The Study of the Inhibition of the Recombinant TACE Prodomain to Endotoxemia in Mice
Abstract
:Objective:
Methods:
Results:
Conclusions:
1. Introduction
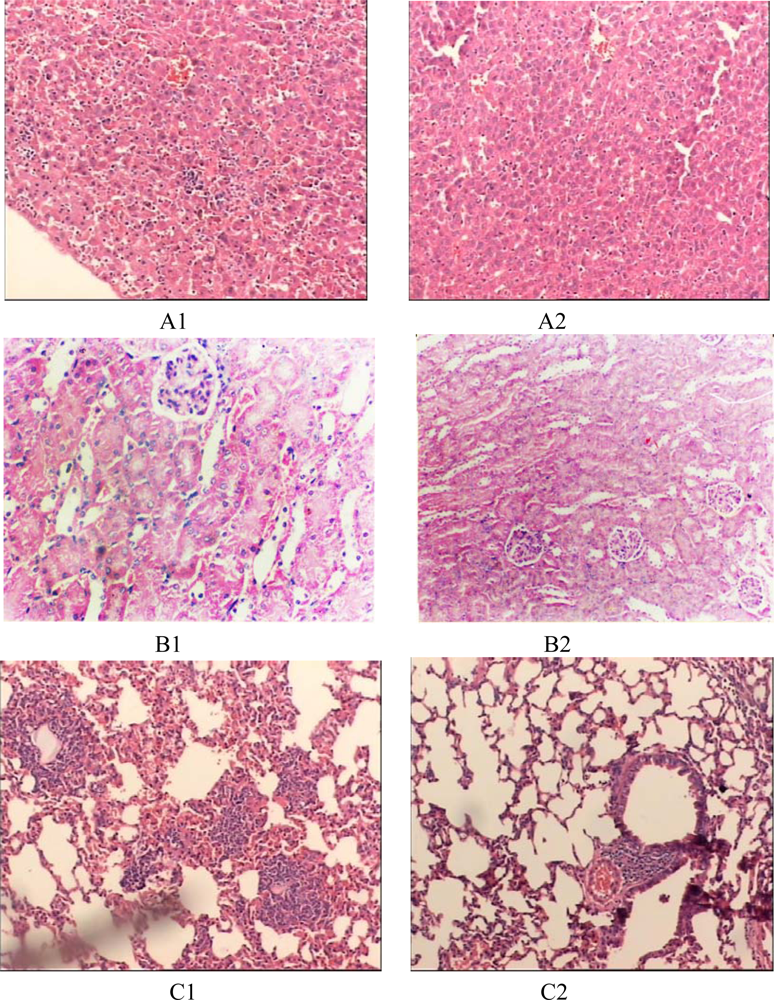
2. Results and Discussion
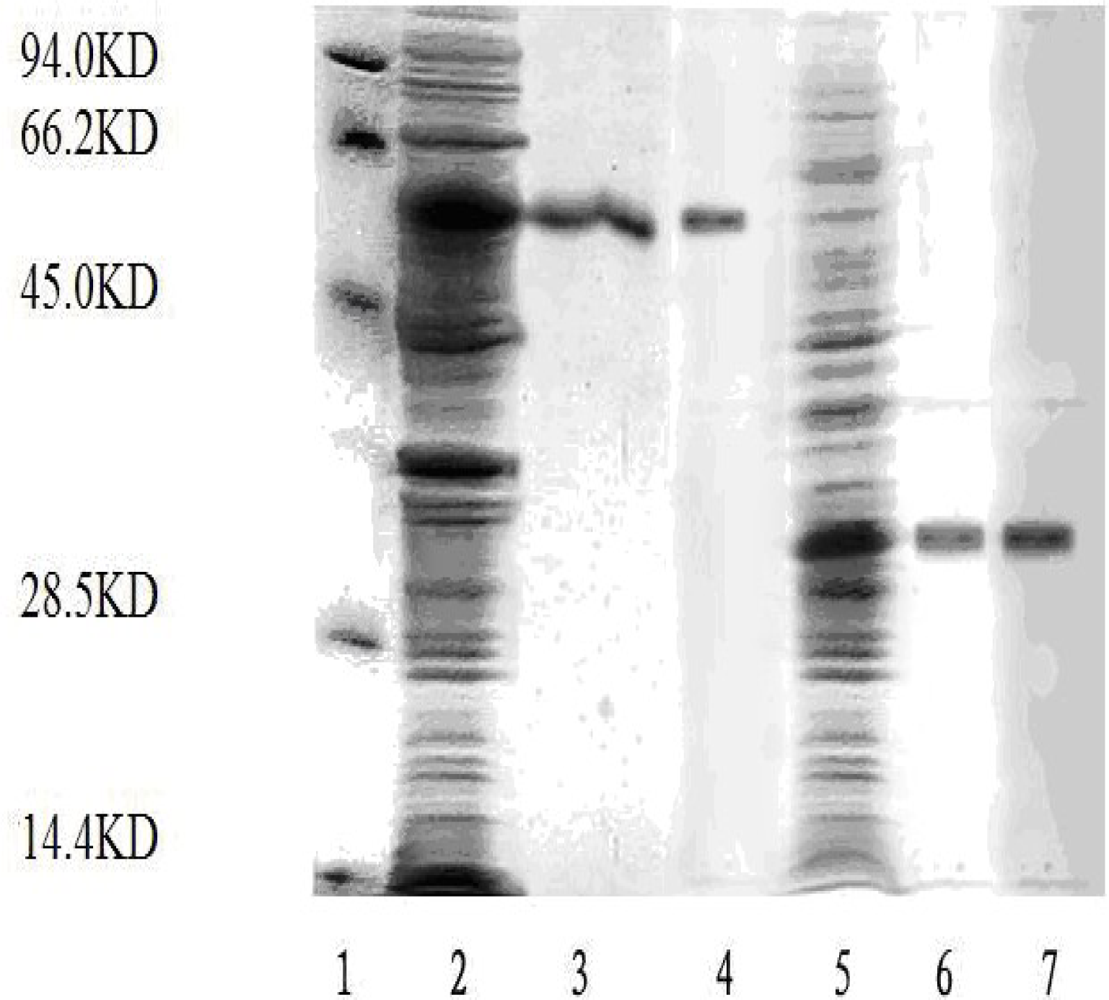
2.1. Expression and Purification of TACE Prokaryotic Expression Plasmids
2.2. The Binding of the TACE Prodomain Protein to TACE
2.3. Establishment of the Endotoxemia Model
2.4. The Prodomain Protein Mediated Accumulation of mTNF-α on Murine Peritoneal Macrophages
3. Experimental Section
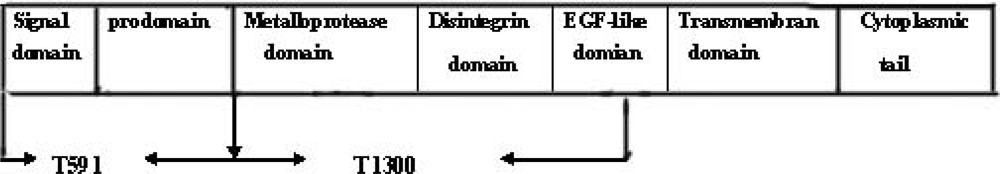
3.1. Construction of Prokaryotic Expression Plasmids of TACE
3.2. Expression and Purification of TACE Ecotodomain and Prodomain
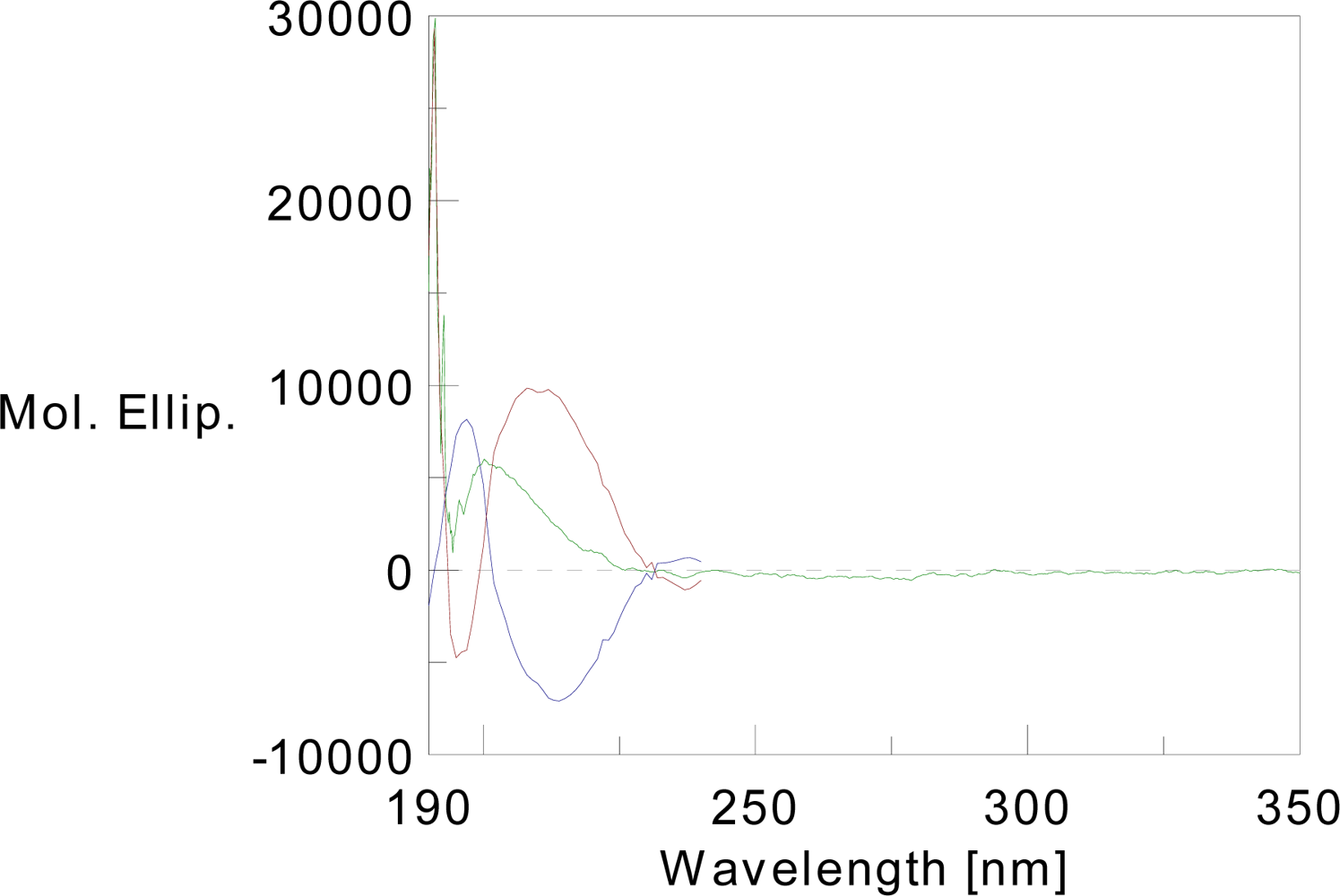
3.3. Circular Dichroism
3.4. Binding Analysis of TACE Prodomain Protein to TACE
3.5. LPS-Induced Endotoxaemia Model
3.6. Flow Cytometric Analysis of mTNF-α Level
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Stone, AL; Kroeger, M; Sang, QX. Structure-function analysis of the ADAM family of disintegrin-like an metalloproteinase-containing protein. J. Protein Chem 1999, 18, 447–465. [Google Scholar]
- Wolfsberg, TG; Peimakoff, P; Myles, DG; White, JM. ADAM, a novel family of membrane protein containing a disintegrin and metalloprotease domain. Cell Biol 1995, 131, 275–278. [Google Scholar]
- Schlondorff, J; Blobel, CP. Metalloprotease-disintegrins: Modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci 1999, 112, 3603–3617. [Google Scholar]
- Kang, T; Zhao, YG; Pei, Q; Sucic, JF; Sang, QX. Intracellular activation of human ADAM19 by furin occurs via one of the two consecutive recognitions sites. Eur. J. Biochem 2003, 270, 2386–2393. [Google Scholar]
