inhibitors.
The fourth group of potential MT inhibitors (DMSO, dimethyl sulfide, dimethyl sulfone, ethanol) comprised compounds that are commonly used to dissolve MT inhibitors and/or may be potential contaminants in DMSO preparations (Table 1). IC50 values for ethanol were similar for all MT samples tested. Little to no inhibition was observed for dimethyl sulfide up to 500 μM using any of the MT samples tested. Perez-Gilabert and Garcia-Carmona reported that dimethyl sulfide is a slow binding inhibitor of tyrosinase, although they did not report IC50 values [38]. We also observed relatively little inhibition of tyrosinase up to 2 mM dimethyl sulfone.
Inhibition by DMSO did not appear to be much different for crude or commercial MT even though the IC50 values were larger that using the purified MT. Our IC50 values for DMSO using commercial and purified MT were somewhat lower than that reported by Chen et al. [31]. We did find that inhibition by DMSO might be dependent on the source and/or purity of the DMSO used (data not shown). We also observed that high concentrations of DMSO caused a precipitate to form in the assay solution, presumably inorganic compounds that are less soluble as the DMSO increased in concentration. DMSO has also been shown to cause protein precipitation and denaturation by Arakawa et al. [39] at higher DMSO concentrations. In addition, Tjernberg et al. [40] reported that even low concentrations of DMSO (< 5 %) can destabilize proteins. These effects may have to be considered when examining inhibitory effects of organic solvents or inhibitors dissolved in organic solvents, like DMSO, on tyrosinase activity. In general, and with the exception of DMSO, the fourth group of potential tyrosinase inhibitors displayed similar IC50 values for the different sources of tyrosinase.
3. Experimental Section
3.1. Materials
Commercial mushroom tyrosinase was purchased from Worthington Biochemical Corp. (Lakewood, NJ, USA). Substrates and inhibitors were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anisaldehyde was obtained form J.T. Baker Chemical Co. (Phillipsburg, NJ, USA). Dimethyl sulfoxide (DMSO) was reagent grade from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Methods
3.2.1. Crude Extract Tyrosinase Preparation
White button mushrooms were purchased from local stores and blended in 100 mM sodium phosphate (pH 6.0) containing 1 mM EDTA, 1 mM PMSF, 5 mM ascorbic acid and 1 M sucrose. The homogenates were filtered and stored at −15 °C in small aliquots. Commercial mushroom tyrosinase. Mushroom tyrosinase was dissolved in the above buffer without ascorbic acid at 2 mg dry weight/mL and stored at 5 °C in small aliquots.
3.2.2. Enzyme Purification
Tyrosinase was partially purified from commercial mushroom tyrosinase preparations using ammonium sulfate precipitation (35–60%), ion exchange on DEAE Sepharose CL-6B, hydrophobic interaction on Phenyl Sepharose in 1 M NaCl, and adsorption on hydroxyapatite [41,42].
3.2.3. Enzyme Assays
Each one mL assay contained a final concentration of 100 mM sodium phosphate (pH 6.5) and 2 mM DOPA. Crude, commercial or partially purified tyrosinase was added to initiate the reaction. Oxidation of DOPA into dopachrome was monitored at 475 nm. One unit of activity was defined as 1 μmole dopachrome formed per min per ml enzyme using an extinction coefficient of 3600 M−1 cm−1. Rates were determined from the linear portion of the absorbance vs. time curves. Crude enzyme was use at a concentration of 0.1 units (8.4 μg protein) per mL in the assay and assayed in the presence of 0.1% SDS. Commercial tyrosinase was used at a concentration of 0.038 units (3.3 μg protein) per mL in the assay. The purified enzyme was used at a concentration of 0.0067 units (0.58 μg protein) per mL in the assay.
