Abstract
The protocol of lithiation by means of lithium and a catalytic (5% molar) amount of DTBB (4,4’-di-tert-butylbiphenyl), applied to ω-chloro ortho ester 6 under Barbier-type conditions gives, after final acid-catalyzed methanolysis, the corresponding functionalized esters 8 or 9 (for chlorotrimethylsilane as electrophile) or, after ortho ester deprotection and acid catalyzed treatment, the δ-lactones 11. The procedure is also practical for bicyclic ortho esters 14: the β-chloro OBO ester derivate generates the γ-lactones 15 and the γ-chloro OBO ester gives corresponding esters 8.
Introduction
Among organometallic reagents, organolithium compounds are the most reactive ones due to the high ionic nature of the carbon-lithium bond [1]. Functionalized organolithium compounds are very useful in synthetic organic chemistry in order to transfer their functionality to electrophiles in a single synthetic step, thus organolithium derivatives bearing a carboxylic moiety would transfer a carboxylic group to an electrophilic reagent. The corresponding α-derivatives (carboxylate enolate, 1) [2] have been prepared by double deprotonation [2,3] using lithium dialkylamides [4] as bases (e.g. LDA). Recently, Parra et al. [5] have reported the generation of this intermediate (and the subsequent reaction with carbonyl reagents) by using a sub-stoichiometric amount of an amine (Et2NH or AZA: 1,3,3-trimethyl-6-azabicyclo-[3.2.1]-octane) and n-butyllithium to regenerate the amide. Our laboratory has approached the preparation of this organolithium reagent starting from α-chloroacetic acid by chlorine-lithium exchange using an excess of lithium in the presence of catalytic amount of DTBB (4,4’-di-tert-butylbiphenyl, 5% molar) [6].
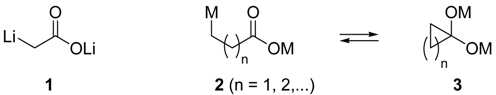
In general homoenolates (i.e. β-enolates, 2 with n = 1), bishomoenolates (i.e. γ-enolates, 2 with n = 2) and higher-order enolates (δ-, ε-, …) 2 are far more unstable because they can undergo cyclization to generate intermediates 3. Carboxylate homoenolates can be prepared by deprotonation only when a stabilizing group is present at the β-position [7]. Another procedure to prepare these organolithium intermediates is via halogen-lithium exchange, either bromo-lithium [8] or chloro-lithium, the later being performed in our group using the same methodology as for the corresponding α-enolate (arene-catalyzed lithiation) [6]. To the best of our knowledge, there are few examples in the literature starting from the corresponding carboxylic acids, in all cases, being prepared by deprotonation with strong bases, at a carbon which bears a stabilizing group [9].
Our laboratory has been studying, during more than a decade, the preparation of functionalized organolithium intermediates [10] starting from different substrates (chlorinated, non-halogenated, or heterocyclic precursors [11]) by using an arene-catalytic lithiation under very mild reaction conditions [12,13,14]. Employing this methodology organolithium compounds bearing a masked carbonyl function (masked lithium ω-enolates) have been prepared [12,15]. We report herein the preparation of masked β-, γ-, and δ-lithium ester enolates starting from the corresponding ω-chloro ortho esters, by a chlorine-lithium exchange using the arene-catalyze lithiation process, and their reaction with electrophilic compounds [16].
Results and Discussion
Monocyclic ortho esters 6 (2-ethoxy-1,3-dioxolane derivatives) were easily prepared from the corresponding nitriles by the Pinner synthesis [17]. The reaction of 4-chlorobutyronitrile (4a) or 5‑chlorovaleronitrile (4b) with ethanol in the presence of hydrogen chloride at -5°C gave the corresponding imidates 5, which by treatment with ethylene glycol in hexane yielded the corresponding ω-chloro ortho esters 6 (Scheme 1).
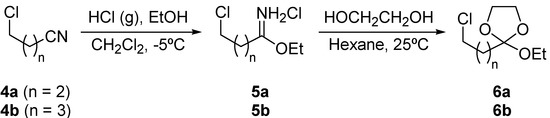
Scheme 1.
Preparation of ω-chloro ortho esters 6
The chlorine-lithium exchange from chlorinated ortho esters 6, using an excess of lithium powder (5 eq) and a catalytic amount of 4,4’-di-tert-butylbiphenyl (DTBB, 5% molar), to generate the organolithium intermediates 7 and their subsequent reaction with different electrophiles [E = ButCHO, PhCHO, (CH2)5CO, Et2CO, PhCOMe, PhCH=NPh, MeSiCl] had to be carried out under Barbier-type conditions (lithiation in the presence of the electrophile [18]) in THF at -78°C in order to avoid decomposition of intermediate 7 under the tested reaction conditions. After hydrolysis with phosphate buffer and final treatment in dry methanol and a catalytic amount of p-toluensulfonic acid (PTSA), the corresponding functionalized methyl esters 8 were obtained (Scheme 2, Table 1) in all the cases, except when chlorotrimethylsilane was used as electrophile. In those cases, ω-trimethylsilyl hydroxyethyl alkanoates 9a and 9b (Table 1, entries 7 and 14) were the isolated products, their formation being explained by considering the anchimeric assistance of the silicon present in the structure during the hydrolysis step (see intermediate 10).
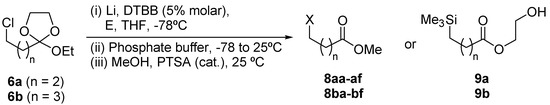
Scheme 2.
Preparation of compounds 8 and 9 from chloro ortho esters 6
Performing the reaction with the ortho ester 6a and benzaldehyde as electrophile at higher temperature (0°C) or under Grignard-type conditions (a two-step process), lower yields were obtained (Table 1, entry 2, footnotes c and d, respectively).
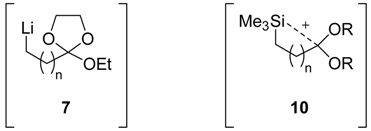

Table 1.
Preparation of compounds 8 and 9 from chloro ortho esters 6
| Entry | Starting material | Electrophile (E) | Product 8 or 9a | |||
| No. | n | X | Yield (%)b | |||
| 1 | 6a | ButCHO | 8aa | 2 | ButCHOH | 58 |
| 2 | 6a | PhCHO | 8ab | 2 | PhCHOH | 63 (54)c (47)d |
| 3 | 6a | (CH2)5CO | 8ac | 2 | (CH2)5COH | 59 |
| 4 | 6a | Et2CO | 8ad | 2 | Et2COH | 51 |
| 5 | 6a | PhCOMe | 8ae | 2 | PhC(OH)Me | 52 |
| 6 | 6a | PhCH=NPh | 8af | 2 | PhCHNHPh | 57 |
| 7 | 6a | Me3SiCl | 9ae | 2 | Me3Si | 40 |
| 8 | 6b | ButCHO | 8ba | 3 | ButCHOH | 62 |
| 9 | 6b | PhCHO | 8bb | 3 | PhCHOH | 66 |
| 10 | 6b | (CH2)5CO | 8bc | 3 | (CH2)5COH | 56 |
| 11 | 6b | Et2CO | 8bd | 3 | Et2COH | 52 |
| 12 | 6b | PhCOMe | 8be | 3 | PhC(OH)Me | 49 |
| 13 | 6b | PhCH=NPh | 8bf | 3 | PhCHNHPh | 54 |
| 14 | 6b | Me3SiCl | 9be | 3 | Me3Si | 47 |
a All compounds 8 and 9 were ≥95% pure (GLC and/or 300 MHz 1H-NMR); b Isolated yield after column chromatography (silica gel, hexane/ethyl acetate) based on the starting chloro ortho ester 6; c Yield corresponding to the reaction at 0°C. d Yield corresponding to the Grignard-type reaction; e The corresponding 2-hydroxyethyl ester was isolated; see Scheme 2.
In the literature there are some examples for the synthesis of δ-lactones from the corresponding δ‑hydroxy esters by treatment with catalytic amounts of an organic acid [e.g. camphorsulphonic acid, trifluoroacetic acid (TFA), etc.] in an inert solvent [19]. Thus, we decided to prepare the corresponding δ-lactones directly from the lithiation resulting mixture by acid catalyzed rearrangement. For the substrates 8ab, 8ac and 8ad we tested different organic and inorganic acids (i.e. catalytic amounts of PTSA, TFA, HCl or H2SO4) in different solvents (i.e. CH2Cl2, benzene, toluene, THF) and at different temperatures (from room temperature to reflux). None of the different experiments gave the expected lactones 11b-d in more than 10% yield, a wide range of decomposition byproducts being obtained in the reaction mixtures.
