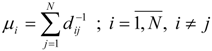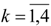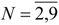Molecular van der Waals Space and Topological Indices from the Distance Matrix
Abstract
:Introduction
Description of Selected Topological Distance Indices

(a) Wiener index
(b) Polarity number
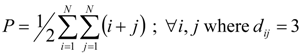
(c) Platt index
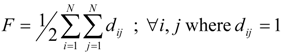
(d) Balaban index


(e) Graph eigenvalues or eigenvector –based indices


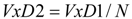

| Alkane | BP | W | P | F | J | VAD1 | VAD2 | VAD3 | VED1 | VED2 | VED3 | VRD |
| C2 | -88.5 | 1 | 0 | 0 | 1.0000 | 1.0000 | 0.5000 | -1.6094 | 1.4142 | 0.7071 | -1.2629 | 1.4142 |
| C3 | -44.5 | 4 | 0 | 2 | 1.6330 | 2.7321 | 0.9107 | -0.1989 | 1.7156 | 0.5719 | -0.6642 | 3.7224 |
| C4 | -0.5 | 10 | 1 | 4 | 1.9747 | 5.1623 | 1.2906 | 0.7251 | 1.9742 | 0.4935 | -0.2361 | 6.5255 |
| 2-M-C3 | -10.5 | 9 | 0 | 6 | 2.3238 | 4.6458 | 1.1614 | 0.6197 | 1.9723 | 0.4931 | -0.2371 | 6.9009 |
| C5 | 36.5 | 20 | 2 | 6 | 2.1906 | 8.2882 | 1.6576 | 1.4217 | 2.2036 | 0.4407 | 0.0970 | 9.7395 |
| 2M-C4 | 27.9 | 18 | 2 | 8 | 2.5395 | 7.4593 | 1.4919 | 1.3163 | 2.2020 | 0.4404 | 0.0962 | 10.1583 |
| 22MM-C3 | 9.5 | 16 | 0 | 12 | 3.0237 | 6.6056 | 1.3211 | 1.1948 | 2.2040 | 0.4408 | 0.0971 | 10.7414 |
| C6 | 68.7 | 35 | 3 | 8 | 2.3391 | 12.1093 | 2.0182 | 1.9832 | 2.4118 | 0.4020 | 0.3696 | 13.3165 |
| 3M-C5 | 63.2 | 31 | 4 | 10 | 2.7542 | 10.7424 | 1.7904 | 1.8634 | 2.4085 | 0.4014 | 0.3682 | 13.8800 |
| 2M-C5 | 60.2 | 32 | 3 | 10 | 2.6272 | 11.0588 | 1.8431 | 1.8924 | 2.4117 | 0.4020 | 0.3695 | 13.6798 |
| 23MM-C4 | 58.1 | 29 | 4 | 12 | 2.9935 | 10.0000 | 1.6667 | 1.7918 | 2.4121 | 0.4020 | 0.3697 | 14.1487 |
| 22MM-C4 | 49.7 | 28 | 3 | 14 | 3.1685 | 9.6702 | 1.6117 | 1.7582 | 2.4111 | 0.4019 | 0.3693 | 14.4073 |
| C7 | 98.4 | 56 | 4 | 10 | 2.4475 | 16.6254 | 2.3751 | 2.4543 | 2.6036 | 0.3720 | 0.6002 | 17.2230 |
| 3E-C5 | 93.5 | 48 | 6 | 12 | 2.9923 | 14.8636 | 2.1234 | 2.3422 | 2.6009 | 0.3716 | 0.5992 | 17.7855 |
| 3M-C6 | 91.8 | 50 | 5 | 12 | 2.8318 | 14.2970 | 2.0424 | 2.3034 | 2.5975 | 0.3711 | 0.5979 | 18.0592 |
| 2M-C6 | 90.0 | 52 | 4 | 12 | 2.6783 | 13.0698 | 1.8671 | 2.2136 | 2.6005 | 0.3715 | 0.5990 | 18.5519 |
| 23MM-C5 | 89.8 | 46 | 6 | 14 | 3.1442 | 15.4048 | 2.2007 | 2.3780 | 2.6050 | 0.3721 | 0.6008 | 17.5136 |
| 33MM-C5 | 86.0 | 44 | 6 | 16 | 3.3604 | 14.1760 | 2.0251 | 2.2949 | 2.6067 | 0.3724 | 0.6014 | 17.8657 |
| 223MMM-C4 | 80.9 | 42 | 6 | 18 | 3.5412 | 13.6346 | 1.9478 | 2.2559 | 2.6027 | 0.3718 | 0.5999 | 18.1940 |
| 24-MMC5 | 80.5 | 48 | 4 | 14 | 2.9532 | 13.6353 | 1.9479 | 2.2560 | 2.6038 | 0.3720 | 0.6003 | 18.2007 |
| 22MM-C5 | 79.2 | 46 | 4 | 16 | 3.1545 | 12.3945 | 1.7706 | 2.1606 | 2.6066 | 0.3724 | 0.6014 | 15.8479 |
| C8 | 125.8 | 84 | 5 | 12 | 2.5301 | 21.8364 | 2.7295 | 2.8604 | 2.7824 | 0.3478 | 0.8002 | 21.4335 |
| 3E-C6 | 118.9 | 72 | 7 | 14 | 3.0744 | 19.5420 | 2.4428 | 2.7494 | 2.7787 | 0.3473 | 0.7989 | 22.0645 |
| 3M-C7 | 118.8 | 76 | 6 | 14 | 2.8621 | 19.7628 | 2.4704 | 2.7607 | 2.7810 | 0.3476 | 0.7997 | 21.9365 |
| 34MM-C6 | 118.7 | 68 | 8 | 16 | 3.2925 | 18.7788 | 2.3474 | 2.7096 | 2.7762 | 0.3470 | 0.7979 | 22.3387 |
| 3E-3M-C5 | 118.2 | 64 | 9 | 18 | 3.5832 | 16.6705 | 2.0838 | 2.5905 | 2.7768 | 0.3471 | 0.7982 | 23.1188 |
| 4M-C7 | 117.7 | 75 | 6 | 14 | 2.9196 | 17.4187 | 2.1773 | 2.6344 | 2.7789 | 0.3474 | 0.7989 | 22.7102 |
