Molecular Characterization of Tb, a New Approach for an Ancient Brucellaphage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Tb morphology
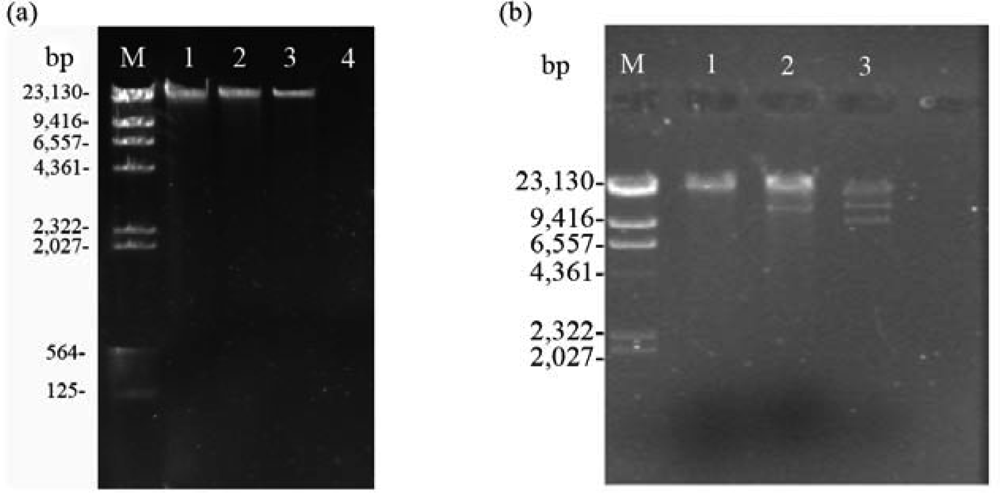
2.2. Nucleic acid characterization
2.3. RAPD analysis for identification between phage Tb and host strain B. abortus S 19
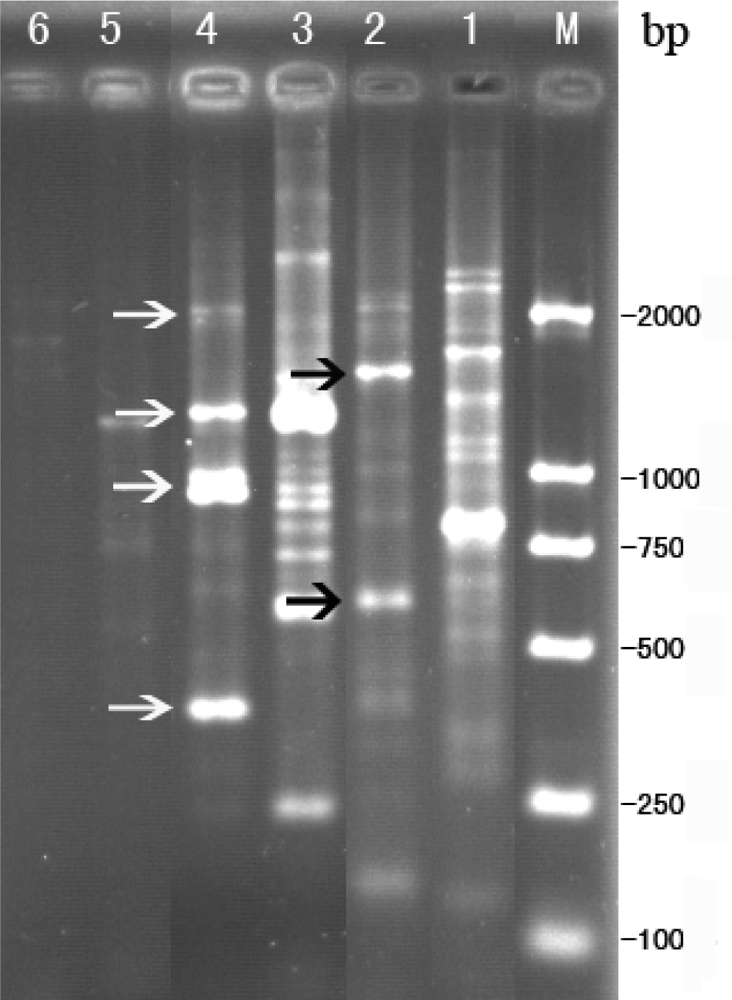
2.4. Sequence analysis for RAPD products of phage Tb
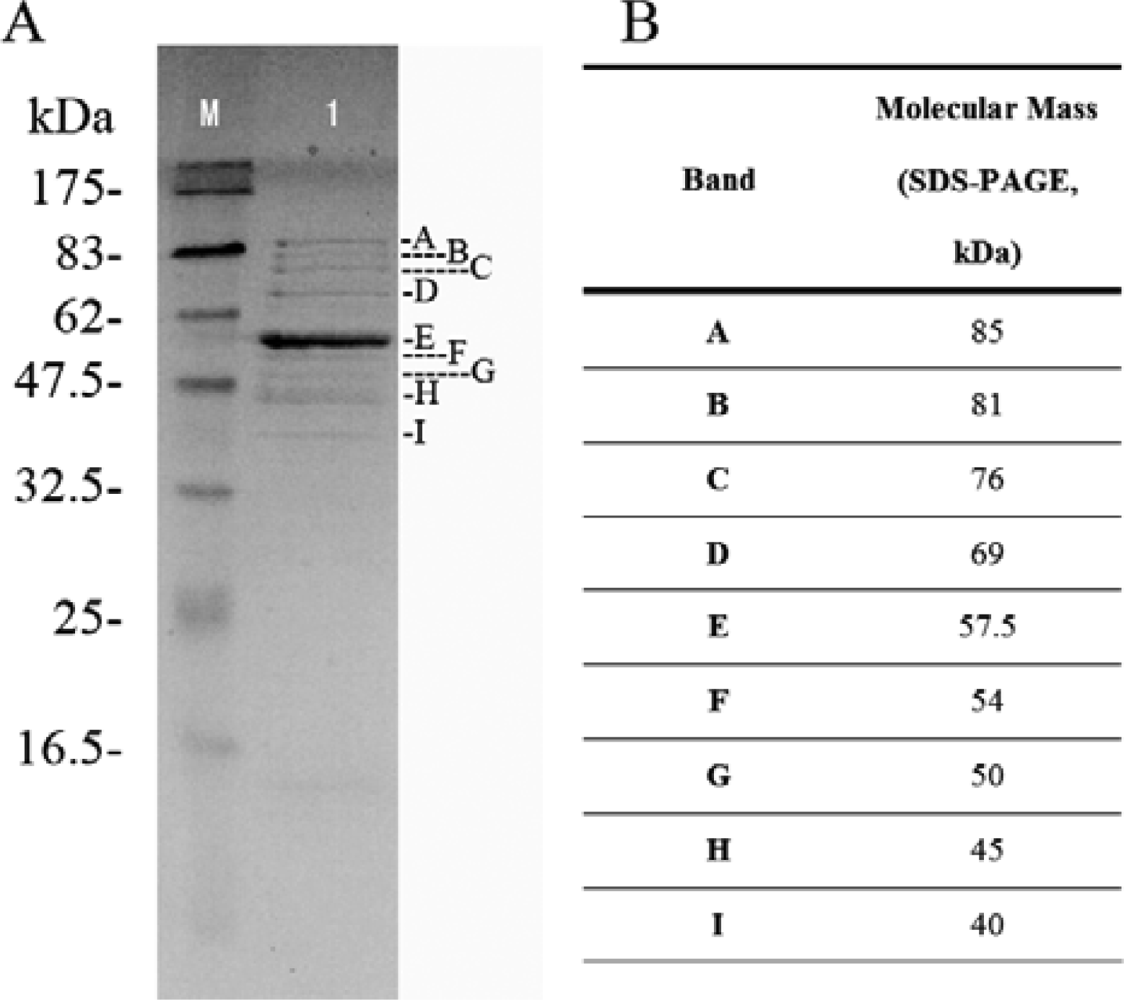
2.5. Structural protein of phage Tb
2.6. Discussion
3. Materials and Methods
3.1. Phage and bacteria
3.2. Propagation of Brucella phages
3.3. Nucleic acid extraction from the isolated bacteriophage
3.4. Electron microscopy
3.5. Nucleic acid characterization
3.6. Restriction endonuclease analysis
3.7. RAPD
3.8. Subcloning of RAPD products
3.9. Computer-assisted Sequence Analysis
3.10. Analysis of Phage Tb Structural Proteins
4. Conclusions
Acknowledgments
References and Notes
- Carrere, L; Roux, J; Mandin, J. Obtention of lysogenic strains of Corynebacterium parvum and Brucella melitensis. CR Seances Soc. Biol. Fil 1956, 150, 1599–1600. [Google Scholar]
- Drozhevkina, MS. Secondary phago-resistant cultures of Brucella. Zh Mikrobiol. Epidemiol. Immunobiol 1956, 27, 63–68. [Google Scholar]
- Parnas, J; Feltynowski, A; Bulikowski, W. Anti-brucella phage. Nature 1958, 182, 1610–1611. [Google Scholar]
- Pickett, MJ; Nelson, EL. Observations on the problem of Brucella blood cultures. J. Bacteriol 1951, 61, 229–237. [Google Scholar]
- Vershilova, PA. Certain problems of epidemiology of brucellosis. Zh Mikrobiol. Epidemiol. Immunobiol 1956, 27, 53–57. [Google Scholar]
- Ackermann, HW. Frequency of morphological phage descriptions in the year 2000. Brief review. Arch. Virol 2001, 146, 843–857. [Google Scholar]
- Ackermann, HW; Simon, F; Verger, JM. A survey of Brucella phages and morphology of new isolates. Intervirology 1981, 16, 1–7. [Google Scholar]
- Altschul, SF; Madden, TL; Schaffer, AA; Zhang, J; Zhang, Z; Miller, W; Lipman, DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 1997, 25, 3389–3402. [Google Scholar]