We therefore prepared the δ-lactones 11a-d following the standard procedure for hydrolysis of the ortho ester protecting group [20] to generate the corresponding δ-hydroxy acid and converting this to the final δ‑lactone. Hence, the ortho ester 6a was lithiated as described above and, after hydrolysis, the reaction mixture was treated successively with KHSO4 in a mixture of dimethoxyethane/water (5:1) at 0°C and lithium hydroxide (basic pH) in the same solvent mixture at room temperature. Refluxing the mixture obtained in benzene in the presence of a catalytic amount of PTSA resulted in formation of the corresponding δ-lactones 11a-d with modest overall yields (Scheme 3, Table 2).
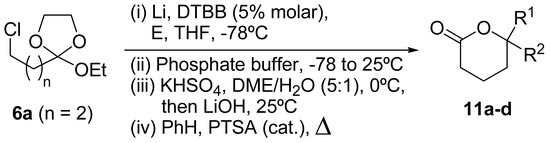
Scheme 3.
Preparation of δ-lactones 11 from ortho ester 6a

Table 2.
Preparation of δ-lactones 11 from ortho ester 6a
| Entry | Carbonyl compound (E) | Product 11a | |||
| No. | R1 | R2 | Yield (%)b | ||
| 1 | ButCHO | 11a | But | H | 47 |
| 2 | PhCHO | 11b | Ph | H | 33 |
| 3 | (CH2)5CO | 11c | (CH2)5 | 37 | |
| 4 | Et2CO | 11d | Et | Et | 35 |
a All the lactones 11 were ≥95% pure (GLC and/or 300 MHz 1H‑NMR); b Isolated yield after chromatography (silica gel, hexane/ethyl acetate) based on the starting chloro ortho ester 6a.
In order to generate the masked corresponding homoenolates (7, n = 1) we planned to use the same strategy starting from the corresponding β-chloro ortho ester, nevertheless, we could not obtain it in pure form. By employing the synthetic methodology described by Pinner and starting from 3‑chloronitrile we obtained an inseparable mixture of the desired ortho ester and the corresponding dehydrochlorinated ortho ester (ca. 1:1 mixture). For this reason, we prepared the β‑chloro bicyclic ortho ester 14a (OBO ester: 2,6,7-trioxabicyclo[2.2.2]octane ester [17a, 20]). The ortho ester 14a was prepared according to the synthetic path outlined in Scheme 4, starting from the commercially available 3-chloropropionyl chloride (12a), which was coupled with 3-hydroxymethyl-3-methyloxetane in the presence of pyridine to yield the ester 13a (80%). Treatment of this oxetane ester with boron trifluoride etherate gave the isomeric bridged ortho ester 14a in 63% yield. The corresponding γ-chloro OBO ester (14b) was also prepared following the same synthetic pathway, starting in this case from 4-chlorobutanoyl chloride (12b), which subsequently was coupled to generate the corresponding ester 13b (92% isolated yield) and finally rearranged to the ortho ester 14b (65% isolated yield from the ester 13b).
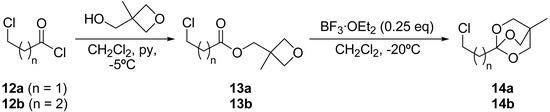
Scheme 4.
Preparation of ortho esters 14
Performing the lithiation reaction with the OBO ester 14a by means of the protocol used above for compounds 6 [using different carbonyl compounds as electrophiles: i.e. ButCHO, PhCHO, (CH2)5CO, Et2CO, PhCOMe], after hydrolysis and final treatment with a catalytic amount of PTSA in THF the corresponding lactones 15 were the only isolated products (Scheme 5, Table 3). If the final treatment with a catalytic amount of PTSA was carried out in dry methanol a mixture of the corresponding methyl esters and the corresponding γ-butyrolactones were obtained.

Scheme 5.
Preparation of γ-lactones 15 from ortho ester 14a

Table 3.
Preparation of γ-lactones 15 from ortho ester 14a
| Entry | Carbonyl compound (E) | Product 15a | |||
| No. | R1 | R2 | Yield (%)b | ||
| 1 | ButCHO | 15a | But | H | 45 |
| 2 | PhCHO | 15b | Ph | H | 43 |
| 3 | (CH2)5CO | 15c | (CH2)5 | 39 | |
| 4 | Et2CO | 15d | Et | Et | 38 |
| 5 | PhCOMe | 15e | Ph | Me | 37 |
a All the γ-lactones 15 were ≥96% pure (GLC and/or 300 MHz 1H‑NMR); b Isolated yield after chromatography (silica gel, hexane/ethyl acetate) based on the starting chloro ortho ester 14a.
When the corresponding γ-chloro OBO ester 14b was lithiated using ButCHO, PhCHO, PhCOMe as electrophiles and treated afterwards under the conditions depicted in Scheme 2, but at 0°C, the corresponding methyl esters 8aa (38% yield), 8ab (41% yield) and 8ae (37% yield) were isolated, respectively. For this type of ortho ester the lithiation process gave lower yield when the reaction was performed at lower temperature (-78°C). For instance, the ester 8ab was obtained in 30% yield, after final methanolysis, performing the lithiation at -78°C. For the reaction carried out via a two-step process (Grignard conditions), and using benzaldehyde as electrophile, a similar yield of the corresponding ester 8ab was obtained (39%).
With the intention of studying the stability of the organolithium intermediates involved in these reactions we decided to carry out deuterolysis experiments with ortho esters 6a and 14b. After generating the corresponding organolithium intermediate through the DTBB-catalyzed methodology at -78°C (for intermediate 7a) or 0°C (for intermediate 15b), the final hydrolysis was carried out with deuterium oxide, yielding the corresponding ortho esters 16 and 17 with the yields and deuterium incorporation shown in Scheme 6.
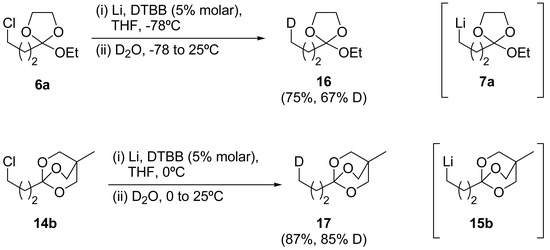
Scheme 6.
Deuterolysis of lithiated intermediates 7a and 15b
These observations indicate that ω-lithium OBO ester 15b is more stable under the reaction conditions assayed than the corresponding monocyclic 7a, which abstracts a proton from the reaction media more easily. These results are in agreement with the fact that monocyclic ortho esters gave better results under Barbier-type conditions whereas for OBO esters, regardless the conditions (Barbier-type or Grignard-type), gave the final product with almost the same yield.
Conclusions
We have demonstrated that the DTBB-catalyzed lithiation methodology can be applied to generate synthons of β-, γ-, and δ-lithio carboxylic acids by using an ortho ester protective group to mask the carboxylic moiety. These intermediates react with different electrophilic compounds (mainly carbonyl compounds) to generate remote functionalized carboxylic acid derivatives (i.e. hydroxy esters and/or lactones). Regarding to the stability we have verified that ω-lithium OBO ester intermediates are more stable than the corresponding monocyclic ortho esters ones. Albeit in general, better yields of the final products have been obtained starting from 2-ethoxy-1,3-dioxolanes performing the reaction under Barbier-type conditions.
Acknowledgements
This work was generously supported by the Dirección General de Enseñanza Superior (DGES) of the Spanish Ministerio de Ciencia y Tecnología (MCyT, grant no. BQU2001-0538) and the Generalitat Valenciana (GV, grant no. GRUPOS 03/135). R. T. thanks the GV for a scholarship.