3.2.4. IC50 Determinations
Various amounts of inhibitors were added into assays to determine a range of inhibitors needed to encompass the IC50 value. Then assays were repeated within this range of inhibitor concentrations. Rates were determined as above. If any inhibitor caused a significant change in the shape of the A vs. time curve, rates were calculated from steady state rate regions (linear portions) in the curves. The concentration of inhibitor needed to inhibit 50% of the tyrosinase activity was extrapolated from % activity vs. [inhibitor] curves. For some inhibitors, we also used Statistical Data Analysis Software (SDAS, version 2008A) using an exponential equation to obtain a best fit curve and then to calculate IC50 values. For the crude and commercial MT, experiments were repeated at least twice and the average IC50 value reported. Because of the limited amount of purified tyrosinase, not all experiments could be repeated twice. In experiments requiring DMSO to dissolve the inhibitor, control assays contained the assay mixture with DMSO but minus inhibitor. Final DMSO concentrations were 5% or less in the assays.
4. Conclusions
IC50 values for tyrosinase inhibitors can vary for a number of reasons, including choice of assay conditions, rate determinations, and solvent considerations. Therefore, it is sometimes difficult to reproduce data reported in the literature. Our results seem to indicate the IC50 values can also vary depending on the purity of the tyrosinase. However, this observation also seems to be related to the type of inhibitor used. Crude MT extracts and commercial MT sources contain a variety of proteins, enzymes, carbohydrate, and organic compounds. Any one of these substances can interfere with IC50 value estimations through chemical or enzymatic reactions. We suggest that MT preparations need to be partially purified to eliminate these potential problems in IC50 value determinations.
Acknowledgments
We would like to thank Indiana State University and the John W. Anderson Foundation for providing funding for undergraduates participating in the SURE program in the Chemistry Department.
Abbreviations:
| ATTM | ammonium tetrathiomolybdate |
| biphenol | 4,4’-dihydroxy-biphenyl |
| DOPA | dihydroxyphenylalanine |
| DMSO | dimethyl sulfoxide |
| EDTA | ethylenediaminetetraacetic acid |
| 4HR | 4-hexylresorcinol |
| MT | mushroom tyrosinase |
| PMSF | phenylmethylsulfonyl fluoride |
| SDS | sodium dodecylsulfate |
| SHAM | salicyl-hydroxamic acid |
References and Notes
- Flurkey, WH; Inlow, JK. Proteolytic processing of polyphenol oxidase from plants and fungi. J. Inorg. Biochem 2008, 102, 2160–2170. [Google Scholar]
- Ando, H; Kondoh, H; Ichihashi, M; Hearing, VJ. Approaches to identify inhibitors of melanin biosynthesis via quality control of tyrosinase. J. Invest. Dermatol 2007, 127, 751–761. [Google Scholar]
- Mayer, AM. Polyphenol oxidases in plants and fungi: Going places? Phytochemistry 2006, 67, 2318–2331. [Google Scholar]
- Selinheimo, E; NiEidhin, D; Steffenson, C; Nielson, J; Lomascolo, A; Halaouli, S; Record, E; O’Beirne, D; Buchert, J; Kruus, K. Comparison of the characteristics of fungal and plant tyrosinases. J. Biotechnol 2007, 130, 471–480. [Google Scholar]
- Gandia-Herrero, F; Escribano, J; Garcia-Carmona, F. Betaxanthins as substrates for tyrosinase. An approach to the role of tyrosinase in the biosynthetic pathway of betalains. Plant Physiol 2005, 138, 421–432. [Google Scholar]