- Galazka, G; Windsor, LJ; Birkedal-Hansa, H. APMA (4-Aminophenylmercu acetate) activation of stromelysin-1 involves protein interactions in addition to those with cystine-75 in the propeptider. Biochemistry 1999, 8, 316–1322. [Google Scholar]
- Nakayama, K. Furin: A mammaliam subtilisin/kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J 1997, 327, 625–635. [Google Scholar]
- Anders, A; Gilbart, S; Garten, W; Postina, R; Fahrennolz, H. Regulation of the α-secretase ADAM10 by its prodomain and proprotein convertase. FASEB J 2001, 5, 1837–1839. [Google Scholar]
- Kang, T; Zhao, YG; Pei, D; Sucic, JF; Sang, QX. Intracellular activation of human adamalysin 19/disintegrin and metalloproteinase 19 by furin occurs via one of the two consecutive recognition sites. J. Biol. Chem 2002, 77, 25583–25591. [Google Scholar]
- Loechel, F; Gilpin, BJ; Engvall, E; Albrechtsen, R; Wewer, UM. Human ADAM 12 (meltrin) is an active metalloprotease. J. Biol. Chem 1998, 273, 16993–16997. [Google Scholar]
- Locksley, RM; Killeen, N; Lenardo, MJ. The TNF and TNF receptor supurfamilies integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar]
- Stone, AL; Kroeqer, M; Sang, QX. Structure-function analysis of the ADAM family of disintegrin-like and metalloproteinase-containing protein. Prot. Chem 1999, 18, 447–465. [Google Scholar]
- Wolfsberg, TG; Primakoff, P; Myles, DG; White, JM. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain. Cell Biol 1995, 131, 275–278. [Google Scholar]
- Black, RA; Rauch, CT; Kozlosky, CJ; Peschon, JJ; Slack, JL; Wolfson, MF; Castner, BJ; Stocking, KL; Reddy, P; Srinivasan, S; Nelson, N; Boiani, N; Schooley, KA; Gerhart, M; Davis, R; Fitzner, JN; Johnson, RS; Paxton, RJ; March, CJ; Cerretti, DP. A metalloproteinase disintegrin that releases tumor-necrosis factor-alapha from cells. Nature 1997, 385, 729–733. [Google Scholar]
