Anomalously Strong Effect of the Ion Sign on the Thermochemistry of Hydrogen Bonded Aqueous Clusters of Identical Chemical Composition
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
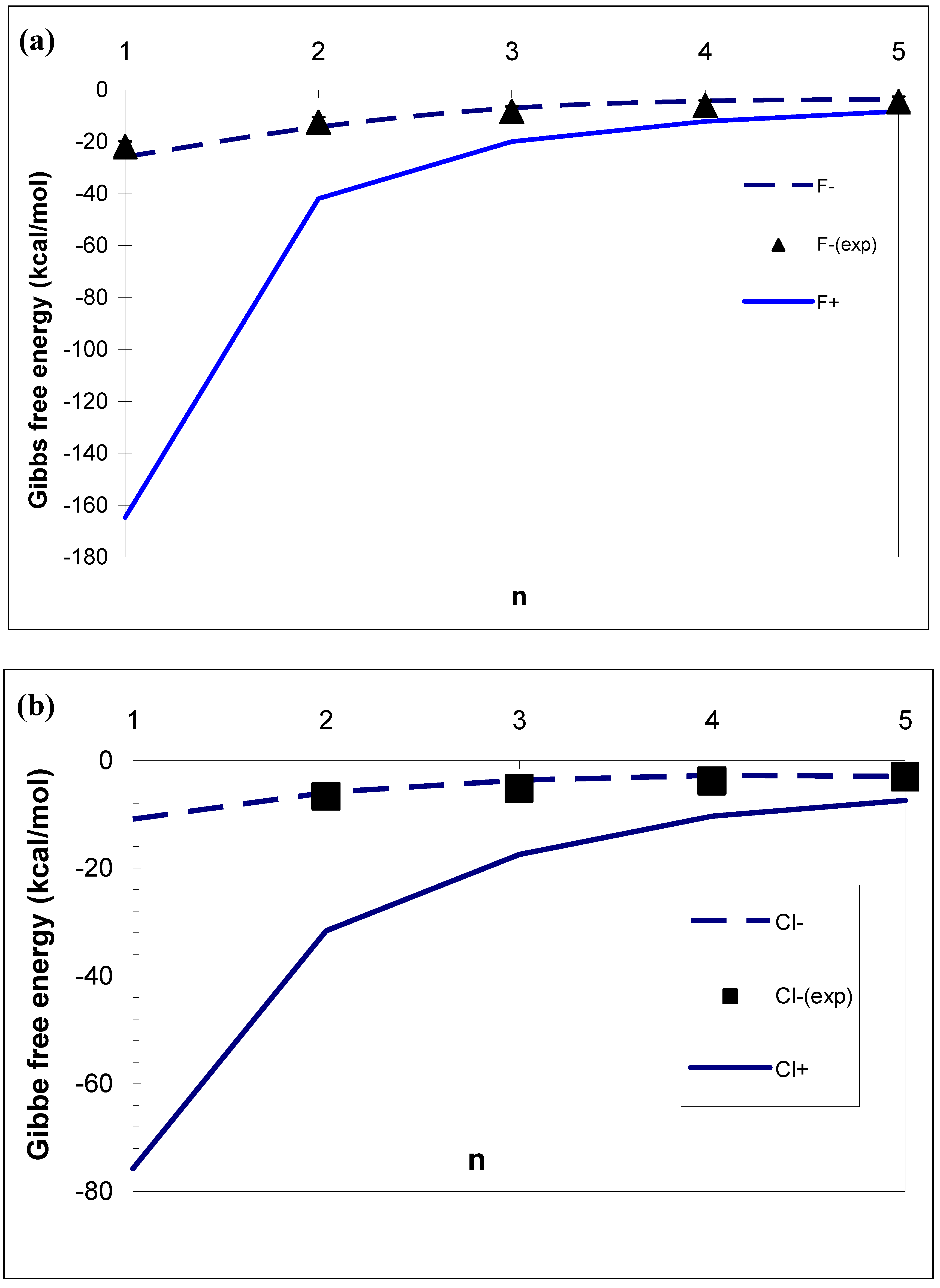
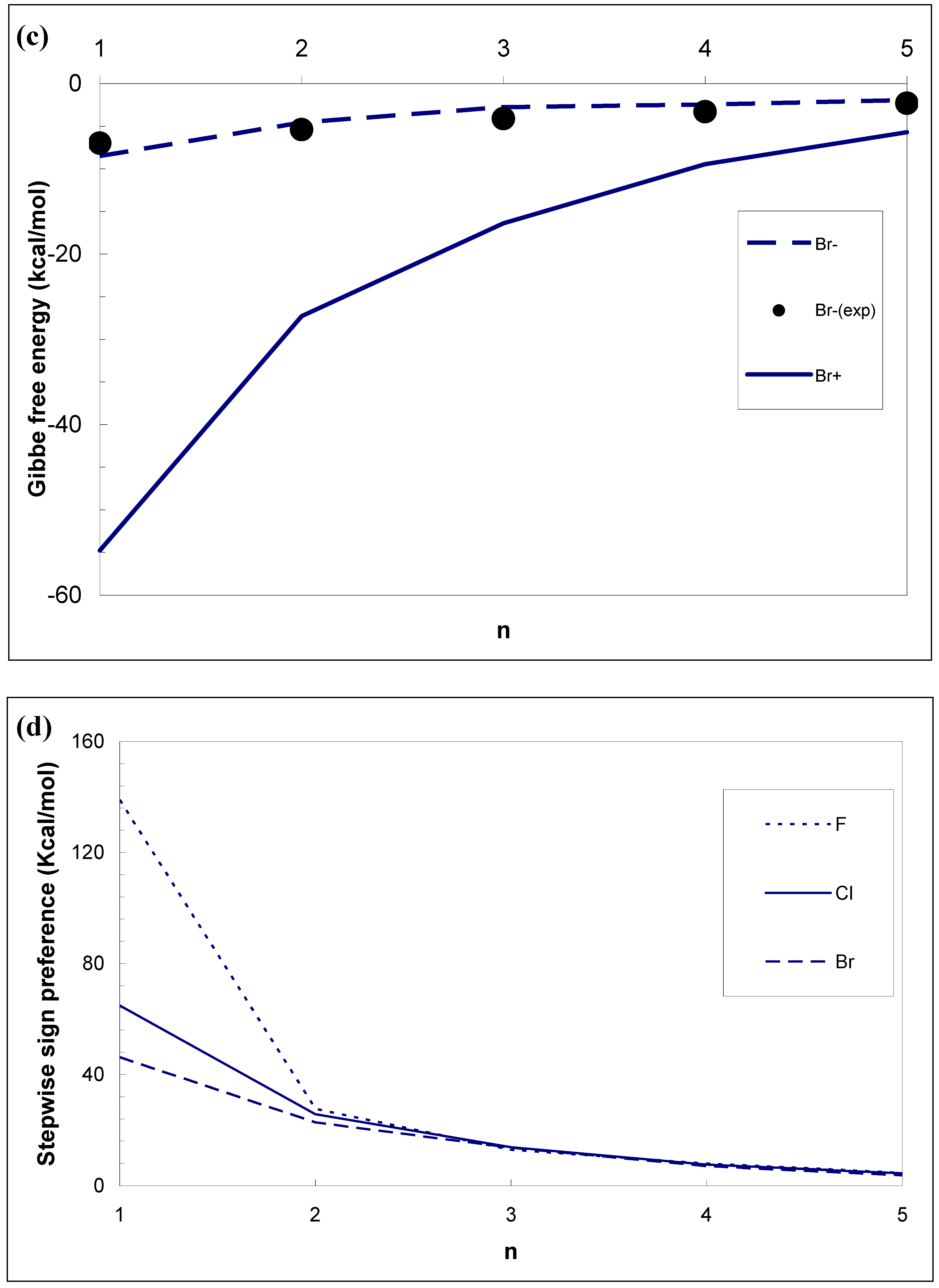
3.1. Difference in Formation Free Energies Between Cations and Anions
3.2. Vibrational Anharmonicity
3. Conclusions
- The effect of ion sign on the formation free energies of aqueous ionic clusters of identical chemical composition is very strong. For example, the difference in the stepwise Gibbs free energy changes ΔG0,1 for F±(H2O)i, Cl±(H2O)i and Br±(H2O)i reaches ∼ 140, 65 and 45 kcal mole−1, respectively. It is important to note that the positive sign preference found for unary water vapours does not contradict with the opposite (negative) sign preference observed in recent experiments in binary sulfuric acid-water vapours [20–22]. In contrast to unary clusters, whose stability is controlled by the affinity of water monomers to ions, the stability of more complex binary clusters studied by Froyd and Lovejoy [20,21] and Sorokin et