- Yoruk, R; Marshall, M. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem 2003, 27, 361–422. [Google Scholar]
- Walker, JRL; Ferrar, PH. The control of enzymic browning in foods. Chem. Indust 1995, 16, 836–839. [Google Scholar]
- Kim, J; Marshall, MR; Wei, C. Polyphenoloxidase. In Sea Food Enzymes; Haard, NF, Simpson, BK, Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 271–315. [Google Scholar]
- Rescigno, A; Zucca, P; Flurkey, A; Inlow, J; Flurkey, WH. Identification and discrimination between some contaminant enzyme activities in commercial preparations of mushroom tyrosinase. Enzyme Micro. Tech 2007, 41, 620–627. [Google Scholar]
- Flurkey, A; Cooksey, J; Reddy, A; Spoonmore, K; Rescigno, A; Inlow, J; Flurkey, WH. Enzyme, protein, carbohydrate, and phenolic contaminants in commercial tyrosinase preparations; potential problems affecting tyrosinase activity and inhibition studies. J. Agric. Food Chem 2008, 56, 4760–4768. [Google Scholar]
- Flurkey, WH; Ratcliff, B; Lopez, L; Kuglin, J; Dawley, RM. Differentiation of fungal tyrosinase and laccases using selective inhibitors and substrates. In Enzymatic Browning and Its Prevention; Lee, CY, Whitaker, JR, Eds.; American Chemical Society: Washington, DC, USA, 1995; Series 600, Chapter 6, pp. 81–90. [Google Scholar]
- Sugumaran, M; Bolton, JL. Laccase-and not tyrosinase- is the enzyme responsible for quinone methide production from 2,6-dimethoxy-4-allyl-phenol. Arch. Biochem. Biophys 1998, 353, 207–212. [Google Scholar]
- Jacobsohn, GM; Jacobsohn, MK. Incorporation and binding of estrogens into melanin: Comparison of mushroom and mammalian tyrosinases. Biochim. Biophys. Acta 1992, 1116, 173–182. [Google Scholar]
- Galindo, JD; Martinez, JH; Lopez-Ballester, JA; Penafiel, R; Solano, F; Lozano, JA. The effect of polyamines on tyrosinase activity. Biochem. Int 1987, 15, 1151–1158. [Google Scholar]
- Rescigno, A; Sollai, F; Pisu, B; Rinaldi, A; Sanjust, E. Tyrosinase inhibition: General and applied aspects. J. Enzyme. Inhib. Med. Chem 2002, 17, 207–218. [Google Scholar]
- Seo, SY; Sharma, VK; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem 2003, 51, 2837–2853. [Google Scholar]
- Kim, YJ; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Molec. Life Sci 2005, 62, 1707–1723. [Google Scholar]
- Marrero-Ponce, Y; Khan, MTH; Martin, GMC; Ather, A; Sultankhodzhaev, MN; Torrens, F; Rotondo, R. Prediction of tyrosinase inhibition activity using atom-based bilinear indices. Chem. Med. Chem 2007, 2, 449–478. [Google Scholar]
- Kahn, V; Zakin, V. Effect of salicylhydroxamic acid (SHAM) on dl-DOPA oxidation by mushroom tyrosinae and by NaIO4. J. Food Biochem 2000, 24, 399–415. [Google Scholar]
- Chen, QX; Ke, LN; Song, KK; Huang, H; Liu, XD. Inhibitory effects of hexylresorcinol and dodecylresorcinaol on mushroom (Agaricus bisporus) tyrosinase. Prot. J 2004, 23, 135–141. [Google Scholar]
- Kahn, V; Andrawis, A. Inhibition of mushroom tyrosinase by tropolone. Phytochemistry 1985, 24, 905–908. [Google Scholar]
- Andrawis, A; Kahn, V. Effect of methimazole on the activity of mushroom tyrosinase. Biochem. J 1986, 235, 91–96. [Google Scholar]
- Park, KH; Park, YD; Lee, JR; Hahn, HS; Lee, SJ; Bae, CD; Yang, JM; Kim, DE; Hahn, MJ. Inhibition kinetics of mushroom tyrosinase by copper-chelating ammonium tetrathiomolybdate. Biochim. Biophys. Acta 2005, 1726, 115–120. [Google Scholar]