| 2M-C7 | 117.6 | 79 | 5 | 14 | 2.7158 | 17.6759 | 2.2095 | 2.6491 | 2.7799 | 0.3475 | 0.7993 | 22.5967 |
| 3E-2M-C5 | 115.6 | 67 | 8 | 16 | 3.3549 | 17.4427 | 2.1803 | 2.6358 | 2.7789 | 0.3474 | 0.7989 | 22.7488 |
| 23MM-C6 | 115.3 | 70 | 7 | 16 | 3.1708 | 20.4792 | 2.5599 | 2.7963 | 2.7849 | 0.3481 | 0.8011 | 21.6556 |
| 233MMM-C5 | 114.6 | 62 | 9 | 20 | 3.7083 | 19.1115 | 2.3889 | 2.7272 | 2.7878 | 0.3485 | 0.8021 | 21.9131 |
| 234MMM-C5 | 113.4 | 65 | 8 | 18 | 3.4642 | 18.3964 | 2.2996 | 2.6890 | 2.7838 | 0.3480 | 0.8007 | 22.2412 |
| 33MM-C6 | 112.0 | 67 | 7 | 18 | 3.3734 | 18.1815 | 2.2727 | 2.6773 | 2.7815 | 0.3477 | 0.7999 | 22.3829 |
| 223MMM-C5 | 110.5 | 63 | 8 | 20 | 3.6233 | 16.8079 | 2.1010 | 2.5987 | 2.7851 | 0.3481 | 0.8011 | 22.7627 |
| 24MM-C6 | 109.4 | 71 | 6 | 16 | 3.0988 | 16.0683 | 2.0085 | 2.5537 | 2.7826 | 0.3478 | 0.8002 | 23.1970 |
| 25MM-C6 | 108.4 | 74 | 5 | 16 | 2.9278 | 18.4133 | 2.3017 | 2.6899 | 2.7843 | 0.3480 | 0.8009 | 22.2507 |
| 22MM-C6 | 107.0 | 71 | 5 | 18 | 3.1118 | 17.0338 | 2.1292 | 2.6121 | 2.7878 | 0.3485 | 0.8021 | 22.6056 |
| 2233MMMM-C4 | 106.0 | 58 | 9 | 24 | 4.0204 | 16.3152 | 2.0394 | 2.5690 | 2.7838 | 0.3480 | 0.8007 | 23.0345 |
| 224MMM-C5 | 99.3 | 66 | 5 | 20 | 3.3889 | 14.9373 | 1.8672 | 2.4807 | 2.7892 | 0.3487 | 0.8026 | 23.5670 |
| C9 | 150.6 | 120 | 6 | 14 | 2.5951 | 27.7422 | 3.0825 | 3.2176 | 2.9505 | 0.3278 | 0.9766 | 25.9281 |
| 33EE-C5 | 146.2 | 88 | 12 | 20 | 3.8247 | 25.0208 | 2.7801 | 3.1143 | 2.9471 | 0.3275 | 0.9755 | 26.5488 |
| 3E-C7 | 143.0 | 104 | 8 | 16 | 3.0922 | 23.6799 | 2.6311 | 3.0593 | 2.9429 | 0.3270 | 0.9740 | 26.9873 |
| 3M-C8 | 143.0 | 110 | 7 | 16 | 2.8766 | 21.7527 | 2.4170 | 2.9744 | 2.9458 | 0.3273 | 0.9750 | 27.4391 |
| 4M-C8 | 142.5 | 108 | 7 | 16 | 2.9548 | 22.6204 | 2.5134 | 3.0135 | 2.9491 | 0.3277 | 0.9761 | 27.0595 |
| 2M-C8 | 142.5 | 114 | 6 | 16 | 2.7467 | 22.2705 | 2.4745 | 2.9979 | 2.9448 | 0.3272 | 0.9747 | 27.3590 |
| 3E-23MM-C5 | 141.6 | 86 | 12 | 22 | 3.9192 | 25.4119 | 2.8236 | 3.1299 | 2.9505 | 0.3278 | 0.9766 | 26.3543 |
| 2334MMMM-C5 | 141.5 | 84 | 12 | 24 | 4.0137 | 24.0988 | 2.6776 | 3.0768 | 2.9457 | 0.3273 | 0.9750 | 26.7923 |
| 4E-C7 | 141.2 | 102 | 8 | 16 | 3.1753 | 21.3349 | 2.3705 | 2.9550 | 2.9439 | 0.3271 | 0.9744 | 27.6869 |
| 3E-3M-C6 | 140.6 | 92 | 10 | 20 | 3.6174 | 22.2198 | 2.4689 | 2.9956 | 2.9461 | 0.3273 | 0.9751 | 27.2876 |
| 23MM-C7 | 140.5 | 102 | 8 | 18 | 3.1553 | 20.7438 | 2.3049 | 2.9269 | 2.9501 | 0.3278 | 0.9765 | 27.6329 |
| 334MMM-C6 | 140.5 | 88 | 11 | 22 | 3.8024 | 19.8563 | 2.2063 | 2.8832 | 2.9481 | 0.3276 | 0.9758 | 28.0907 |
| 2233MMMM-C5 | 140.3 | 82 | 12 | 26 | 4.1447 | 23.0687 | 2.5632 | 3.0331 | 2.9507 | 0.3279 | 0.9767 | 26.8886 |
| 34MM-C7 | 140.1 | 98 | 9 | 18 | 3.3248 | 22.6789 | 2.5199 | 3.0161 | 2.9473 | 0.3275 | 0.9755 | 27.1235 |
| 234MMM-C6 | 139.0 | 92 | 10 | 20 | 3.5758 | 22.6772 | 2.5197 | 3.0160 | 2.9482 | 0.3276 | 0.9759 | 27.1184 |
| 233MMM-C6 | 137.7 | 90 | 10 | 22 | 3.7021 | 20.3172 | 2.2575 | 2.9061 | 2.9490 | 0.3277 | 0.9761 | 27.8661 |
| 33MM-C7 | 137.3 | 98 | 8 | 20 | 3.3301 | 26.2722 | 2.9191 | 3.1632 | 2.9537 | 0.3282 | 0.9777 | 26.0911 |
| 3E-24MM-C5 | 136.7 | 90 | 10 | 20 | 3.6776 | 24.7896 | 2.7544 | 3.1051 | 2.9575 | 0.3286 | 0.9790 | 26.2728 |
| 35MM-C7 | 136.0 | 100 | 8 | 18 | 3.2230 | 23.9292 | 2.6588 | 3.0697 | 2.9542 | 0.3282 | 0.9779 | 26.5647 |
| 25MM-C7 | 136.0 | 104 | 7 | 18 | 3.0608 | 23.5441 | 2.6160 | 3.0535 | 2.9506 | 0.3278 | 0.9767 | 26.7803 |