Experimental
General
All lithiation reactions were carried out under argon atmosphere in oven-dried glassware. All commercially available reagents (Acros, Aldrich, Fluka) were used without further purification, except in the case of electrophiles, which were used freshly distilled. Commercially available anhydrous THF (99.9%, water content ≤0.006%, Acros) was used as solvent in all the lithiation reactions. IR spectra (thin film) were measured with a Nicolet Impact 400 D-FT Spectrometer. NMR spectra were recorded with a Bruker AC-300 using CDCl3 as solvent (unless another solvent is indicated) and TMS as internal standard; chemical shifts are given in ppm and coupling constants (J) are given in Hz. LRMS were measured with Shimadzu GC/HS QP-5000 and Hewlett-Packard EM/CG-5973A spectrometers, and HRMS were measured with Finnigan MAT95 S spectrometer, fragment ions in m/z with relative intensities (%) in parentheses. The purity of volatile products and the chromatographic analyses (GLC) were determined with a flame ionization detector and a 30 m capillary column (0.32 mm diam.; 0.25 μm film thickness), using nitrogen (2 mL/min) as carrier gas, Tinjector = 275°C, Tdetector = 300°C, Tcolumn = 60°C (3 min) and 60 to 270°C (15°C/min), P = 40 KPa. Thin layer chromatography (TLC) was carried out on Merck plastic sheets coated with silica gel 60 F254. Lithium powder was prepared from commercially available lithium granules (99%, high sodium content, Aldrich) as previously reported by us [21].
Preparation of ortho esters 6.
Hydrogen chloride was bubbled through a cooled (-5°C) solution of the starting nitrile 4 (0.1 mol) and ethanol (0.12 mol, 7 mL) in CH2Cl2 (150 mL) until the solution was saturated. The resulting solution was then allowed to stand at 0°C for 4 days. The reaction mixture was concentrated to 2/3 of the original volume under vacuum and the precipitated imidate salt 5 was filtered, washed with several portions of diethyl ether (5 x 20 mL) and dried. Imidic ester hydrochlorides can be kept for several weeks if carefully protected from atmospheric moisture. To a solution of part of the corresponding imidate (50 mmol) in dry hexane (150 mL) was added ethylene glycol (150 mmol, 8.5 mL), and the reaction was allowed to stir for 2 days. The resulting solid (ammonium chloride) was filtered out and the solvent was concentrated under vacuum. The ortho ester 6 was pure obtained as an oil by fractional distillation from anhydrous K2CO3.
2-(3-Chloropropyl)-2-ethoxy-1,3-dioxolane (6a): b.p.: 108°C (0.1 mmHg); 1H-NMR δ: 1.18 (3H, t, J = 7.0, CH3), 1.96 (4H, m, CCH2CH2), 3.55 (2H, q, J = 7.0, CH2CH3), 3.60 (2H, m, CH2Cl), 3.97, 4.11 (2H and 2H, 2m, OCH2CH2O); 13C-NMR δ: 15.2 (CH3), 27.3, 32.7 (CCH2CH2), 44.9 (CH2Cl), 57.4 (CH2CH3), 64.95 (2C, OCH2CH2O), 122.3 (C).; IR cm-1: 1193 (C-O); GC-MS (m/z): 151 (M++2-OEt, 32%), 149 (M+-OEt, 94), 117 (29), 105 (44), 99 (24), 77 (27), 69 (30), 55 (45), 45 (57), 43 (27), 42 (34), 41 (100).
2-(3-Chlorobutyl)-2-ethoxy-1,3-dioxolane (6b): b.p.: 122°C (0.1 mmHg); 1H-NMR δ: 1.17 (3H, t, J = 7.0, CH3), 1.58, 1.80 [2H and 4H, 2m, C(CH2)3], 3.53 (4H, m, CH2CH3 and CH2Cl), 3.95, 4.09 (2H and 2H, 2m, OCH2CH2O; 13C-NMR δ: 15.2 (CH3), 21.2, 32.3, 34.6 [C(CH2)3], 44.8 (CH2Cl), 57.3 (CH2CH3), 64.9 (2C, OCH2CH2O), 122.4 (C); IR cm-1: 1191 (C-O); GC-MS (m/z): 165 (M++2-OEt, 17%), 163 (M+-OEt, 52), 117 (27), 99 (42), 91 (16), 89 (57), 55 (100), 45 (43), 43 (19), 42 (20), 41 (22).
DTBB-catalyzed lithiation of ortho esters 6: Preparation of esters 8 and 9.
A solution of the ortho ester 6 (2 mmol) and the electrophile (2.2 mmol) in THF (3 mL) was slowly added (ca. over 2 h) to a stirred green suspension of lithium powder (17.1 mmol, 120 mg) and DTBB (0.2 mmol, 53.2 mg) in THF (10 mL) at -78°C under an argon atmosphere. The reaction mixture was stirred for 30 additional minutes at the same temperature and then quenched with phosphate buffer (10 mL). The reaction mixture was extracted with ethyl acetate (3 x 10 mL), the organic phase was washed with brine (10 mL) and water (10 mL) and was dried over anhydrous sodium sulphate. The solvent was concentrated under vacuum (15 Torr) and the resulting crude was solved in dry methanol (20 mL), a catalytic amount of p-toluenesulphonic acid (10 mg) was added and the reaction was stirred at room temperature for 12 h. The reaction was hydrolyzed by adding water (20 mL) and then extracted with diethyl ether (4x20 mL). The organic phase was washed with NaOH (0.1 M, 10 mL) and water (10 mL), dried over anhydrous magnesium sulphate and concentrated under vacuum (15 Torr). Compounds 8 and 9 were isolated after column chromatography (silica gel, hexane/ethyl acetate mixtures).
Methyl 5-hydroxy-6,6-dimethylheptanoate (8aa): 1H-NMR δ: 0.97 [9H, s, C(CH3)3], 1.43-1.59, 1.75-1.95 (2H and 2H, 2m, CHCH2CH2), 1.96 (1H, br s, OH), 2.37 (1H, m, CHHCO2), 2.43 (1H, m, CHHCO2), 3.47 (3H, s, OCH3), 3.95 (1H, dd, J = 11.9, 2.7, HOCH); 13C-NMR δ: 18.5, 22.5, 34.15 (3 x CH2), 25.4 [3C, C(CH3)3], 33.8 (C), 51.4 (OCH3), 79.3 (CH), 174.3 (CO2); IR cm-1: 3590-3201 (OH), 1737 (C=O), 1240 (C-O); GC-MS (m/z): 170 (M+-H2O, 0.2%), 131 (26), 99 (100), 74 (25), 71 (40), 57 (32), 55 (27), 43 (41), 41 (37).
Methyl 5-hydroxy-5-phenylpentanoate (8ab) [22]: 1H-NMR δ: 1.57-1.83 (4H, m, CHCH2CH2), 2.31 (2H, def t, J = 6.4, CH2CO2), 2.37 (1H, br s, OH), 3.62 (3H, s, CH3), 4.63 (1H, m, CH), 7.31 (5H, m, ArH); 13C-NMR δ: 21.1, 33.6, 38.3 (3 x CH2), 51.3 (CH3), 73.8 (CH), 125.7, 127.3, 128.3, 144.6 (6C, ArC), 173.9 (CO2); IR cm-1: 3619-3173 (OH), 3086, 3061, 3028, 1434 (C=CH), 1722 (C=O), 1025 (C-O); GC-MS (m/z): 208 (M+, 10%), 117 (20), 107 (99), 105 (16), 102 (22), 91 (17), 79 (76), 77 (47), 59 (100), 51 (19), 43 (33), 42 (25).
Methyl 4-(1-hydroxycyclohexyl)butanoate (8ac): 1H-NMR δ: 1.37-1.61, 1.66-1.79 (11H and 4H, 2m, 7 x CH2 and OH), 2.33 (2H, t, J = 7.3, CH2CO2), 3.67 (3H, s, OCH3); 13C-NMR δ: 18.45, 22.1, 25.7, 34.3, 37.3, 41.5 (8C, 8 x CH2), 51.4 (CH3), 71.1 (C), 174.1 (CO2); IR cm-1: 3644-3254 (OH), 1730 (C=O), 1240 (C-O); GC-MS (m/z): 200 (M+, 0.2%), 125 (46), 112 (33), 99 (100), 98 (34), 97 (47), 84 (17), 83 (27), 81 (72), 79 (20), 74 (35), 67 (19), 59 (18), 55 (94), 43 (55), 42 (30), 41 (58); HRMS (CI) for C11H20O3 (M+): Calcd 200.1412; Found 200.1406.