al. [22] is controlled by two somewhat competing factors : affinities of H2O and H2SO4 to the ions. While the affinity of water to cations is stronger than that to anions, the affinity of H2SO4, the key binary nucleation precursor, to ions exhibits the opposite behavior. This leads, due to the very large difference in the affinity of H2SO4 between anions and cations, to the negative sign preference observed in the experiments [23].
- The harmonic approximation implemented in the framework of the DFT works well in the case of aqueous ionic clusters. Both DFT and ab initio MP2 studies show that the effect of vibrational anharmonicity is mild, and is unlikely a source of large uncertainties in computed free energies.
Acknowledgments
References
- Wilson, CTR. Condensation of water vapour in the presence of dust-free air and other gases. Phil. Trans. R. Soc. London A 1897, 189, 265–307. [Google Scholar]
- Yu, F; Turco, RP. From molecular clusters to nanoparticles: The role of ambient ionization in tropospheric aerosol formation. Geophys. Res. Lett. 2000, 27, 883–887. [Google Scholar]
- Lee, S-H; Reeves, JM; Wilson, JC; Hunton, DE; Viggiano, AA; Miller, TM; Ballenthin, JO; Lait, LR. Particle formation by ion nucleation in the upper troposphere and lower stratosphere. Science 2003, 301, 1886–1889. [Google Scholar]
- Nadykto, AB; Yu, F. Dipole of condensing monomers: A new parameter controlling ion-induced nucleation. Phys. Rev. Lett. 2004, 93, 016101–016104. [Google Scholar]
- Nadykto, AB; Al Natsheh, A; Yu, F; Mikkelsen, KV; Russkanen, J. Qunatum nature of the sign preference in ion-induced nucleation. Phys. Rev. Lett. 2006, 96, 125701–125704. [Google Scholar]
- Castleman, AW, Jr; Tang, IN. Role of small clusters in nucleation about ions. J. Chem. Phys. 1972, 57, 3629–3638. [Google Scholar]
- Kathmann, SM; Schenter, GK; Garrett, BC. Comment. Phys. Rev. Lett. 2007, 98, 109603. [Google Scholar]
- Nadykto, AB; Al Natsheh, A; Yu, F; Mikkelsen, KV; Russkanen, J. Reply Comment. Phys. Rev. Lett. 2007, 98, 109604. [Google Scholar]
- Frisch, MJ; Trucks, GW; Schlegel, HB; Scuseria, GE; Robb, MA; Cheeseman, JR; Montgomery, JA, Jr; Vreven, T; Kudin, KN; Burant, JC; et al. . Gaussian 03, Gaussian. Inc., Walingford, CT, 2004.