- Moss, ML; Jin, LC; Milla, ME; Burkhart, W; Carter, HL; Chen, WJ; Clay, WC; Didsbury, JR; Hassler, D; Hoffman, CR; Kost, TA; Lambert, MH; Leesnitzer, MA; McCauley, P; McGeehan, G; Mitchell, J; Moyer, M; Pahel, G; Rocque, W; Overton, LK; Schoenen, F; Seaton, T; Su, JL; Becherer, JD. Cloning of a disintegrin metalloproteinase that processes precursor tumor-necrosis factor-alpha. Nature 1997, 385, 733–736. [Google Scholar]
- Philip, R; Epstein, L. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature 1986, 323, 86–89. [Google Scholar]
- Heller, R. A; Kronke, M. Tumor necrosis factor receptormediated signaling pathways. J. Cell Biol 1994, 126, 5–9. [Google Scholar]
- Huang, W; Li, LB; Huang, L; Yang, YZ. Screening of TACE peptide inhibition from phage display peptide library. J. Huazhong Univ. Sci. Technol 2005, 25, 473–476. [Google Scholar]
- Ting, TB; Li, LB; Zhu, KL; Huang, W; Yang, YZ. Relationship between the increase of secretion of sTNF-α induced by L ipopolysacchar ides and the enhanced expression of TACEmRNA in HL-60 cells and adhesive cells from human spleen. J. Tongji Med. Univ 2001, 21, 265–268. [Google Scholar]
- Loechel, F; Overgaard, MT; Oxvig, G. Regulation of human ADAM12 protease by the prodomain. J. Biol 1999, 274, 13427–13433. [Google Scholar]
- Amin, AR. Regulation of tumor necrosis factor-alpha and tumor necrosis factor-alpha converting enzyme in human osteoarthris. Osteoarthritis Cartilage 1999, 79, 392–394. [Google Scholar]
- Moss, ML; Jin, SL; Milla, ME; Bickett, DM; Burkhart, W; Carter, HL; Chen, WJ; Clay, WC; Didsbury, JR; Hassler, D; Hoffman, CR; Kost, TA; Lambert, MH; Leesnitzer, MA; McCauley, P; McGeehan, G; Mitchell, J; Moyer, M; Pahel, G; Rocque, W; Overton, LK; Schoenen, F; Seaton, T; Su, JL; Becherer, JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 1997, 385, 733–736. [Google Scholar]