Methyl 5-ethyl-5-hydroxyheptanoate (8ad) [23]: 1H-NMR δ: 0.88 (6H, t, J = 7.3, 2xCH2CH3), 1.47 (4H, q, J = 7.3, 2 x CH2CH3), 1.53 (1H, br s, OH), 1.60-1.74 (4H, m, CCH2CH2), 2.33 (2H, t, J = 7.3, CH2CO2), 3.67 (3H, s, OCH3); 13C-NMR δ: 7.6 (2C, 2 x CH2CH3), 18.9, 30.85, 34.3, 37.4 (5C, 5 x CH2), 51.4 (OCH3), 74.3 (C), 174.1 (CO2); IR cm-1: 3624-3254 (OH), 1737 (C=O), 1172 (C-O); GC‑MS (m/z): 187 (M+-1, 0.2%), 127 (100), 99 (59), 87 (79), 74 (37), 69 (19), 57 (91), 55 (53), 45 (57), 43 (46), 41 (39).
Methyl 5-hydroxy-5-phenylhexanoate (8ae) [24]: 1H-NMR δ: 1.56 (3H, s, CCH3), 1.42-1.71, 1.79-1.88 (2H and 2H, 2m, CCH2CH2), 1.90 (1H, br s, OH), 2.26 (2H, t, J = 7.3, CH2CO2), 3.63 (3H, s, OCH3), 7.23, 7.33, 7.43 (1H, 2H and 2H, 3m, ArH); 13C-NMR δ: 19.5, 34.0, 43.3 (3 x CH2), 30.3 (CCH3), 51.5 (OCH3), 74.4 (C), 124.7, 126.6, 128.2, 147.6 (6C, ArC), 174.0 (CO2); IR cm-1: 3600-3154 (OH), 3052, 3046, 1446 (C=CH), 1739 (C=O), 1025 (C-O); GC-MS (m/z): 207 (M+-Me, 0.8%), 121 (43), 43 (100).
Methyl 5-anilino-5-phenylpentanoate (8af): 1H-NMR δ: 1.76-1.91 (4H, m, CH2CH2CH), 2.33 (2H, t, J = 6.7, CH2CO2), 3.65 (3H, s, CH3), 3.67 (1H, s, NH), 4.32 (1H, t, J = 6.5, CH), 6.51 (2H, dd, J = 8.6, 0.9, ArH), 6.63 (1H, def t, J = 7.4, ArH), 7.07, 7.19-7.27, 7.32 (2H, 2H and 3H, 3m, ArH); 13C-NMR (CDCl3) δ: 21.7, 33.6, 38.1, (3 x CH2), 51.6 (CH3), 57.9 (CH), 113.2, 115.1, 117.2, 126.3, 127.0, 128.6, 129.1, 129.3, 143.7, 147.3 (12C, ArC), 173.8 (CO2); IR cm-1: 3651-3100 (NH), 3018, 1433 (C=CH), 1728 (C=O); GC-MS (m/z): 283 (M+, 4%), 183 (14), 182 (100), 117 (20), 104 (19), 93 (12), 91 (23), 77 (38), 55 (16), 51 (15); HRMS (CI) for C18H21NO2 (M+): Calcd 283.1572; Found 283.1536.
2-Hydroxyethyl 4-trimethylsilylbutanoate (9a): 1H-NMR δ: -0.02 [9H, s, Si(CH3)3], 0.50 (2H, m, CH2Si), 1.63 (2H, m, CH2CH2Si), 2.15 (1H, br s; OH), 2.36 (2H, t, J = 7.3, CH2CO2), 3.80, 4.19 (2H and 2H, 2m, OCH2CH2OH); 13C-NMR (CDCl3) δ: -1.8 [3C, Si(CH3)3], 16.4 (CH2Si), 19.7 (CH2), 37.7 (CH2CO2), 61.2, 65.8 (OCH2CH2OH), 174.1 (CO2); IR cm-1: 3600-3190 (OH), 1737 (C=O), 1118, 1025 (C-O); GC-MS (m/z): 189 (M+-Me, 3%), 145 (20), 117 (36), 75 (100), 73 (98), 45 (54), 44 (29), 43 (86), 42 (31).
Methyl 6-hydroxy-7,7-dimethyloctanoate (8ba): 1H-NMR δ: 0.89 [3C, C(CH3)3], 1.21-1.43, 1.47-1.75 [2H and 4H, 2m, CH(CH2)3], 1.69 (1H, br s, OH), 2.33 (2H, t, J = 7.3, CH2CO2), 3.18 (1H, dd, J = 10.4, 1.8, CH), 3.67 (3H, s, OCH3); 13C-NMR δ: 25.6 [3C, C(CH3)3], 24.9, 26.55, 31.0, 34.0 [(CH2)4], 34.9 (C), 51.4 (OCH3), 79.6 (CH), 174.2 (CO2); IR cm-1: 3614-3214 (OH), 1739 (C=O), 1090 (C-O); GC-MS (m/z): 184 (M+-H2O, 0.4%), 145 (32), 113 (100), 95 (19), 87 (49), 85 (22), 67 (59), 57 (51), 55 (28), 43 (41), 41 (56).
Methyl 6-hydroxy-6-phenylhexanoate (8bb): 1H-NMR δ: 1.26-1.53, 1.61-1.88 [2H and 4H, 2m, (CH2)3], 1.57 (1H, br s, OH), 2.30 (2H, t, J = 7.3, CH2CO2), 3.65 (3H, s, CH3), 4.67 (1H, dd, J = 7.6, 5.8, CH), 7.33 (5H, m, ArH); 13C-NMR δ: 24,7, 25.3, 33.9, 38.55 (4 x CH2), 51.4 (CH3), 74.2 (CH), 125.8, 127.5, 128.4, 144.7 (6C, ArC), 174.1 (CO2); IR cm-1: 3637-3207 (OH), 3093, 3060, 3026, 1454, 1441 (C=CH), 1737 (C=O), 1179 (C-O); GC-MS (m/z): 222 (M+, 9%), 130 (39), 129 (16), 116 (31), 91 (28), 87 (100), 79 (59), 77 (38), 55 (25); HRMS (CI) for C13H18O3 (M+): Calcd 222.1256; Found 222.1264.
Methyl 5-(1-hydroxycyclohexyl)pentanoate (8bc) [25]: 1H-NMR δ: 1.35-1.68 (17H, m, 8xCH2 and OH), 2.33 (2H, t, J = 7.3, CH2CO2), 3.67 (3H, s, OCH3; 13C-NMR δ: 22.1, 22.3, 25.4, 25.7, 33.9, 37.3, 41.8 (9C, 9 x CH2), 51.3 (CH3), 71.1 (C), 174.1 (CO2); IR cm-1: 3617-3193 (OH), 1740 (C=O), 1106, 1023 (C-O); GC-MS (m/z): 196 (M+-H2O, 2%), 111 (16), 99 (93), 98 (39), 97 (22), 93 (19), 87 (39), 81 (67), 79 (17), 67 (27), 59 (21), 57 (18), 55 (100), 43 (67), 42 (23), 41 (81).
Methyl 6-ethyl-6-hydroxyoctanoate (8bd) [23]: 1H-NMR δ: 0.85 (6H, t, J = 7.6, 2xCH2CH3), 1.25-1.49 (9H, m, 2 x CH2CH3, CCH2CH2CH2 and OH), 1.64 (2H, m, CCH2), 2.33 (2H, t, J = 7.3, CH2CO2), 3.67 (3H, s, OCH3); 13C-NMR δ: 7.7 (2C, 2 x CH2CH3), 22.9, 25.5, 30.9, 34.0, 37.8 (6C, 6 x CH2), 51.4 (OCH3), 74.4 (C), 174.1 (CO2); IR cm-1: 3629-3213 (OH), 1736 (C=O), 1169 (C-O); GC-MS (m/z): 184 (M+-H2O, 2%), 141 (34), 123 (22), 95 (90), 87 (100), 69 (21), 57 (80), 55 (46), 45 (46), 43 (35), 41 (39).