- Park, YD; Kim, SY; Lyou, YJ; Lee, JY; Yang, JM. A new type of uncompetitive inhibition of tyrosinase by Cl− binding. Biochimie 2005, 87, 931–937. [Google Scholar]
- Masamoto, Y; Ando, H; Murata, Y; Shimoishi, Y; Tada, M; Takahata, K. Mushroom tyrosinase inhibitory activtiy of esculetin isolated from seeds of Euphorbia lathyris, L. Biosci. Biotechnol. Biochem 2003, 67, 631–634. [Google Scholar]
- Kim, YJ; No, JK; Lee, JH; Chung, HY. 4,4′-Dihydroxybiphenyl as a new potent tyrosinase inhibitor. Biol. Pharm. Bull 2005, 28, 323–327. [Google Scholar]
- Wang, Q; Qiu, L; Chen, X; Song, KK; Shi, Y; Chen, QX. Inhibitory effects of phloridzin dehydrate on the activity of mushroom (Agaricus bisporus) tyrosinase. Bioorg. Med. Chem 2007, 15, 1568–1571. [Google Scholar]
- Li, B; Huang, Y; Paskewitz, SM. Hen egg white lysozyme as an inhibitor of mushroom tyrosinase. FEBS Lett 2006, 580, 1877–1882. [Google Scholar]
- Han, HY; Zou, HC; Jeon, JY; Wang, JY; Xu, WA; Yang, JM; Park, YD. The inhibition kinetics and thermodynamic changes of tyrosinase via zinc ion. Biochim. Biophys. Acta 2007, 1774, 822–827. [Google Scholar]
- Ha, TJ; Tamura, S; Kubo, I. Effects of mushroom tyrosinase on anisaldehyde. J. Agric. Food Chem 2005, 53, 7024–7028. [Google Scholar]
- Chen, QX; Liu, XD; Huang, H. Inactivation kinetics of mushroom tyrosinase in the dimethyl sulfoxide solution. Biochemistry (Moscow) 2003, 68, 644–649. [Google Scholar]
- Jimenez, M; Garcis-Carmona, F. 4-Substituted resorcinols (sulfite alternatives) as slow binding inhibitors of tyrosinase catecholase activity. J. Agric. Food Chem 1997, 45, 2061–2065. [Google Scholar]
- Munoz-Munoz, JL; Garcia-Molina, F; Varon, R; Rodriquez-Lopez, JN; Garcia-Canovas, F; Tudela, J. Kinetic chara-cterization of the oxidation of esculetin by polyphenol oxidase and peroxidase. Biosci. Biotechnol. Biochem 2007, 71, 390–396. [Google Scholar]
- Sollai, F; Zucca, P; Sanjust, E; Steri, D; Rescigno, A. Umbelliferone and esculetin: Inhibitors or substrates for polyphenol oxidases? Biol. Pharm. Bull 2008, 31, 2187–2193. [Google Scholar]
- Kasraee, B. Peroxidase-mediated mechanisms are involved in the melanocytotoxic and melanogenesis-inhibiting effects of chemical agents. Dermatology 2002, 205, 329–339. [Google Scholar]
- Passi, S; Nazzaro-Porro, M. Molecular basis of substrate and inhibitory specificity of tyrosinase: Phenolic compounds. Br. J. Dermatol 1981, 104, 659–665. [Google Scholar]
- Lee, HS. Tyrosinase inhibitors of Pulsatila cernua root-derived materials. J. Agric. Food Chem 2002, 50, 1400–1403. [Google Scholar]
- Perez-Gilabert, M; Garcia-Carmona, F. Dimethyl sulfide, a volatile flavor constituent, is a slow binding inhibitor of tyrosinase. Biochem. Biophys. Res. Commun 2001, 285, 257–261. [Google Scholar]
- Arakawa, T; Kita, Y; Timasheff, SN. Protein precipitation and denaturation by dimethyl sulfoxide. Biophys. Chem 2007, 131, 62–70. [Google Scholar]
- Tjernberg, A; Markova, N; Griffiths, WJ; Hallen, D. DMSO-related effects in protein characterization. J. Biomolec. Screen 2006, 11, 131–137. [Google Scholar]
- Fan, Y; Flurkey, WH. Purification and characterization of tyrosinase from gill tissue of Portabella mushrooms. Phytochemistry 2004, 65, 671–678. [Google Scholar]
- Ingebrigtsen, J; Flurkey, WH. Affinity and hydrophobic chromatography of mushroom tyrosinase. Phytochemistry 1988, 27, 1593–1599. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).