- Arshadi, M; Yamdagni, R; Kebarle, P. Hydration of halide negative ions in the gas phase. II. Comparison of hydration energies for the alkali positive and halide negative ions. J. Phys. Chem. 1970, 74, 1475–1489. [Google Scholar]
- Hiraoka, K; Mizuse, S; Yamabe, S. Solvation of halide ions with H2O and CH3CN in the gas phase. J. Phys. Chem. 1988, 92, 3943–3957. [Google Scholar]
- Kathmann, SM; Schenter, GK; Garrett, BC. The critical role of anharmonicity in aqueous ionic clusters relevant to nucleation. J. Phys. Chem. C 2007, 111, 4977–4983. [Google Scholar]
- Kathmann, SM; Schenter, GK; Garrett, BC. Ion-induced nucleation: The importance of chemistry. Phys. Rev. Lett. 2005, 94, 116104–116107. [Google Scholar]
- Diri, K; Myshakin, EM; Jordan, KD. On the contribution of vibrational anharmonicity to the binding energies of water clusters. J. Phys. Chem. A 2005, 109, 4005–4009. [Google Scholar]
- Chaban, GM; Jung, JO; Gerber, RB. Anharmonic vibrational spectroscopy of hydrogen-bonded systems directly computed from ab initio potential surfaces: (H2O)n, n = 2, 3; Cl−(H2O)n, n = l, 2; H+(H2O)n, n = l, 2; H2O-CH3OH. J. Phys. Chem. A 2000, 104, 2772–2779. [Google Scholar]
- Xantheas, SS. Anharmonic vibrational spectra of hydrogen bonded clusters: Comparison between higher energy derivative and mean-field grid based methods. Intern. Rev. Phys. Chem. 2006, 25, 719–733. [Google Scholar]
- Roscioli, JR; Diken, EG; Johnson, MA; Horvath, S; McCoy, AB. Prying apart a water molecule with anionic H-bonding: A comparative spectroscopic study of the X-H2O (X = OH, O, F, Cl, and Br) binary complexes in the 600–3800 cm-1 region. J. Phys. Chem. A 2006, 110, 4943–4952. [Google Scholar]
- Ayotte, P; Weddie, GH; Kim, J; Johnson, MA. Vibrational spectroscopy of the ionic hydrogen bond: Fermi resonances and ion-molecule stretching frequencies in the binary X-·H2O (X = Cl, Br, I) complexes via argon predissociation spectroscopy. J Am ChemSoc 1998, 120, 12361–12362. [Google Scholar]