Methyl 6-hydroxy-6-phenylheptanoate (8be): 1H-NMR δ: 1.12-1.36, 1.57 (2H and 2H, 2m, CH2CH2), 1.54 (3H, s, CCH3), 1.80 (2H, m, CCH2), 1.91 (1H, br s, OH), 2.24 (2H, t, J = 7.3, CH2CO2), 3.62 (3H, s, OCH3), 7.23, 7.32, 7.41 (1H, 2H and 2H, 3m, ArH); 13C-NMR δ: 23.5, 25.1, 33.9, 43.7 (4 x CH2), 30.1 (CCH3), 51.4 (OCH3), 74.5 (C), 124.7, 126.5, 128.1, 147.8 (6C, ArC), 174.1 (CO2; IR cm-1: 3624-3228 (OH), 3073, 3026, 1448 (C=CH), 1741 (C=O), 1172 (C-O; GC-MS (m/z): 218 (M+-H2O, 10%), 144 (31), 129 (52), 121 (81), 118 (32), 105 (19), 91 (28), 77 (19), 44 (23), 43 (100), 40 (33; HRMS (CI) for C14H20O3 (M+): Calcd 236.1412; Found 236.1402.
Methyl 6-anilino-6-phenylhexanoate (8bf): 1H-NMR δ: 1.24-1.53, 1.66, 1.83 [3H, 2H and 2H, 3m, CH(CH2)3 and NH], 2.30 (2H, t, J = 7.3, CH2CO2), 3.66 (3H, s, CH3), 4.31 (1H, t, J = 6.7, CH), 6.55 (2H, d, J = 7.9, 2 x NArH), 6.65 (1H, t, J = 7.3, NArH), 7.08, 7.25, 7.33 (2H, 1H and 4H, 3m, 2 x NArH and ArH); 13C-NMR δ: 24.7, 25.8, 33.8, 38.3 (4 x CH2), 51.5 (CH3), 58.2 (CH), 113.4, 115.1, 117.4, 126.35, 126.95, 128.5, 129.0, 143.7, 147.1 (12C, ArC), 173.9 (CO2); IR cm-1: 3400 (NH), 3052, 3024, 1452, 1434 (C=CH), 1736 (C=O), 1154 (C-O); GC-MS (m/z): 297 (M+, 4%), 182 (100), 104 (18), 91 (21), 77 (25); HRMS (CI) for C19H23NO2 (M+): Calcd 297.1729; Found 297.1732.
2-Hydroxyethyl 5-trimethylsilylpentanoate (9b): 1H-NMR δ: -0.02 (9H, s, 3xCH3), 0.50 (2H, m, CH2Si), 1.31, 1.69 (2H and 2H, 2m, CH2CH2CH2Si), 2.36 (2H, t, J = 7.5, CH2CO2), 3.85, 4.22 (2H and 2H, 2m, OCH2CH2O); 13C-NMR δ: -1.76 (3C, 3 x CH3), 16.3, 23.5, 28.6, 33.9 [(CH2)4], 61.2, 65.8 (OCH2CH2O), 174.2 (CO2); IR (thin film) cm-1: 3671-3154 (OH), 1739 (C=O), 1248, 1189 (C-O); GC-MS (m/z): 203 (M+-Me, 4%), 99 (25), 75 (89), 73 (100), 57 (18), 56 (17), 55 (50), 45 (49), 44 (16), 43 (27).
Preparation of δ-lactones 11.
A solution of the ortho ester 6a (2 mmol) and the electrophile (2.2 mmol) in THF (3 mL) was slowly added (ca. over 2 h) to a stirred green suspension of lithium powder (17.1 mmol, 120 mg) and DTBB (0.2 mmol, 53.2 mg) in THF (10 mL) at -78°C under an argon atmosphere. The reaction was stirred for an additional 30 minutes at the same temperature and then quenched with phosphate buffer (10 mL). The reaction mixture was extracted with ethyl acetate (3 x 10 mL), the organic phase was washed with brine (10 mL) and water (10 mL) and was dried over anhydrous sodium sulphate. The solvent was concentrated under vacuum (15 Torr) and the resulting crude was treated with a 5:1 mixture of dimethoxyethane/KHSO4 (sat. sol.) (12 mL) at 0°C for 20 min. After this, lithium hydroxide was added to a basic pH and the reaction was stirred for 2 hours at room temperature. The aqueous phase was treated with HCl (3M) to an acidic pH, then it was extracted with diethyl ether (3 x 15 mL) and the resulting organic phase was washed with water (10 mL), dried over anhydrous magnesium sulphate and concentrated under vacuum (15 Torr). The resulting crude was dissolved in benzene (10 mL), a catalytic amount of p-toluenesulphonic acid (10 mg) was added and the reaction mixture was refluxed with stirring for 2 hours. Anhydrous potassium carbonate (15 mg) was added to the solution, then it was filtered out and the solvent was concentrated under vacuum (15 Torr). Lactones 11 were isolated after column chromatography (neutral silica gel, hexane/ethyl acetate mixtures).
6-(tert-Butyl)tetrahydro-2H-2-pyranone (11a) [26]: 1H-NMR δ: 0.97 (9H, s, 3xCH3), 1.41-1.59, 1.75-1.86, 1.88-1.99, 2.31-2.47, 2.56-2.66 (1H, 1H, 2H, 1H and 1H, 5m, 3 x CH2), 3.93-3.98 (1H, dd, J = 11.8, 2.7, CH); 13C-NMR δ: 18.5, 22.4, 29.3 (3C, 3 x CH2), 25.3 (3C, 3 x CH3), 34.1 (C), 88.0 (CH), 172.5 (CO2); IR (thin film) cm-1: 1732 (C=O), 1242 (C-O); GC-MS (m/z): 156 (M+, 0.4%), 100 (51), 99 (100), 71 (51), 57 (45), 55 (29).
6-Phenyltetrahydro-2H-2-pyranone (11b) [26]: 1H-NMR δ: 1.79-2.04, 2.12-2.21 (3H and 1H, 2m, 2 x CH2), 2.51-2.76 (2H, m, CH2CO), 5.33-5.37 (1H, dd, J = 10.4, 3.4, CH), 7.36 (5H, s, ArH); 13C‑NMR δ: 18.5, 29.4, 30.4 (3 x CH2), 81.6 (CH), 125.6, 128.2, 128.5, 139.6 (6C, ArC), 171.3 (CO2); IR cm-1: 3064, 3034, 1456 (C=CH), 1731 (C=O), 1241 (C-O); GC-MS (m/z): 176 (M+, 21%), 105 (51), 104 (100), 78 (16), 77 (26), 70 (40), 51 (16).
1-Oxaspiro[5.5]undecan-2-one (11c) [15d]: 1H-NMR δ: 1.33-1.91 (14 H, m, 7 x ring CH2), 2.50 (2H, def t, J = 6.9, CH2CO); 13C-NMR δ: 15.8, 21.4, 25.1, 29.3, 32.2, 37.1 (8C, 8x ring CH2), 82.9 (C), 171.2 (CO2); IR cm-1: 1731 (C=O), 1241 (C-O); GC-MS (m/z): 168 (M+, 22%), 126 (17), 125 (54), 112 (54), 98 (30), 97 (79), 96 (17), 84 (37), 83 (62), 81 (47), 79 (16), 70 (28), 67 (28), 55 (100).
6,6-Diethyltetrahydro-2H-2-pyranone (11d) [15d]: 1H-NMR δ: 0.91 (6H, t, J = 7.4, 2 x CH3), 1.60-1.78, 1.80-1.89 (6H and 2H, 2m, 4xCH2), 2.48 (t, J = 6.9, CH2CO); 13C-NMR δ: 7.7 (2C, 2 x CH3), 16.4, 28.8, 29.5, 30.7 (5C, 5xCH2), 86.8 (C), 171.7 (CO2); IR cm-1: 1731 (C=O), 1252 (C-O); GC-MS (m/z): 156 (M+, 0.3%), 127 (100), 99 (84), 69 (19), 57 (83), 55 (37).
Preparation of ortho esters 14: Preparation of esters 13.
To an ice-cooled solution of 3-hydroxymethyl-3-methyloxetane (20 mmol, 2 mL) and pyridine (24 mmol, 1.95 mL) in CH2Cl2 (10 mL) was added the acid chloride 12 (20 mmol). After the addition the reaction was allowed to stand at 0°C for 2 days. The reaction mixture was dropped over crushed ice (5 g) and then extracted with CH2Cl2 (3x10 mL). The organic phase was dried over anhydrous sodium sulphate and concentrated under vacuum (15 Torr). The esters 13 were obtained pure after column chromatography (neutral silica gel, hexane/ethyl acetate mixtures).