- Peschon, JJ; Slecck, JL; Reddy, P. Enhanced: A cellular striptease act. Science 1998, 282, 1279–1280. [Google Scholar]
- Zhu, KL; Yang, YZ; Han, L. The study on different acting mechanisms of three types of TACE inhibitors in converting of pro-TNFα into sTNFα [in Chinese]. Chin J Immunol 2003, 19, 711–752. [Google Scholar]
- Milla, ME; Leesnitzer, MA; Moss, ML. Specific sequence elements are required for the expression of functional tumor. J. Biol. Chem 1999, 274, 30563–30570. [Google Scholar]
- Raetz, CR; Ulevitch, RJ; Wright, SD; Sibley, CH; Ding, A; Nathan, CF. Gram-negative endotoxin. An extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 1991, 5, 2652–2660. [Google Scholar]
- Ulevitch, RJ; Tobias, PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol 1995, 13, 437–457. [Google Scholar]
- Pajkrt, D; Doran, JE; Koster, F. Anti-inflammatory effects of reconstituted high-density lipoprotein during human endotoxaemia. J. Cell Biol 1996, 184, 1601–1608. [Google Scholar]
- Deitch, EA. Role of bacterial translocation in necrotizing enterocolitis. Acta Paediatr. Suppl 1994, 396, 33–36. [Google Scholar]
- Nadler, EP; Upperman, JS; Dickinson, EC; Ford, HR. Nitric oxide and intestinal barrier failure. Semin. Pediatr. Surg 1999, 8, 148–154. [Google Scholar]
- Choe, YH; Lee, SW. Effect of lactoferrin on the production of tumor necrosis factor-alpha and nitric oxide. J. Cell Biochem 1999, 76, 30–36. [Google Scholar]
- Sahin, U; Weskamp, G; Zhou, HM; Higashiyama, S; Peschon, J; Hartmann, D; Saftig, P; Blobel, CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol 2004, 164, 769–779. [Google Scholar]
- Weskamp, G; Schlondorff, J; Lun, L; Becherer, JD; Kim, TW; Saftig, P; Hartmann, D; Murphy, G; Blobel, CP. Evidence for a critical role of the tumor necrosis factor alpha convertase (TACE) in ectodomain shedding of the p75 neurotrophin receptor (p75NTR). J. Biol. Chem 2004, 279, 4241–4249. [Google Scholar]