| 26MM-C7 | 135.2 | 108 | 6 | 18 | 2.9147 | 21.1839 | 2.3538 | 2.9479 | 2.9525 | 0.3281 | 0.9773 | 27.4273 |
| 44MM-C7 | 135.2 | 96 | 8 | 20 | 3.4311 | 23.5541 | 2.6171 | 3.0539 | 2.9507 | 0.3279 | 0.9767 | 26.7833 |
| 4E-2M-C6 | 133.8 | 98 | 8 | 18 | 3.3074 | 22.0627 | 2.4514 | 2.9885 | 2.9549 | 0.3283 | 0.9781 | 27.0476 |
| 3E-22MM-C5 | 133.8 | 88 | 10 | 22 | 3.7929 | 21.1970 | 2.3552 | 2.9485 | 2.9515 | 0.3279 | 0.9770 | 27.4362 |
| 24MM-C7 | 133.5 | 102 | 7 | 18 | 3.1513 | 20.7945 | 2.3105 | 2.9293 | 2.9490 | 0.3277 | 0.9761 | 27.7030 |
| 2234MMMM-C5 | 133.0 | 86 | 10 | 24 | 3.8776 | 19.3005 | 2.1445 | 2.8548 | 2.9542 | 0.3283 | 0.9779 | 28.1080 |
| 22MM-C7 | 132.7 | 104 | 6 | 20 | 3.0730 | 23.9635 | 2.6626 | 3.0712 | 2.9540 | 0.3282 | 0.9778 | 26.5871 |
| 223MMM-C6 | 131.7 | 92 | 9 | 22 | 3.5887 | 22.4662 | 2.4962 | 3.0067 | 2.9585 | 0.3287 | 0.9793 | 26.8263 |
| 235MMM-C6 | 131.3 | 96 | 8 | 20 | 3.3766 | 21.6063 | 2.4007 | 2.9676 | 2.9548 | 0.3283 | 0.9781 | 27.1920 |
| 244MMM-C6 | 126.5 | 92 | 8 | 22 | 3.5768 | 20.1263 | 2.2363 | 2.8967 | 2.9602 | 0.3289 | 0.9799 | 27.5398 |
| 224MMM-C6 | 126.5 | 94 | 7 | 22 | 3.4673 | 21.2250 | 2.3583 | 2.9498 | 2.9512 | 0.3279 | 0.9769 | 27.4608 |
| 225MMM-C6 | 124.0 | 98 | 6 | 22 | 3.2807 | 19.7257 | 2.1917 | 2.8766 | 2.9565 | 0.3285 | 0.9786 | 27.8260 |
Reciprocal Distance – Based Indices
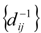 , i,j = 1,N, where N is the total number of graph vertices. This is a symmetrical matrix whose elements are reciprocal of the topological distance [5,16,17,20,33]. The first TDIs proposed on the basis of RD have been developed by a two-steps process as follows [5,16,17].
, i,j = 1,N, where N is the total number of graph vertices. This is a symmetrical matrix whose elements are reciprocal of the topological distance [5,16,17,20,33]. The first TDIs proposed on the basis of RD have been developed by a two-steps process as follows [5,16,17].
- (i)
- The LOVI of each vertex in a molecular graph Γ, denoted later by μi, was defined from the RD using the following relation [5,16]:In relation (13) dij is the topological distance between the vertices i and j, N represents the total number of vertices (i.e. non-hydrogen atoms) in Γ, and summation is made over all possible paths, from dij = 1 to dij = max(dij). Thus, each vertex is well characterized; it contains global information of the topological structure of Γ, the topological interaction between vertices i and j decreasing as distance dij is increasing. That is, for each vertex i, the quantity μi may be viewed as a measure if the influence of all others vertices in a given graph Γ on the vertex i.
- (ii)
- The LOVIs μi were condensed into a TDI, hδ, with the aid of the Randić-type formula [34], the generalized molecular connectivity [35], as follows [5,16]:These topological distances connectivity indices (TDCIs) [5,16], also called topological distance measure connectivity indices (TDMCIs) [17], of order higher than three, have not been used in correlation due to the expected small contributions to the molecular properties.

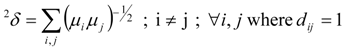



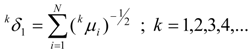
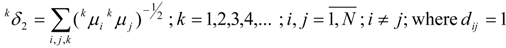
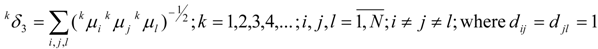
van der Waals Molecular Descriptors
(a) Molecular van der Waals Volume
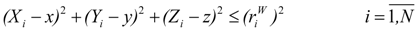
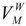 ) of the molecule M.