3-Methyl-3-oxetanylmethyl 3-chloropropanoate (13a): 1H-NMR δ: 1.35 (3H, s, CH3), 2.85 (2H, t, J = 6.6, CH2CH2Cl), 3.78 (2H, t, J = 6.6, CH2Cl), 4.24 (2H, s, CO2CH2), 4.39 (2H, d, J = 6.0, 2 x ring CHH), 4.52 (2H, d, J = 6.0, 2x ring CHH); 13C-NMR δ: 21.0 (CH3), 37.4, 38.9 (CH2CH2Cl), 39.0 (C), 69.0 (CO2CH2), 79.4 (2C, 2 x ring CH2), 170.2 (CO2); IR cm-1: 1740 (C=O), 1247, 1147 (C-O); GC‑MS (m/z): 193 (M++1, 0.1%), 93 (19), 91 (53), 72 (100), 65 (23), 63 (60), 55 (60), 54 (29), 43 (19), 41 (31).
3-Methyl-3-oxetanylmethyl 4-chlorobutanoate (13b) [27]: 1H-NMR δ: 1.34 (3H, s, CH3), 2.12 (2H, m, ClCH2CH2), 2.57 (2H, t, J = 7.1, CH2CO2), 3.62 (2H, t, J = 6.2, ClCH2), 4.19 (2H, s, OCH2C), 4.39 (2H, d, J = 6.0, 2 x ring CHH), 4.51 (2H, d, J = 6.0, 2 x ring CHH); 13C-NMR δ: 21.0 (CH3), 27.5 (CH2CH2Cl), 31.0 (CH2CO2), 38.95 (CCH3), 43.9 (CH2Cl), 68.7 (CO2CH2), 79.4 (2C, 2 x ring CH2), 172.6 (CO2); IR cm-1: 1740 (C=O), 1244, 1148 (C-O; GC-MS (m/z): 206 (M+, 0.2%), 91 (43), 72 (100), 65 (19), 63 (61), 55 (62), 54 (24), 43 (19).
Rearrangement of esters 13: Preparation of ortho esters 14.
BF3·OEt2 (4 mmol, 0.5 mL) was added to a cooled (-20°C) solution of the corresponding ester 13 (16 mmol) in CH2Cl2 (15 mL) under an argon atmosphere. The reaction was stirred for 24 h at the same temperature and a solution of Et3N (16 mmol, 2.2 mL) in diethyl ether (20 mL) was then added to quench the reaction. The reaction mixture was filtered and the solvent was concentrated under vacuum (15 Torr). The resulting crude was filtered through a pad of neutral silica gel eluting with CH2Cl2 (150 mL), the solvent was concentrated under vacuum (15 Torr) to give the corresponding pure ortho ester 14.
1-(2-Chloroethyl)-4-methyl-2,6,7-trioxabicyclo[2.2.2]octane (14a) [28]: 1H-NMR (benzene-d6) δ: 0.02 (3H, s, CH3), 2.41 (2H, m, CCH2), 3.46 (6H, s, 3 x CH2O), 3.74 (2H, m, CH2Cl); 13C-NMR (benzene-d6) δ: 13.8 (CH3), 29.8 (CCH3), 39.1, (CH2Cl), 40.8 (CH2C), 72.4 (3C, 3 x CH2O), 107.9 [C(OCH2)3]; IR cm-1: 1151, 1043 (C-O); GC-MS (m/z): 191 (M+-H, 0.2%), 91 (39), 72 (100), 63 (47), 55 (52), 54 (24).
1-(3-Chloropropyl)-4-methyl-2,6,7-trioxabicyclo[2.2.2]octane (14b) [29]: 1H-NMR δ: 0.80 (3H, s, CH3), 1.81, 1.93 (2H and 2H, 2m, CH2CH2CH2Cl), 3.56 (2H, t, J = 6.6, CH2Cl), 3.88 (6H, s, 3 x CH2O); 13C-NMR δ: 14.4 (CH3), 26.7, 30.1, 44.8 [(CH2)3], 33.7 (CCH3), 72.5 (3C, 3 x CH2O), 108.6 [C(OCH2)3; IR cm-1: 1150, 1040 (C-O); GC-MS (m/z): 206 (M+, 0.1%), 176 (26), 107 (65), 105 (100), 79 (16), 77 (41), 72 (57), 55 (44), 54 (27), 43 (32), 41 (88).
DTBB-catalyzed lithiation of ortho esters 14: Preparation of γ-lactones 15.
A solution of the ortho ester 14a (2 mmol) and the electrophile (2.2 mmol) in THF (3 mL) was slowly added to a stirred green suspension of lithium powder (17.1 mmol, 120 mg) and DTBB (0.2 mmol, 53.2 mg) in THF (10 mL) at -78°C (ca. for 2 h) under an argon atmosphere. The reaction mixture was stirred for 30 additional minutes at the same temperature and then quenched with phosphate buffer (10 mL). The reaction mixture was extracted with ethyl acetate (3 x 10 mL), the organic phase was washed with brine (10 mL) and water (10 mL) and dried over anhydrous sodium sulphate. The solvent was concentrated under vacuum (15 Torr) and the resulting crude product was dissolved in dry THF (20 mL), a catalytic amount of p-toluenesulphonic acid (10 mg) was added and the reaction was stirred at room temperature for 12 h. The reaction was hydrolyzed by adding water (20 mL) and then extracted with diethyl ether (4 x 20 mL). The organic phase was washed with NaOH (0.1 M, 10 mL) and water (10 mL), dried over anhydrous magnesium sulphate and concentrated under vacuum (15 Torr). Compounds 15 were isolated after column chromatography (silica gel, hexane/ethyl acetate mixtures).
5-(tert-Butyl)tetrahydro-2-furanone (15a): 1H-NMR δ: 0.95 (9H, s, 3 x CH3), 1.90-2.17, 2.44-2.56, 2.73-2.80 (2H, 1H and 1H, 3m, 2 x CH2), 4.17-4.23 (1H, m, CH); 13C-NMR δ: 26.4 (3C, 3 x CH3), 29.3, 34.6 (2xCH2), 35.4 (C), 86.9 (CH), 177.4 (CO2).; IR cm-1: 1744 (C=O), 1229, 1209 (C-O); GC-MS (m/z): 142 (M+, 1.2%), 87 (27), 86 (24), 85 (83), 57 (100), 56 (18), 55 (24), 44 (24), 43 (60), 41 (58; HRMS (CI) for C8H14O2 (M+): Calcd 142.0994; Found 142.0978.
5-Phenyltetrahydro-2-furanone (15b): 1H-NMR δ: 2.12-2.28, 2.62-2.68 (1H and 3H, 2m, 2 x CH2), 5.51 (1H, dd, J = 7.9, 6.1, CH), 7.31-7.42 (5H, m, ArH); 13C-NMR δ: 28.9, 30.9 (2 x CH2), 81.2 (CH), 125.2, 128.4, 128.6, 128.7, 139.3 (6C, ArC), 176.9 (CO2); IR cm-1: 3063, 3033, 1454 (C=CH), 1770 (C=O), 1024 (C-O); GC-MS (m/z): 162 (M+, 64%), 117 (34), 107 (72), 105 (52), 91 (17), 79 (20), 78 (20), 77 (40), 56 (100), 51 (43), 50 (20); HRMS (CI) for C10H10O2 (M+): Calcd 162.0681; Found 162.0672.
1-Oxaspiro[4.5]decan-2-one (15c) [30]: 1H-NMR δ: 1.37-1.84 (10H, m, 5 x ring CH2), 2.00 (2H, def t, J = 8.5, CCH2CH2CO), 2.58 (2H, def t, J = 8.5, CCH2CH2CO); 13C-NMR δ: 22.6, 25.0, 28.6, 32.9, 37.0 (7C, 7 x CH2), 86.4 (C), 176.8 (CO2); IR cm-1: 1769 (C=O), 1193 (C-O); GC-MS (m/z): 154 (M+, 18%), 112 (22), 111 (100), 98 (32), 83 (17), 67 (16), 56 (20), 55 (39), 42 (19), 41 (36).