- Barone, V. Anharmonic vibrational properties by a fully automated second-order perturbative approach. J Chem Phys 2005, 122, 1–10. [Google Scholar]
- Froyd, KD; Lovejoy, ER. Experimental thermodynamics of cluster ions composed of H2SO4 and H2O. 1. Positive ions. J. Phys. Chem. A 2003, 107, 9800–9811. [Google Scholar]
- Froyd, KD; Lovejoy, ER. Experimental thermodynamics of cluster ions composed of H2SO4 and H2O. 2. Measurements and ab initio structures of negative ions. J. Phys. Chem. A 2003, 107, 9812–9824. [Google Scholar]
- Sorokin, A; Arnold, F; Wiedner, D. Formation and growth of sulfuric acid-water cluster ions: Experiments, modelling, and implications for ion-induced aerosol formation. Atmospheric Environment 2006, 40, 2030–2045. [Google Scholar]
- Nadykto, AB; Yu, F; Herb, J. Towards understanding the sign preference in binary atmospheric nucleation. Phys. Chem. Chem. Phys. 2008, 10, 7073–7078. [Google Scholar]

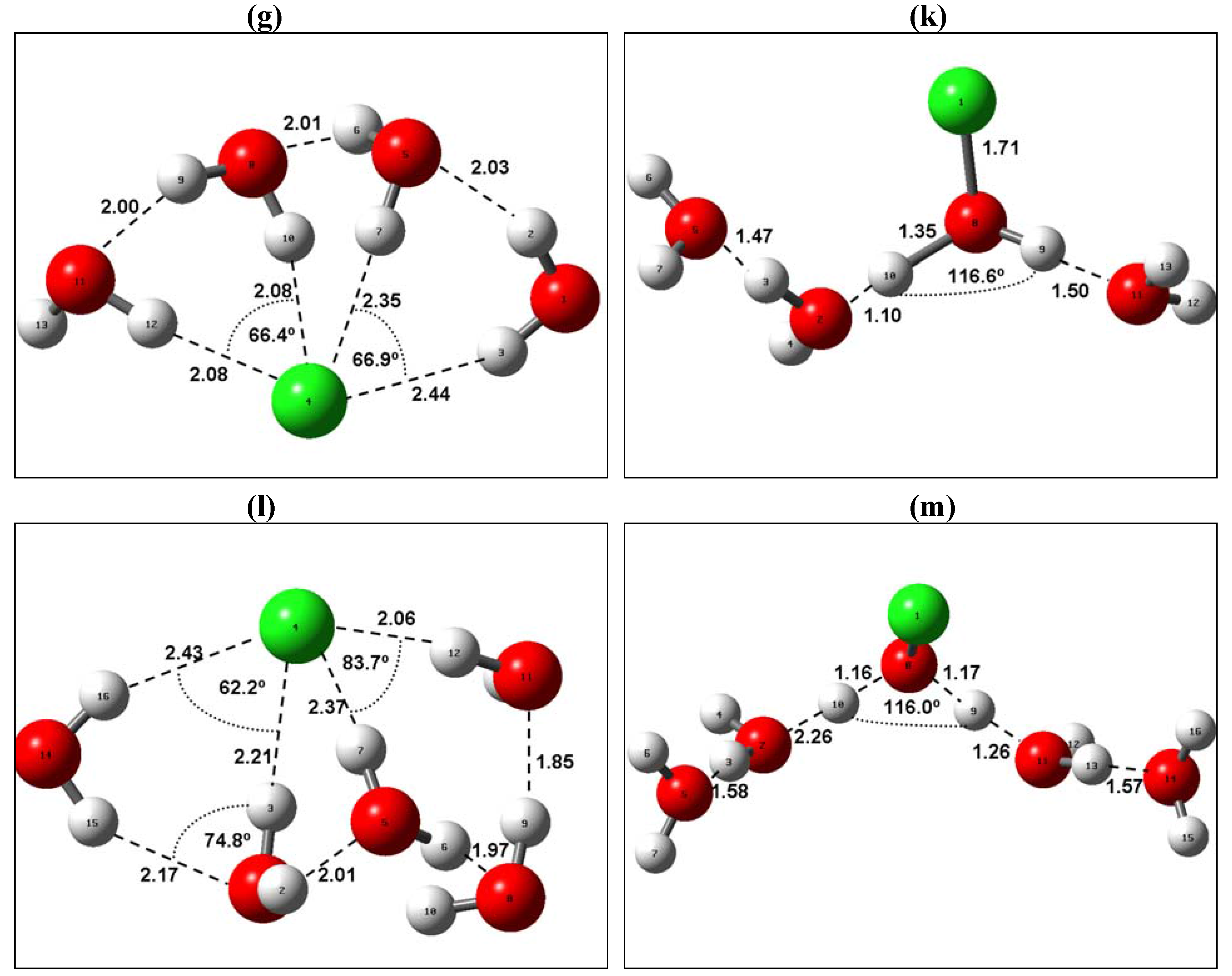

| F−(H2O)
| F−(H2O)2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PW91H | PW91A | MP2H | MP2A | Expa | Expb | PW91H | PW91A | Expa | |
| 1 | 3768 | 3556 | 3952 | 3770 | 3690 | 3687 | 3776 | 3578 | 3700 |
| 2 | 1844 | 1783 | 2069 | 953 | |||||
| 3 | 1623 | 1609 | 1715 | 1625 | 1650 | 2717 | 2375 | 2520 | |
| 4 | 1157 | 1178 | 1242 | 1260 | 1083–1250 | 2506 | 2236 | 2435 | |
| 5 | 569 | 598 | 595 | 586 | |||||
| 6 | 436 | 401 | 412 | 441 | |||||
| Cl−(H2O)
| Cl−(H2O)2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PW91H | PW91H | MP2H | MP2A | Exp. | PW91H | PW91A | exp.1 | exp.2 | |
| 1 | 3770 | 3567 | 3952 | 3764 | 3698a, 3690a, 3699c | ||||
| 2 | 3069 | 2740 | 3376 | 3161 | 3285a, 3130a, 3130c | 3618 | 3431 | 3700a | 3686a |
| 3 | 1626 | 1612 | 1678 | 1743 | 1650b | 3418 | 3092 | 3317a | 3375a |
| 4 | 763 | 782 | 794 | 795 | 745c | 3037 | 2720 | 3245a | 3130a |
| 5 | 394 | 352 | 387 | 366 | |||||