) of the molecule M.| Alkane | 1δο | 2δο | 3δο | 4δο | 1δ1 | 2δ1 | 3δ1 | 4δ1 | 1δ2 | 2δ2 | 3δ2 | 4δ2 | 1δ3 | 2δ3 | 3δ3 | 4δ3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C2 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 2.0000 | 4.0000 | 4.0000 | 4.0000 | 4.0000 |
| C3 | 5.0000 | 4.5000 | 4.2500 | 4.1250 | 2.3401 | 2.4960 | 2.5927 | 2.6474 | 2.3094 | 2.5298 | 2.6667 | 2.7440 | 5.4042 | 6.3143 | 6.9139 | 7.2644 |
| C4 | 8.6667 | 7.2222 | 6.5741 | 6.2747 | 2.7420 | 3.0476 | 3.2273 | 3.3217 | 2.6684 | 3.1746 | 3.4867 | 3.6562 | 5.9369 | 7.7158 | 8.8912 | 9.5537 |
| 2-M-C3 | 9.0000 | 7.5000 | 6.7500 | 6.3750 | 2.6987 | 3.0268 | 3.2606 | 3.4058 | 2.4495 | 2.8284 | 3.0984 | 3.2660 | 6.6104 | 8.5612 | 10.1027 | 11.1232 |
| C5 | 12.8333 | 10.0694 | 8.9294 | 8.4322 | 3.1512 | 3.6103 | 3.8698 | 4.0001 | 3.0184 | 3.8281 | 4.3204 | 4.5786 | 6.4421 | 9.1923 | 11.0331 | 12.0504 |
| 2M-C4 | 13.3333 | 10.4444 | 9.1481 | 8.5494 | 3.1005 | 3.5870 | 3.9085 | 4.0918 | 2.8014 | 3.4922 | 3.9664 | 4.2431 | 6.7078 | 9.4432 | 11.5347 | 12.8389 |
| 22MM-C3 | 14.0000 | 11.0000 | 9.5000 | 8.7500 | 3.0298 | 3.5237 | 3.9112 | 4.1707 | 2.5298 | 3.0237 | 3.4112 | 3.6707 | 7.6649 | 10.6547 | 13.3420 | 15.3090 |
| C6 | 17.4000 | 12.9967 | 11.3007 | 10.5929 | 3.5580 | 4.1756 | 4.5145 | 4.6794 | 3.3552 | 4.4798 | 5.1562 | 5.5026 | 6.9256 | 10.6698 | 13.1919 | 14.5611 |
| 3M-C5 | 18.1667 | 13.5139 | 11.5775 | 10.7316 | 3.4944 | 4.1486 | 4.5609 | 4.7810 | 3.1171 | 4.1359 | 4.8312 | 5.2222 | 6.8584 | 10.4033 | 13.1037 | 14.7234 |
| 2M-C5 | 18.0000 | 13.4167 | 11.5347 | 10.7147 | 3.5062 | 4.1508 | 4.5533 | 4.7718 | 3.1536 | 4.1548 | 4.8105 | 5.1729 | 7.1146 | 10.8819 | 13.7111 | 15.4109 |
| 23MM-C4 | 18.6667 | 13.8889 | 11.7963 | 10.8488 | 3.4495 | 4.1170 | 4.5856 | 4.8619 | 2.9495 | 3.8299 | 4.4719 | 4.8606 | 7.1742 | 10.8019 | 13.8032 | 15.7592 |
| 22MM-C4 | 19.0000 | 14.1667 | 11.9722 | 10.9491 | 3.4206 | 4.0819 | 4.5653 | 4.8647 | 2.8604 | 3.6769 | 4.2923 | 4.6781 | 7.5167 | 11.2053 | 14.4129 | 16.6341 |
| C7 | 22.3000 | 15.9794 | 13.6813 | 12.7551 | 3.9603 | 4.7412 | 5.1599 | 5.3589 | 3.6800 | 5.1280 | 5.9921 | 6.4268 | 7.3893 | 12.1384 | 15.3525 | 17.0740 |
| 3E-C5 | 23.5000 | 16.7083 | 14.0382 | 12.9216 | 3.8752 | 4.7084 | 5.2169 | 5.4731 | 3.3959 | 4.7553 | 5.6906 | 6.2028 | 6.9966 | 11.3769 | 14.7713 | 16.7592 |
| 3M-C6 | 23.2333 | 16.5661 | 13.9801 | 12.9001 | 3.8924 | 4.7120 | 5.2071 | 5.4618 | 3.4464 | 4.7880 | 5.6738 | 6.1525 | 7.2633 | 11.8383 | 15.2911 | 17.3058 |
| 2M-C6 | 22.9667 | 16.4239 | 13.9220 | 12.8786 | 3.9090 | 4.7153 | 5.1983 | 5.4513 | 3.4927 | 4.8103 | 5.6491 | 6.0982 | 7.5534 | 12.3402 | 15.8714 | 17.9278 |
| 23MM-C5 | 24.0000 | 17.0833 | 14.2569 | 13.0388 | 3.8348 | 4.6756 | 5.2385 | 5.5521 | 3.2543 | 4.4670 | 5.3366 | 5.8418 | 7.3034 | 11.7589 | 15.4142 | 17.7128 |
| 33MM-C5 | 24.5000 | 17.4583 | 14.4757 | 13.1560 | 3.7987 | 4.6369 | 5.2219 | 5.5613 | 3.1509 | 4.3008 | 5.1632 | 5.6840 | 7.4491 | 11.8284 | 15.5991 | 18.1020 |
| 223MMM-C4 | 25.0000 | 17.8333 | 14.6944 | 13.2731 | 3.7592 | 4.6011 | 5.2358 | 5.6326 | 3.0182 | 4.0264 | 4.8121 | 5.3131 | 7.7694 | 12.2923 | 16.3997 | 19.2855 |
| 24-MMC5 | 23.6667 | 16.8889 | 14.1713 | 13.0050 | 3.8562 | 4.6864 | 5.2345 | 5.5428 | 3.3081 | 4.4977 | 5.3100 | 5.7725 | 7.6854 | 12.5026 | 16.3675 | 18.7859 |
| 22MM-C5 | 24.1667 | 17.2639 | 14.3900 | 13.1222 | 3.8200 | 4.6450 | 5.2116 | 5.5459 | 3.2087 | 4.3428 | 5.1438 | 5.6140 | 7.8246 | 12.5789 | 16.5845 | 19.2393 |
| C8 | 27.4857 | 19.0030 | 16.0677 | 14.9182 | 4.3576 | 5.3063 | 5.8056 | 6.0386 | 3.9943 | 5.7728 | 6.8275 | 7.3510 | 7.8354 | 13.5967 | 17.5119 | 19.5871 |