5,5-Diethyltetrahydro-2-furanone (15d) [31]: 1H-NMR δ: 0.94 (6H, t, J = 7.3, 2xCH3), 1.66-1.74 (4H, m, 2 x CH2CH3), 2.01 (2H, def t, J = 8.5, CH2CH2CO), 2.58 (2H, def t, J = 8.5, CH2CH2CO); 13C-NMR δ: 7.8 (2C, 2 x CH3), 29.2, 29.8, 30.85 (4C, 4 x CH2), 89.6 (C), 177.0 (CO2); IR cm-1: 1770 (C=O), 1203, 1163 (C-O); GC-MS (m/z): 143 (M++1, 0.4%), 113 (100), 95 (21), 87 (27), 85 (16), 69 (16), 57 (60), 56 (23), 55 (21), 41 (34).
5-Methyl-5-phenyltetrahydro-2-furanone (15e): 1H-NMR δ: 1.72 (3H, s, CH3), 2.36-2.68 (4H, m, CH2CH2), 7.31, 7.37 (1H and 4H, 2m, ArH); 13C-NMR δ: 28.9, 36.1 (2 x CH2), 29.4 (CH3), 86.9 (C), 124.05, 127.6, 128.6, 144.3 (6C, ArC), 176.4 (CO2).; IR cm-1: 3060, 3029, 1446 (C=CH), 1770 (C=O), 1130, 1068 (C-O; GC-MS (m/z): 176 (M+, 9%), 161 (100), 121 (35), 105 (44), 77 (29), 51 (21), 43 (52; HRMS (CI) for C11H12O2 (M+): Calcd 176.0837; Found 176.0845.
Preparation of esters 8aa, 8ab and 8ae.
The same procedure as described above was used, but starting with ortho ester 14b and carrying out the lithiation at 0°C instead of -78°C. The final step was performed in methanol. Compounds 8aa, 8ab and 8ae were isolated after column chromatography (silica gel, hexane/ethyl acetate mixtures).
DTBB-catalyzed lithiation of ortho esters 6a and 14b.
Deuterolysis of lithiated intermediates. Preparation of compounds 16 and 17.
The ortho ester 6a or 14b (2 mmol) was slowly added to a stirred green suspension of lithium powder (17.1 mmol, 120 mg) and DTBB (0.2 mmol, 53.2 mg) in THF (10 mL) at -78°C (for 6a) or at 0°C (for 14b) under an argon atmosphere. The reaction mixture was stirred for 30 additional minutes at the same temperature and then quenched with D2O (0.2 mL) and the reaction mixture was allowed to warm up until room temperature. Phosphate buffer (10 mL) was added and the resulting mixture was extracted with diethyl ether (3 x 10 mL), the organic phase was washed with brine (10 mL) and water (10 mL) and dried over anhydrous magnesium sulphate. The solvent was removed under vacuum (15 Torr). Compounds 16 and 17 were isolated after column chromatography (neutral silica gel, hexane/ethyl acetate mixtures).
2-(3-Deuteropropyl)-2-ethoxy-1,3-dioxolane (16): 1H-NMR δ: 0.92 (2H, m, CH2D), 1.18 (3H, t, J = 7.02, CH3), 1.46 (2H, m, CCH2), 1.79 (2H, m, CCH2CH2), 3.54 (2H, q, J = 7.1, CH2CH3), 3.96, 4.10 (2H and 2H, 2m, OCH2CH2O); 13C-NMR δ: 13.6 (t, J = 19.2, CH2D), 15.2 (CH3), 17.0, 37.3 (2C, CH2CH2), 57.3 (CH2CH3), 64.8 (2C, OCH2CH2O), 122.6 (C); IR cm-1: 1148, 1047 (C-O); GC-MS (m/z): 161 (M+, 0.02%), 117 (42), 116 (100), 115 (63), 99 (36), 89 (73), 87 (10), 72 (34), 71 (18), 55 (43).
1-(4-Deuteropropyl)-4-methyl-2,6,7-trioxabicyclo[2.2.2]octane (17): 1H-NMR δ: 0.79 (3H, s, CH3), 0.90 (2H, m, CH2D), 1.45, 1.65 (2H and 2H, 2m, CH2CH2), 3.89 (6H, s, 3 x CH2O); 13C-NMR δ: 13.7 (t, J = 19.2, CH2D), 14.5 (CH3), 16.5, 38.7 (CH2CH2), 30.2 (CCH3), 72.5 (3C, 3xCH2O), 109.0 [C(OCH2)3]; IR cm-1: 1148, 1047 (C-O); GC-MS (m/z): 172 (M+-H, 0.3%), 72 (100), 71 (21), 55 (20), 44 (73), 43 (27), 42 (21).
References and Notes
- Negishi, E. Organometallics in Organic Chemistry; Wiley: New York, 1980. [Google Scholar] Boudier, A.; Bromm, L. O.; Lotz, M.; Knochel, P. New applications of polyfunctional organometallic compounds in organic synthesis. Angew. Chem. Int. Ed. 2000, 39, 4414–4435. [Google Scholar]
- Review: Petragnani, N.; Yonashiro, M. The reactions of dianions of carboxylic acids and ester enolates. Synthesis 1982, 521–578. [Google Scholar]
- Review: Gil, S.; Parra, M. Dienediolates of carboxylic acids in synthesis. Recent advances. Curr. Org. Chem. 2002, 6, 283–302. [Google Scholar]
- Mekelburger, H. B.; Wilcos, C. S. Formation of enolates. In Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 2, pp. 99–131. [Google Scholar]
- Parra, M.; Sotoca, E.; Gil, S. A convenient generation of acetic acid dianion. Eur. J. Org. Chem. 2003, 1386–1388. [Google Scholar]
- Pastor, I. M.; Yus, M. Lithium α-lithioacetate and β-lithiopropionate: useful intermediates in organic synthesis. Tetrahedron Lett. 2000, 41, 5335–5339. [Google Scholar]
- Kuwajima, I.; Nakamura, E. Metal homoenolates. In Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 2, pp. 441–454. [Google Scholar] Ahlbrecht, H.; Beyer, U. Stereoselective of chiral homoenolate equivalents. Synthesis 1999, 365–390. [Google Scholar] Beak, P.; Gallagher, D. J.; Park, J. S.; Thayumanavan, S. Regioselective, diastereoselective, and enantioselective lithiation-substitution sequences: reactions pathways and synthetic applications. Acc. Chem. Res. 1999, 29, 552–560. [Google Scholar]
- Caine, D.; Frobese, A. S. A synthesis of γ-lactones by reaction of lithium β-lithiopropionate with aldehydes and ketones. Tetrahedron Lett. 1978, 883–886. [Google Scholar]
- Usually a sulfur-containing moiety, see: Thompson, C. M.; Green, D. L. C. Recent advances in dianion chemistry. Tetrahedron 1991, 47, 4223–4285. [Google Scholar] Thompson, C. M.; Frick, J. A. 4-(Phenylsulfonyl)butanoic acid. Preparation, dianion generation, and application to four-carbon chain extension. J. Org. Chem. 1989, 54, 890–896. [Google Scholar] Green, D. L. C.; Kiddle, J. J.; Thompson, C. M. Stereochemistry of remote dianion addition to imines. application to the synthesis of (1S, 8aS)-1-hydroxyindolizidine. Tetrahedron 1995, 51, 2865–2874. [Google Scholar] Thompson, C. M. “Remote” dianion-I. Utility of 4-phenylsulfonylbutanoic acid in the mild conversion of aldehydes and ketones to lactones. Tetrahedron Lett. 1987, 28, 4243–4246. [Google Scholar]
- For reviews see: Nájera, C.; Yus, M. Functionalized organolithium compounds in synthetic organic chemistry. Trends Org. Chem. 1991, 2, 155–181. [Google Scholar] Nájera, C.; Yus, M. Recent developments in the chemistry of functionalized organolithium compounds. Recent Res. Devel. Org. Chem. 1997, 1, 67–96. [Google Scholar] Nájera, C.; Yus, M. Functionalized organolithium compounds: New synthetic adventures. Curr. Org. Chem. 2003, 7, 867–926. [Google Scholar] Nájera, C.; Sansano, J. M.; Yus, M. Recent synthetic uses of functionalised aromatic and heteroaromatic organolithium reagents prepared by non-deprotonating methods. Tetrahedron 2003, 59, 9255–9303. [Google Scholar] Chinchilla, R.; Nájera, C.; Yus, M. Metallated heterocycles and their applications in synthetic organic chemistry. Chem. Rev. 2004, in press (cr020101a). [Google Scholar]