- Letellier, L; Boulanger, P; Plancon, L; Jacquot, P; Santamaria, M. Main features on tailed phage, host recognition and DNA uptake. Front. Biosci 2004, 9, 1228–1339. [Google Scholar]
- Scholl, D; Rogers, S; Adhya, S; Merril, CR. Bacteriophage K1–5 encodes two different tail fiber proteins, allowing it to infect and replicate on both K1 and K5 strains of Escherichia coli. J. Virol 2001, 75, 2509–2515. [Google Scholar]
- Daniels, DL; Schroeder, JL; Szybalski, W; Sanger, F; Coulson, AR; Hong, GF; Hill, DF; Petersen, GB; Blattner, FR. Appendix II Complete Annotated Lambda Sequence; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1983. [Google Scholar]
- Emrich, J; Streisinger, G. The role of phage lysozyme in the life cycle of phage T4. Virology 1968, 36, 387–391. [Google Scholar]
- Kao, SH; McClain, WH. Baseplate protein of bacteriophage T4 with both structural and lytic functions. J. Virol 1980, 34, 95–103. [Google Scholar]
- Yamazaki, Y. Enzymatic activities on cell walls in bacteriophage T4. Biochim. Biophys. Acta 1969, 178, 542–550. [Google Scholar]
- Chibani-Chennoufi, S; Bruttin, A; Dillmann, ML; Brussow, H. Phage-host interaction: An ecological perspective. J. Bacteriol 2004, 186, 3677–3686. [Google Scholar]
- Corbel, MJ. Properties of Brucella-phages lytic for non-smooth Brucella strains. Dev. Biol. Stand 1984, 56, 55–62. [Google Scholar]
- Jones, LM; Mc, DC; Wilson, JB. Phenotypic alterations in the colonial morphology of Brucella abortus due to a bacteriophage carrier state. J. Bacteriol 1962, 83, 860–866. [Google Scholar]
- Corbel, MJ; Morris, JA. Investigation of the effect of brucella-phage on the course of experimental infection with Brucella abortus. Br. Vet. J 1980, 136, 278–289. [Google Scholar]
- Calderone, JG; Pickett, MJ. Characterization of Brucellaphages. J. Gen. Microbiol 1965, 39, 1–10. [Google Scholar]
- Corbel, MJ; Tolari, F; Yadava, VK. Characterisation of a new phage lytic for both smooth and non-smooth Brucella species. Res. Vet. Sci 1988, 44, 45–49. [Google Scholar]
- Ikram, G; Dellagi, K; Ismail, RB. Random amplified polymorphic DNA technique for identification and differentiation of old world Leishmania species. Am J Trop Med Hyg 2002, 66, 152–156. [Google Scholar]