| 3E-C6 | 28.9667 | 19.8406 | 16.4568 | 15.0933 | 4.2637 | 5.2701 | 5.8641 | 6.1546 | 3.7022 | 5.3951 | 6.5311 | 7.1334 | 7.3777 | 12.7893 | 16.9615 | 19.3494 |
| 3M-C7 | 28.5333 | 19.6289 | 16.3767 | 15.0655 | 4.2885 | 5.2756 | 5.8524 | 6.1415 | 3.7698 | 5.4364 | 6.5110 | 7.0777 | 7.7008 | 13.2928 | 17.4546 | 19.8252 |
| 34MM-C6 | 29.7333 | 20.3578 | 16.7336 | 15.2320 | 4.2109 | 5.2319 | 5.8921 | 6.2430 | 3.5361 | 5.0896 | 6.1974 | 6.8225 | 7.4466 | 12.7169 | 17.0353 | 19.6753 |
| 3E-3M-C5 | 30.5000 | 20.8750 | 17.0104 | 15.3707 | 4.1627 | 5.1871 | 5.8802 | 6.2603 | 3.4063 | 4.8953 | 6.0230 | 6.6881 | 7.4129 | 12.4814 | 16.8736 | 19.6994 |
| 4M-C7 | 28.6333 | 19.6739 | 16.3920 | 15.0702 | 4.2834 | 5.2746 | 5.8538 | 6.1428 | 3.7575 | 5.4330 | 6.5155 | 7.0829 | 7.6393 | 13.2499 | 17.4739 | 19.8881 |
| 2M-C7 | 28.2000 | 19.4622 | 16.3119 | 15.0424 | 4.3072 | 5.2797 | 5.8435 | 6.1309 | 3.8189 | 5.4601 | 6.4857 | 7.0227 | 7.9891 | 13.7942 | 18.0287 | 20.4407 |
| 3E-2M-C5 | 29.8333 | 20.4028 | 16.7488 | 15.2366 | 4.2051 | 5.2305 | 5.8943 | 6.2452 | 3.5201 | 5.0765 | 6.1945 | 6.8241 | 7.3998 | 12.6984 | 17.1022 | 19.8072 |
| 23MM-C6 | 29.4667 | 20.2156 | 16.6755 | 15.2105 | 4.2265 | 5.2371 | 5.8846 | 6.2330 | 3.5770 | 5.1171 | 6.1800 | 6.7729 | 7.6750 | 13.1693 | 17.5974 | 20.2978 |
| 233MMM-C5 | 31.0000 | 21.2500 | 17.2292 | 15.4878 | 4.1270 | 5.1511 | 5.8919 | 6.3299 | 3.2923 | 4.6369 | 5.6781 | 6.3186 | 7.7163 | 12.9264 | 17.6277 | 20.8159 |
| 234MMM-C5 | 30.3333 | 20.7778 | 16.9676 | 15.3538 | 4.1686 | 5.1968 | 5.9131 | 6.3223 | 3.3990 | 4.8047 | 5.8459 | 6.4638 | 7.6951 | 13.0744 | 17.7163 | 20.7199 |
| 33MM-C6 | 30.0667 | 20.6356 | 16.9095 | 15.3323 | 4.1883 | 5.1979 | 5.8690 | 6.2431 | 3.4731 | 4.9521 | 6.0113 | 6.6197 | 7.7662 | 13.1951 | 17.7780 | 20.7155 |
| 223MMM-C5 | 30.8333 | 21.1528 | 17.1863 | 15.4710 | 4.1365 | 5.1563 | 5.8887 | 6.3236 | 3.3155 | 4.6580 | 5.6766 | 6.2961 | 7.8598 | 13.2227 | 18.0216 | 21.2749 |
| 24MM-C6 | 29.3000 | 20.1183 | 16.6327 | 15.1936 | 4.2360 | 5.2451 | 5.8878 | 6.2329 | 3.5965 | 5.1282 | 6.1730 | 6.7523 | 7.8211 | 13.4491 | 17.9496 | 20.6859 |
| 25MM-C6 | 28.9333 | 19.9311 | 16.5594 | 15.1675 | 4.2564 | 5.2519 | 5.8805 | 6.2228 | 3.6463 | 5.1511 | 6.1455 | 6.6950 | 8.1313 | 13.9817 | 18.5400 | 21.2935 |
| 22MM-C6 | 29.5333 | 20.3511 | 16.7934 | 15.2893 | 4.2183 | 5.2087 | 5.8567 | 6.2256 | 3.5484 | 5.0012 | 5.9848 | 6.5405 | 8.2150 | 14.0130 | 18.7422 | 21.7600 |
| 2233MMMM-C4 | 32.0000 | 22.0000 | 17.6667 | 15.7222 | 4.0599 | 5.0746 | 5.8780 | 6.3994 | 3.0987 | 4.2357 | 5.1633 | 5.7769 | 8.1946 | 13.5658 | 18.7685 | 22.6023 |
| 224MMM-C5 | 30.3333 | 20.8611 | 17.0579 | 15.4203 | 4.1649 | 5.1758 | 5.8899 | 6.3159 | 3.3789 | 4.6995 | 5.6511 | 6.2177 | 8.3047 | 14.1290 | 19.2053 | 22.6104 |
| C9 | 32.9214 | 22.0579 | 18.4580 | 17.0818 | 4.7502 | 5.8708 | 6.4512 | 6.7183 | 4.2996 | 6.4144 | 7.6624 | 8.2752 | 8.2660 | 15.0456 | 19.6696 | 22.1000 |
| 33EE-C5 | 37.0000 | 24.4167 | 19.5764 | 17.5932 | 4.5127 | 5.7311 | 6.5397 | 6.9614 | 3.6315 | 5.4611 | 6.8712 | 7.6900 | 7.3862 | 13.1373 | 18.2140 | 21.4143 |
| 3E-C7 | 34.6000 | 22.9589 | 18.8626 | 17.2603 | 4.6523 | 5.8320 | 6.5097 | 6.8346 | 4.0103 | 6.0358 | 7.3669 | 8.0585 | 7.8024 | 14.2310 | 19.1250 | 21.8706 |
| 3M-C8 | 34.0524 | 22.7080 | 18.7724 | 17.2302 | 4.6811 | 5.8389 | 6.4977 | 6.8212 | 4.0843 | 6.0809 | 7.3466 | 8.0021 | 8.1361 | 14.7428 | 19.6123 | 22.3387 |
| 4M-C8 | 34.2190 | 22.7775 | 18.7944 | 17.2364 | 4.6733 | 5.8372 | 6.4993 | 6.8226 | 4.0669 | 6.0763 | 7.3521 | 8.0080 | 8.0534 | 14.6879 | 19.6343 | 22.4074 |
| 2M-C8 | 33.6714 | 22.5266 | 18.7041 | 17.2063 | 4.7009 | 5.8437 | 6.4889 | 6.8105 | 4.1340 | 6.1054 | 7.3213 | 7.9470 | 8.4147 | 15.2408 | 20.1847 | 22.9532 |