| Groups of coating antigen | Density of coating antigen (mg/L) | Density of T1300 (mg/L) | OD450 |
|---|---|---|---|
| Pro-domain protein | 20 | 100 | 0.69 |
| Pro-domain protein | 20 | 50 | 0.50 |
| Pro-domain protein | 20 | 10 | 0.42 |
| Pro-domain protein | 20 | 5 | 0.28 |
| Pro-domain protein | 20 | 1 | 0.18 |
| BSA | 100 | 0.13 | |
| BSA | 50 | 0.16 | |
| BSA | 10 | 0.14 | |
| BSA | 5 | 0.14 | |
| BSA | 1 | 0.15 | |
| Coating | 10 | 0.08 | |
| liquor(without antigen) |
| Groups | mTNF-α expression (%) |
|---|---|
| basic control | 10.12 ± 8.22 |
| pro-domain alone | 9.88 ± 6.35 |
| LPS-stimulation | 20.85 ± 10.61 |
| pro-domain protein (10 mg/kg) + LPS | 40.34 ± 2.31a |
| pro-domain protein (20 mg/kg) + LPS | 94.56 ± 4.11a |
| pro-domain protein (50 mg/kg) + LPS | 98.14 ± 2.43a |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.; Yan, Y.; Huang, W.; Yang, Y. The Study of the Inhibition of the Recombinant TACE Prodomain to Endotoxemia in Mice. Int. J. Mol. Sci. 2009, 10, 5442-5454. https://doi.org/10.3390/ijms10125442
Li X, Yan Y, Huang W, Yang Y. The Study of the Inhibition of the Recombinant TACE Prodomain to Endotoxemia in Mice. International Journal of Molecular Sciences. 2009; 10(12):5442-5454. https://doi.org/10.3390/ijms10125442
Chicago/Turabian StyleLi, Xiaoou, Yuan Yan, Wei Huang, and Yuzhen Yang. 2009. "The Study of the Inhibition of the Recombinant TACE Prodomain to Endotoxemia in Mice" International Journal of Molecular Sciences 10, no. 12: 5442-5454. https://doi.org/10.3390/ijms10125442
APA StyleLi, X., Yan, Y., Huang, W., & Yang, Y. (2009). The Study of the Inhibition of the Recombinant TACE Prodomain to Endotoxemia in Mice. International Journal of Molecular Sciences, 10(12), 5442-5454. https://doi.org/10.3390/ijms10125442