- Pogue, GP; Koul, S; Lee, NS; Dwyer, DM; Nakhasi, HL. Identification of intra- and interspecific Leishmania genetic polymorphisms by arbitrary primed polymerase chain reactions and use of polymorphic DNA to identify differentially regulated genes. Parasitol. Res 1995, 81, 282–290. [Google Scholar]
- Pogue, GP; Lee, NS; Koul, S; Dwyer, DM; Nakhasi, HL. Identification of differentially expressed Leishmania donovani genes using arbitrarily primed polymerase chain reactions. Gene 1995, 165, 31–38. [Google Scholar]
- Schonian, G; Schweynoch, C; Zlateva, K; Oskam, L; Kroon, N; Graser, Y; Presber, W. Identification and determination of the relationships of species and strains within the genus Leishmania using single primers in the polymerase chain reaction. Mol Biochem Parasitol 1996, 77, 19–29. [Google Scholar]
- Tibayrenc, M; Neubauer, K; Barnabe, C; Guerrini, F; Skarecky, D; Ayala, FJ. Genetic characterization of six parasitic protozoa: Parity between random-primer DNA typing and multilocus enzyme electrophoresis. Proc. Natl. Acad. Sci. USA 1993, 90, 1335–1339. [Google Scholar]
- Waitumbi, JN; Murphy, NB. Inter- and intra-species differentiation of trypanosomes by genomic fingerprinting with arbitrary primers. Mol. Biochem. Parasitol 1993, 58, 181–185. [Google Scholar]
- Procunier, JD; Fernando, MA; Barta, JR. Species and strain differentiation of Eimeria spp. of the domestic fowl using DNA polymorphisms amplified by arbitrary primers. Parasitol. Res 1993, 79, 98–102. [Google Scholar]
- Shirley, MW; Bumstead, N. Intra-specific variation within Eimeria tenella detected by the random amplification of polymorphic DNA. Parasitol. Res 1994, 80, 346–351. [Google Scholar]
- Dias Neto, E; de Souza, CP; Rollinson, D; Katz, N; Pena, SD; Simpson, AJ. The random amplification of polymorphic DNA allows the identification of strains and species of schistosome. Mol. Biochem. Parasitol 1993, 57, 83–88. [Google Scholar]