| 3E-23MM-C5 | 37.5000 | 24.7917 | 19.7951 | 17.7104 | 4.4802 | 5.6951 | 6.5492 | 7.0295 | 3.5326 | 5.2173 | 6.5325 | 7.3221 | 7.6733 | 13.5674 | 18.9279 | 22.4681 |
| 2334MMMM-C5 | 38.0000 | 25.1667 | 20.0139 | 17.8275 | 4.4477 | 5.6592 | 6.5587 | 7.0975 | 3.4337 | 4.9735 | 6.1938 | 6.9541 | 7.9605 | 14.0001 | 19.6539 | 23.5480 |
| 4E-C7 | 34.7667 | 23.0283 | 18.8846 | 17.2666 | 4.6441 | 5.8303 | 6.5118 | 6.8364 | 3.9904 | 6.0269 | 7.3706 | 8.0640 | 7.7226 | 14.1724 | 19.1455 | 21.9393 |
| 3E-3M-C6 | 36.4667 | 24.1322 | 19.4602 | 17.5502 | 4.5420 | 5.7452 | 6.5280 | 6.9427 | 3.7049 | 5.5319 | 6.8675 | 7.6236 | 7.7215 | 13.8285 | 19.0539 | 22.3194 |
| 23MM-C7 | 35.1000 | 23.3339 | 19.0814 | 17.3775 | 4.6176 | 5.7992 | 6.5297 | 6.9128 | 3.8964 | 5.7645 | 7.0174 | 7.6982 | 8.0909 | 14.6099 | 19.7581 | 22.8174 |
| 334MMM-C6 | 37.2333 | 24.6494 | 19.7371 | 17.6889 | 4.4945 | 5.7029 | 6.5450 | 7.0214 | 3.5669 | 5.2523 | 6.5375 | 7.3006 | 7.8283 | 13.8587 | 19.2586 | 22.8134 |
| 2233MMMM-C5 | 38.5000 | 25.5417 | 20.2326 | 17.9447 | 4.4191 | 5.6198 | 6.5332 | 7.0972 | 3.3628 | 4.8363 | 6.0255 | 6.7821 | 8.1344 | 14.1916 | 20.0131 | 24.1679 |
| 34MM-C7 | 35.5333 | 23.5456 | 19.1614 | 17.4052 | 4.5950 | 5.7918 | 6.5385 | 6.9241 | 3.8412 | 5.7309 | 7.0389 | 7.7534 | 7.8077 | 14.1126 | 19.2166 | 22.2610 |
| 234MMM-C6 | 36.4667 | 24.1322 | 19.4602 | 17.5502 | 4.5377 | 5.7501 | 6.5661 | 7.0132 | 3.6731 | 5.4219 | 6.7052 | 7.4444 | 7.8294 | 14.0204 | 19.3366 | 22.6855 |
| 233MMM-C6 | 36.9667 | 24.5072 | 19.6790 | 17.6674 | 4.5090 | 5.7095 | 6.5388 | 7.0119 | 3.6029 | 5.2825 | 6.5257 | 7.2548 | 8.0118 | 14.2671 | 19.7998 | 23.4304 |
| 33MM-C7 | 35.7667 | 23.7783 | 19.3221 | 17.5009 | 4.5789 | 5.7599 | 6.5143 | 6.9230 | 3.7952 | 5.6017 | 6.8504 | 7.5460 | 8.1623 | 14.6262 | 19.9391 | 23.2389 |
| 3E-24MM-C5 | 36.6667 | 24.2222 | 19.4907 | 17.5594 | 4.5272 | 5.7459 | 6.5684 | 7.0162 | 3.6479 | 5.4013 | 6.7009 | 7.4473 | 7.7455 | 13.9657 | 19.4139 | 22.8677 |
| 35MM-C7 | 35.2667 | 23.4033 | 19.1034 | 17.3837 | 4.6087 | 5.8019 | 6.5413 | 6.9232 | 3.8696 | 5.7494 | 7.0335 | 7.7318 | 7.9742 | 14.4001 | 19.5371 | 22.5891 |
| 25MM-C7 | 34.8333 | 23.1917 | 19.0233 | 17.3560 | 4.6310 | 5.8100 | 6.5342 | 6.9130 | 3.9217 | 5.7757 | 7.0071 | 7.6745 | 8.2729 | 14.9280 | 20.1221 | 23.1915 |
| 26MM-C7 | 34.4333 | 23.0006 | 18.9517 | 17.3312 | 4.6515 | 5.8159 | 6.5261 | 6.9026 | 3.9707 | 5.7989 | 6.9809 | 7.6190 | 8.5582 | 15.4324 | 20.6972 | 23.8055 |
| 44MM-C7 | 35.9667 | 23.8683 | 19.3526 | 17.5102 | 4.5695 | 5.7574 | 6.5165 | 6.9252 | 3.7743 | 5.5942 | 6.8580 | 7.5555 | 8.0562 | 14.5324 | 19.9493 | 23.3281 |
| 4E-2M-C6 | 35.4333 | 23.4728 | 19.1253 | 17.3900 | 4.5999 | 5.7998 | 6.5442 | 6.9259 | 3.8467 | 5.7313 | 7.0295 | 7.7335 | 7.9109 | 14.3765 | 19.6153 | 22.7325 |
| 3E-22MM-C5 | 37.1667 | 24.5972 | 19.7095 | 17.6766 | 4.4979 | 5.7064 | 6.5440 | 7.0172 | 3.5737 | 5.2601 | 6.5330 | 7.2796 | 7.9123 | 14.1177 | 19.7050 | 23.3971 |
| 24MM-C7 | 35.0333 | 23.2817 | 19.0538 | 17.3652 | 4.6216 | 5.8060 | 6.5343 | 6.9139 | 3.9032 | 5.7719 | 7.0149 | 7.6829 | 8.1669 | 14.8378 | 20.1251 | 23.2669 |
| 2234MMMM-C5 | 37.6667 | 24.9722 | 19.9282 | 17.7938 | 4.4646 | 5.6723 | 6.5601 | 7.0926 | 3.4691 | 5.0036 | 6.1899 | 6.9201 | 8.1948 | 14.4912 | 20.3010 | 24.2834 |
| 22MM-C7 | 35.1000 | 23.4450 | 19.1925 | 17.4546 | 4.6130 | 5.7721 | 6.5017 | 6.9051 | 3.8764 | 5.6527 | 6.8222 | 7.4653 | 8.6219 | 15.4526 | 20.8955 | 24.2724 |
| 223MMM-C6 | 36.7000 | 24.3650 | 19.6209 | 17.6459 | 4.5226 | 5.7161 | 6.5348 | 7.0046 | 3.6339 | 5.3072 | 6.5210 | 7.2279 | 8.1914 | 14.6038 | 20.1965 | 23.8601 |