- Yus, M.; Foubelo, F. Reductive opening of saturated oxa-, aza- and thia-cycles by means of an arene-promoted lithiation: synthetic applications. Rev. Heteroatom Chem. 1997, 17, 73–107. [Google Scholar] Yus, M.; Foubelo, F. Reductive opening of heterocycles with lithium metal as a source of functionalised organolithium compounds: synthetic applications. In Targets in Heterocyclic Systems; The Royal Society of Chemistry: Cambridge, U.K., 2002; Vol. 6, pp. 136–171. [Google Scholar]
- Reviews on arene-catalyzed lithiation: Yus, M. Arene-catalyzed lithiation reactions. Chem. Soc. Rev. 1996, 25, 155–161. [Google Scholar] Ramón, D. J.; Yus, M. New methodologies based on arene-catalyzed lithiation reactions and their application to synthetic organic chemistry. Eur. J. Org. Chem. 2000, 225–237. [Google Scholar] Yus, M. From arene-catalyzed lithiation to other synthetic adventures. Synlett 2001, 1197–1205. [Google Scholar] Yus, M. Arene-catalyzed lithiation. In The Chemistry of Organolithium Compounds; Rapopport, Z., Marek, I., Eds.; Wiley: Chichester, 2003; chapter 11. [Google Scholar]
- For polymer supported arene-catalyzed version of this methodology, see: Gómez, C.; Ruiz, S.; Yus, M. Polymer supported arene-catalyzed lithiation reactions. Tetrahedron 1999, 55, 7017–7026. [Google Scholar] Yus, M.; Gómez, C.; Candela, P. Polyphenylene as an electron transfer catalyst in lithiation processes. Tetrahedron 2002, 58, 6207–6210. [Google Scholar] Arnauld, T.; Barrett, A. G. M.; Hopkins, B. T. ROMPgel-supported biphenyl and naphthalene: reagents for lithiation reactions with minimal purification. Tetrahedron Lett. 2002, 43, 1081–1083. [Google Scholar]
- For mechanistic studies, see: Herrera, R. P.; Guijarro, A.; Yus, M. Primary alkyl fluorides as regioselective alkylating reagents of lithium arene Dianions. Easy prediction of regioselectivity by MO calculations on the dianion. Tetrahedron Lett. 2003, 44, 1313–1316. [Google Scholar] Herrera, R. P.; Guijarro, A.; Yus, M. On the dichotomy of the SN2/ET reaction pathways: an apparent SN2 reactivity in the reaction of naphthalene dianion with alkyl fluorides. Tetrahedron Lett. 2003, 44, 1309–1312. [Google Scholar] Yus, M.; Herrera, R. P.; Guijarro, A. On the mechanism of arene-catalyzed lithiation: the role of arene dianions-naphthalene radical anion versus naphthalene dianion. Chem. Eur. J. 2002, 8, 2574–2584. [Google Scholar]
- Gil, J. F.; Ramón, D. J.; Yus, M. Intramolecular 1,6-hydride transfer in acyclic 1,6-diols: a mechanistic study. Tetrahedron 1994, 50, 7307–7314. [Google Scholar] Gil, J. F.; Ramón, D. J.; Yus, M. New masked δ-lithio carbonyl compounds: preparation and synthetic applications. Tetrahedron 1993, 49, 4923–4938. [Google Scholar] Ramón, D. J.; Yus, M. One-step synthesis of substituted 6,8-dioxabicyclo[3.2.1]octanes: easy preparation of racemic rontalin, brevicomins, and related systems. J. Org. Chem. 1992, 57, 750–751. [Google Scholar] Ramón, D. J.; Yus, M. Masked lithium bishomoenolates: useful intermediates in organic synthesis. J. Org. Chem. 1991, 56, 3825–3831. [Google Scholar]
- Preliminary communication: Pastor, I. M.; Yus, M. Masked β-, γ- and δ-lithium ester enolates: useful reagents in organic synthesis. Tetrahedron Lett. 2001, 42, 1029–1032. [Google Scholar]
- (a) For a review, see: DeWolfe, R. H. Synthesis of carboxylic and carbonic ortho esters. Synthesis 1974, 153–172. [Google Scholar] (b) See also: Casy, G.; Patterson, J. W.; Taylor, R. J. K. Methyl 7-hydroxyhept-5-ynoate. Org. Synth. 1988, 67, 193–201. [Google Scholar]
- Blomberg, C. The Barbier Reaction and Related One-Step Processes; Spring-Verlag: Berlin, 1993. [Google Scholar] Alonso, F.; Yus, M. Recent developments in Barbier-type reactions. Recent Res. Dev. Org. Chem. 1997, 1, 397–436. [Google Scholar]
- See, for example: Ramachandran, P. V.; Pitre, S.; Brown, H. C. Selective reduction 59. Effective intramolecular asymmetric reductions of α-, β-, and γ-keto acids with diisopino-campheylborane and intermolecular asymmetric reductions of the corresponding esters with β-chlorodiisopinocampheylborane. J. Org. Chem. 2002, 67, 5315–5319. [Google Scholar] Downham, R.; Edwards, P. J.; Entwistle, D. A.; Hughes, A. B.; Kim, K. S.; Ley, S. V. Tetrahedron: Asymmetr 1995, 6, 2403–2440.
- Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis; John Wiley & Sons: New York, 1999; pp. 437–441. [Google Scholar]
- Yus, M.; Martínez, P.; Guijarro, D. DTBB-catalysed dilithiation of styrene and its methyl-derivatives: introduction of two electrophilic reagents. Tetrahedron 2001, 57, 10119–10124. [Google Scholar]
- Borer, B. C.; Taylor, R. J. K. Trimethyl 4-lithioorthobutanoate: preparation and synthetic applications. Synlett 1990, 601–602. [Google Scholar]
- Zahorszhy, U. I. Bifunctional even-electron. III. Fragmentation behavior of aliphatic hydroxonium ions containing an additional carbomethoxy group. Org. Mass Spectrom. 1988, 23, 63–69. [Google Scholar]
- Babcock, B. W.; Dimmel, D. R.; Graves, D. P., Jr.; Mckelvey, R. D. Light-induced free-radical reactions of 2-methoxy-6-methyltetrahydropyran: irreversible ring opening and multisite hydrogen abstraction. J. Org. Chem. 1981, 46, 736–742. [Google Scholar]
- Henri, C.; Plenat, F.; Reliaud, C. Acid-catalyzed oxidation of spirocyclanones using hydrogen peroxide. Bull. Soc. Chim. Fr. 1968, 1566–1571. [Google Scholar]
- Hsu, J. -L.; Chen, C. -T.; Fang, J. -M. Cooperative catalysis of samarium diiodide and mercaptan in a stereoselective one-pot transformation of 5-oxopentanals into δ-lactones. Org. Lett. 1999, 1, 1989–1991. [Google Scholar]
- Baldwin, J. E.; Adlington, R. M.; Robertson, J. Carboxyclic ring expansion reactions via radical chain processes. Part II. Tetrahedron 1991, 47, 6795–6812. [Google Scholar]
- Corey, E. J.; Shimoji, K. Total synthesis of the major human urinary metabolite of prostaglandin D2, a key diagnostic indicator. J. Am. Chem. Soc. 1983, 105, 1662–1664. [Google Scholar]
- Atkins, M. P.; Golding, B. T.; Howes, D. A.; Sellars, P. J. Masking the carboxy group as a 2,6,7-trioxabicyclo[2.2.2]octane: application to the synthesis of alkylcobaloximes containing ester and carboxy groups. J. Chem. Soc., Chem. Commun. 1980, 207–208. [Google Scholar]
- Trost, B. M.; Rhee, Y. H. Ruthenium-catalyzed cycloisomerization-oxidation of homopropargyl alcohols. A new access to γ-butyrolactones. J. Am. Chem. Soc. 1999, 121, 11680–11683. [Google Scholar]
- Machrouhi, F.; Parlea, E.; Namy, J. -L. Barbier-type reactions of cyclic acid anhydrides and keto acids mediated by an SmI2/(NiI2-catalytic) system. Eur. J. Org. Chem. 1998, 2431–2436. [Google Scholar]
- Sample Availability: Not available.
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.