- Laconcha, I; Lopez-Molina, N; Rementeria, A; Audicana, A; Perales, I; Garaizar, J. Phage typing combined with pulsed-field gel electrophoresis and random amplified polymorphic DNA increases discrimination in the epidemiological analysis of Salmonella enteritidis strains. Int. J. Food Microbiol 1998, 40, 27–34. [Google Scholar]
- Liu, J; Dehbi, M; Moeck, G; Arhin, F; Bauda, P; Bergeron, D; Callejo, M; Ferretti, V; Ha, N; Kwan, T; McCarty, J; Srikumar, R; Williams, D; Wu, JJ; Gros, P; Pelletier, J; DuBow, M. Antimicrobial drug discovery through bacteriophage genomics. Nat. Biotechnol 2004, 22, 185–191. [Google Scholar]
- Redmond, WB. Mycobacterial variations as influenced by phage and other genomic factors. Pneumonologie 1970, 142, 191–197. [Google Scholar]
- Adams, JW; McKay, J. Brucella in government-owned livestock in Eastern Nigeria. Nature 1966, 212, 217–218. [Google Scholar]
- McDuff, CR; Jones, LM; Wilson, JB. Characteristics of Brucellaphage. J. Bacteriol 1962, 83, 324–329. [Google Scholar]
- van Drimmelen, DG. Bacteriophage typing applied to strains of Brucella organisms. Nature 1959, 184(Suppl. 14), 1079. [Google Scholar]
- Jones, LM. Comparison of phage typing with standard methods of species differentiation in brucellae. Bull. World Health Organ 1960, 23, 130–133. [Google Scholar]
- Yamamoto, KR; Alberts, BM; Benzinger, R; Lawhorne, L; Treiber, G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 1970, 40, 734–744. [Google Scholar]
- Oakey, HJ; Owens, L. A new bacteriophage, VHML, isolated from a toxin-producing strain of Vibrio harveyi in tropical Australia. J. Appl. Microbiol 2000, 89, 702–709. [Google Scholar]
- Sambrook, J; Russell, DW. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Okuda, J; Ishibashi, M; Abbott, SL; Janda, JM; Nishibuchi, M. Analysis of the thermostable direct hemolysin (tdh) gene and the tdh-related hemolysin (trh) genes in urease-positive strains of Vibrio parahaemolyticus isolated on the West Coast of the United States. J. Clin. Microbiol 1997, 35, 1965–1971. [Google Scholar]
- Laemmli, UK; Quittner, SF. Maturation of the head of bacteriophage T4. IV. The proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology 1974, 62, 483–499. [Google Scholar]


| Fragment | BamHI | EcoRI |
|---|---|---|
| A | 14.9 | 9.7 |