| 235MMM-C6 | 35.9333 | 23.8478 | 19.3441 | 17.5072 | 4.5656 | 5.7667 | 6.5637 | 7.0037 | 3.7352 | 5.4631 | 6.6812 | 7.3734 | 8.1979 | 14.7547 | 20.2447 | 23.6759 |
| 244MMM-C6 | 36.6333 | 24.3128 | 19.5933 | 17.6336 | 4.5258 | 5.7253 | 6.5466 | 7.0132 | 3.6349 | 5.3030 | 6.5169 | 7.2233 | 8.2476 | 14.7385 | 20.4006 | 24.0912 |
| 224MMM-C6 | 36.3667 | 24.1706 | 19.5353 | 17.6121 | 4.5394 | 5.7322 | 6.5428 | 7.0060 | 3.6656 | 5.3281 | 6.5138 | 7.1978 | 8.4249 | 15.0656 | 20.7876 | 24.5142 |
| 225MMM-C6 | 35.9000 | 23.9383 | 19.4467 | 17.5814 | 4.5628 | 5.7423 | 6.5373 | 6.9966 | 3.7170 | 5.3520 | 6.4847 | 7.1383 | 8.7454 | 15.6258 | 21.3973 | 25.1226 |
| 2244MMMM-C5 | 37.5000 | 24.9583 | 19.9757 | 17.8435 | 4.4696 | 5.6607 | 6.5422 | 7.0877 | 3.4658 | 4.9149 | 5.9990 | 6.6664 | 8.8457 | 15.6909 | 22.0017 | 26.4205 |
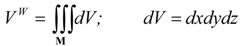
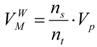
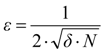
(b) Molecular van der Waals Surface
 :
:
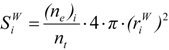
 :
:
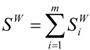
(c) Synthetic van der Waals descriptors of molecular shape
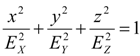
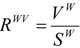
(d) Globularity measures
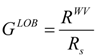

| Alkane | VW | SW | VEL | EX | EY | EZ | GLOB | GLEL | RWV |
|---|---|---|---|---|---|---|---|---|---|
| C2 | 45.672 | 71.059 | 114.658 | 3.021 | 3.145 | 2.881 | 0.613 | 0.880 | 0.643 |
| C3 | 62.678 | 93.293 | 160.367 | 3.516 | 3.780 | 2.881 | 0.533 | 0.709 | 0.672 |
| C4 | 79.733 | 115.287 | 190.706 | 3.763 | 4.200 | 2.881 | 0.494 | 0.615 | 0.692 |
| 2-M-C3 | 79.699 | 114.746 | 217.825 | 3.889 | 3.781 | 3.536 | 0.536 | 0.884 | 0.695 |
| C5 | 96.825 | 137.555 | 247.324 | 4.252 | 4.820 | 2.881 | 0.438 | 0.527 | 0.704 |
| 2M-C4 | 96.719 | 135.905 | 264.984 | 4.182 | 4.248 | 3.561 | 0.503 | 0.825 | 0.712 |
| 22MM-C3 | 96.112 | 135.069 | 254.783 | 3.875 | 3.766 | 4.168 | 0.512 | 0.840 | 0.712 |
| C6 | 113.685 | 159.426 | 284.774 | 4.503 | 5.241 | 2.881 | 0.408 | 0.472 | 0.713 |
| 3M-C5 | 113.597 | 156.104 | 318.161 | 4.307 | 4.899 | 3.599 | 0.446 | 0.646 | 0.728 |
| 2M-C5 | 113.775 | 157.987 | 339.431 | 4.687 | 4.811 | 3.594 | 0.449 | 0.728 | 0.720 |
| 23MM-C4 | 113.295 | 154.842 | 332.325 | 4.503 | 4.220 | 4.175 | 0.488 | 0.869 | 0.732 |
| 22MM-C4 | 113.230 | 155.351 | 311.724 | 4.215 | 4.235 | 4.168 | 0.516 | 0.979 | 0.729 |
| C7 | 131.027 | 181.788 | 352.736 | 4.988 | 5.861 | 2.881 | 0.369 | 0.418 | 0.721 |
| 3E-C5 | 130.432 | 176.805 | 390.419 | 4.661 | 4.931 | 4.055 | 0.449 | 0.777 | 0.738 |
| 3M-C6 | 130.657 | 178.137 | 369.805 | 4.503 | 5.352 | 3.664 | 0.411 | 0.576 | 0.733 |
| 2M-C6 | 130.675 | 180.112 | 389.158 | 4.940 | 5.265 | 3.572 | 0.413 | 0.637 | 0.726 |
| 23MM-C5 | 130.175 | 174.531 | 397.190 | 4.681 | 4.859 | 4.168 | 0.460 | 0.826 | 0.746 |
| 33MM-C5 | 130.438 | 173.979 | 373.215 | 4.344 | 4.921 | 4.168 | 0.457 | 0.748 | 0.750 |
| 223MMM-C4 | 130.086 | 173.065 | 340.481 | 4.587 | 4.251 | 4.169 | 0.492 | 0.842 | 0.752 |
| 24-MMC5 | 130.472 | 177.729 | 363.282 | 4.746 | 5.027 | 3.635 | 0.438 | 0.683 | 0.734 |
| 22MM-C5 | 130.431 | 177.235 | 390.649 | 4.663 | 4.799 | 4.168 | 0.460 | 0.844 | 0.736 |
| C8 | 147.814 | 204.050 | 397.254 | 5.240 | 6.281 | 2.881 | 0.346 | 0.383 | 0.724 |
| 3E-C6 | 147.572 | 199.053 | 423.503 | 4.662 | 5.378 | 4.033 | 0.414 | 0.650 | 0.741 |
| 3M-C7 | 147.843 | 200.529 | 459.253 | 5.026 | 5.961 | 3.660 | 0.371 | 0.518 | 0.737 |
| 34MM-C6 | 146.984 | 194.367 | 422.458 | 4.536 | 5.351 | 4.156 | 0.424 | 0.658 | 0.756 |
| 3E-3M-C5 | 147.192 | 193.627 | 428.593 | 4.792 | 4.950 | 4.314 | 0.461 | 0.844 | 0.760 |
| 4M-C7 | 147.596 | 200.355 | 450.614 | 4.972 | 5.854 | 3.696 | 0.378 | 0.536 | 0.737 |