| 6 | 215 | 204 | 200 | 196 | 210a, 155a | ||||
| PW91H | PW91A | MP2H | MP2A | Expa | |
|---|---|---|---|---|---|
| 1 | 3769 | 3575 | 3948 | 3759 | 3689 |
| 2 | 3223 | 2871 | 3506 | 3257 | 3270 |
| 3 | 1619 | 1578 | 1669 | 1633 | 1642 |
| 4 | 668 | 675 | 699 | 690 | 664 |
| 5 | 323 | 345 | 328 | 310 | |
| 6 | 161 | 159 | 158 | 155 | 158 |
| Cl−(H2O) | 0.979 | Cl−(H2O) MP2 | 0.985 |
| Cl−(H2O)2 | 0.979 | ||
| Br−(H2O) | 0.982 | Br−(H2O) MP2 | 0.983 |
| Br−(H2O)2 | 0.985 | ||
| F−(H2O) | 0.949 | F−(H2O) MP2 | 0.966 |
| F−(H2O)2 | 0.945 | ||
| Cl+(H2O) | 0.984 | Cl+(H2O)MP2 | 0.981 |
| Cl+(H2O)2 | 0.977 | ||
| Br+(H2O) | 0.981 | Br+(H2O)MP2 | 0.984 |
| Br+(H2O)2 | 0.985 | ||
| F+(H2O) | 0.980 | F+(H2O) MP2 | 0.981 |
| F+(H2O)2 | 0.979 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nadykto, A.B.; Yu, F.; Al Natsheh, A. Anomalously Strong Effect of the Ion Sign on the Thermochemistry of Hydrogen Bonded Aqueous Clusters of Identical Chemical Composition. Int. J. Mol. Sci. 2009, 10, 507-517. https://doi.org/10.3390/ijms10020507
Nadykto AB, Yu F, Al Natsheh A. Anomalously Strong Effect of the Ion Sign on the Thermochemistry of Hydrogen Bonded Aqueous Clusters of Identical Chemical Composition. International Journal of Molecular Sciences. 2009; 10(2):507-517. https://doi.org/10.3390/ijms10020507
Chicago/Turabian StyleNadykto, Alexey B., Fangqun Yu, and Anas Al Natsheh. 2009. "Anomalously Strong Effect of the Ion Sign on the Thermochemistry of Hydrogen Bonded Aqueous Clusters of Identical Chemical Composition" International Journal of Molecular Sciences 10, no. 2: 507-517. https://doi.org/10.3390/ijms10020507
APA StyleNadykto, A. B., Yu, F., & Al Natsheh, A. (2009). Anomalously Strong Effect of the Ion Sign on the Thermochemistry of Hydrogen Bonded Aqueous Clusters of Identical Chemical Composition. International Journal of Molecular Sciences, 10(2), 507-517. https://doi.org/10.3390/ijms10020507