| B | 7.2 | 7.1 |
| C | 5.89 | |
| Total | 22.1 | 22.69 |
| Average | 22.4 |
| TA clone | Fragment size (bp) | Best hits (blastn or blastx) analysis to nr database | General function | Score(bits) | E Value | Accession no. |
|---|---|---|---|---|---|---|
| Band-1 | 923 | Microcystin-dependent protein-like from Mesorhizobium sp. BNC1 | Microcystin-dependent protein-like | 157 | 7e-37 | gi:110283346 |
| Bradyrhizobium sp. BTAi1 | putative phage tail Collar Domain | 66.2 | 3e-09 | gi:148251626 | ||
| Stigmatella aurantiaca DW4/3-1 | phage Tail Collar Domain family | 55.8 | 4e-06 | gi:115368091 | ||
| Kordia algicida OT-1 | phage Tail Collar | 53.5 | 2e-05 | gi:161324659 |
| Sr. no. | Primer | Sequence |
|---|---|---|
| 1 | S2 | 5′- TGATCCCTGG -3′ |
| 2 | S57 | 5′- TTTCCCACGG -3′ |
| 3 | S59 | 5′- CTGGGGACTT -3′ |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, C.-Z.; Xiong, H.-Y.; Han, J.; Cui, B.-Y.; Piao, D.-R.; Li, Y.-F.; Jiang, H.; Ren, Q.; Ma, X.-Y.; Chai, Y.-M.; et al. Molecular Characterization of Tb, a New Approach for an Ancient Brucellaphage. Int. J. Mol. Sci. 2009, 10, 2999-3011. https://doi.org/10.3390/ijms10072999
Zhu C-Z, Xiong H-Y, Han J, Cui B-Y, Piao D-R, Li Y-F, Jiang H, Ren Q, Ma X-Y, Chai Y-M, et al. Molecular Characterization of Tb, a New Approach for an Ancient Brucellaphage. International Journal of Molecular Sciences. 2009; 10(7):2999-3011. https://doi.org/10.3390/ijms10072999
Chicago/Turabian StyleZhu, Cai-Zhong, Hong-Yan Xiong, Jing Han, Bu-Yun Cui, Dong-Ri Piao, Ya-Fei Li, Hai Jiang, Qian Ren, Xiang-Yu Ma, Ya-Ming Chai, and et al. 2009. "Molecular Characterization of Tb, a New Approach for an Ancient Brucellaphage" International Journal of Molecular Sciences 10, no. 7: 2999-3011. https://doi.org/10.3390/ijms10072999
APA StyleZhu, C.-Z., Xiong, H.-Y., Han, J., Cui, B.-Y., Piao, D.-R., Li, Y.-F., Jiang, H., Ren, Q., Ma, X.-Y., Chai, Y.-M., Huang, X., Zhao, H.-Y., & Li, L.-Y. (2009). Molecular Characterization of Tb, a New Approach for an Ancient Brucellaphage. International Journal of Molecular Sciences, 10(7), 2999-3011. https://doi.org/10.3390/ijms10072999