| 2M-C7 | 147.868 | 201.974 | 478.805 | 5.432 | 5.861 | 3.591 | 0.375 | 0.568 | 0.732 |
| 3E-2M-C5 | 147.146 | 193.534 | 427.292 | 4.946 | 4.910 | 4.201 | 0.461 | 0.843 | 0.760 |
| 23MM-C6 | 147.319 | 196.670 | 452.909 | 4.852 | 5.351 | 4.165 | 0.420 | 0.706 | 0.749 |
| 233MMM-C5 | 147.226 | 191.854 | 411.251 | 4.769 | 4.944 | 4.164 | 0.466 | 0.813 | 0.767 |
| 234MMM-C5 | 147.420 | 194.534 | 397.214 | 4.780 | 4.962 | 3.998 | 0.458 | 0.776 | 0.758 |
| 33MM-C6 | 147.539 | 195.769 | 422.747 | 4.523 | 5.353 | 4.168 | 0.422 | 0.658 | 0.754 |
| 223MMM-C5 | 147.123 | 193.437 | 401.790 | 4.740 | 4.854 | 4.169 | 0.470 | 0.839 | 0.761 |
| 25MM-C6 | 147.027 | 200.288 | 492.840 | 5.286 | 5.260 | 4.232 | 0.417 | 0.797 | 0.734 |
| 22MM-C6 | 147.747 | 199.191 | 456.496 | 4.961 | 5.270 | 4.168 | 0.422 | 0.744 | 0.742 |
| 2233MMMM-C4 | 146.856 | 188.312 | 338.229 | 4.563 | 4.239 | 4.175 | 0.513 | 0.850 | 0.780 |
| 224MMM-C5 | 147.018 | 196.564 | 410.699 | 4.714 | 5.036 | 4.130 | 0.446 | 0.768 | 0.748 |
| C9 | 164.641 | 226.192 | 476.615 | 5.723 | 6.901 | 2.881 | 0.316 | 0.346 | 0.728 |
| 33EE-C5 | 164.061 | 211.324 | 459.204 | 4.769 | 5.035 | 4.566 | 0.463 | 0.859 | 0.776 |
| 3E-C7 | 164.668 | 221.159 | 532.315 | 5.028 | 6.010 | 4.206 | 0.372 | 0.585 | 0.745 |
| 3M-C8 | 164.821 | 222.363 | 518.339 | 5.259 | 6.396 | 3.680 | 0.348 | 0.473 | 0.741 |
| 4M-C8 | 164.737 | 222.348 | 518.784 | 5.214 | 6.354 | 3.739 | 0.350 | 0.483 | 0.741 |
| 2M-C8 | 164.651 | 224.148 | 535.314 | 5.679 | 6.300 | 3.572 | 0.350 | 0.511 | 0.735 |
| 3E-23MM-C5 | 163.900 | 210.954 | 455.680 | 5.057 | 5.038 | 4.269 | 0.461 | 0.841 | 0.777 |
| 2334MMMM-C5 | 163.486 | 209.776 | 419.044 | 4.812 | 5.003 | 4.155 | 0.467 | 0.799 | 0.779 |
| 4E-C7 | 164.814 | 220.815 | 487.239 | 5.054 | 5.713 | 4.029 | 0.392 | 0.624 | 0.746 |
| 3E-3M-C6 | 164.119 | 215.689 | 472.306 | 4.804 | 5.410 | 4.338 | 0.422 | 0.712 | 0.761 |
| 23MM-C7 | 164.329 | 218.824 | 558.982 | 5.408 | 5.934 | 4.158 | 0.380 | 0.639 | 0.751 |
| 334MMM-C6 | 163.840 | 212.499 | 440.007 | 4.587 | 5.505 | 4.160 | 0.420 | 0.629 | 0.771 |
| 2233MMMM-C5 | 163.704 | 205.939 | 400.956 | 4.676 | 4.902 | 4.176 | 0.486 | 0.813 | 0.795 |
| 34MM-C7 | 163.970 | 215.894 | 522.987 | 5.081 | 5.921 | 4.150 | 0.385 | 0.601 | 0.759 |
| 234MMM-C6 | 164.105 | 214.473 | 460.278 | 4.964 | 5.428 | 4.078 | 0.423 | 0.687 | 0.765 |
| 233MMM-C6 | 164.180 | 213.892 | 455.137 | 4.831 | 5.398 | 4.167 | 0.427 | 0.691 | 0.768 |
| 33MM-C7 | 164.007 | 217.971 | 529.200 | 5.085 | 5.962 | 4.168 | 0.379 | 0.596 | 0.752 |
| 3E-24MM-C5 | 164.363 | 216.103 | 427.188 | 5.057 | 5.064 | 3.982 | 0.451 | 0.785 | 0.761 |
| 35MM-C7 | 164.491 | 218.873 | 533.826 | 5.433 | 5.307 | 4.420 | 0.415 | 0.795 | 0.752 |
| 25MM-C7 | 164.140 | 220.135 | 568.077 | 5.393 | 5.886 | 4.272 | 0.380 | 0.665 | 0.746 |
| 26MM-C7 | 164.715 | 222.197 | 491.514 | 5.443 | 5.995 | 3.596 | 0.371 | 0.545 | 0.741 |
| 44MM-C7 | 164.587 | 217.682 | 501.828 | 4.898 | 5.869 | 4.168 | 0.386 | 0.593 | 0.756 |
| 4E-2M-C6 | 164.549 | 219.575 | 436.658 | 5.125 | 5.414 | 3.757 | 0.415 | 0.657 | 0.749 |
| 3E-22MM-C5 | 164.151 | 214.491 | 446.559 | 5.003 | 5.113 | 4.168 | 0.449 | 0.798 | 0.765 |
| 24MM-C7 | 165.047 | 220.278 | 507.519 | 5.480 | 5.752 | 3.844 | 0.391 | 0.636 | 0.749 |
| 2234MMMM-C5 | 163.603 | 209.897 | 410.677 | 4.731 | 5.047 | 4.106 | 0.463 | 0.763 | 0.779 |
| 22MM-C7 | 164.016 | 221.271 | 554.019 | 5.411 | 5.865 | 4.168 | 0.379 | 0.656 | 0.741 |
| 223MMM-C6 | 163.938 | 215.620 | 456.468 | 4.911 | 5.286 | 4.197 | 0.431 | 0.738 | 0.760 |
| 235MMM-C6 | 163.921 | 216.403 | 486.875 | 5.275 | 5.384 | 4.093 | 0.422 | 0.745 | 0.757 |
| 244MMM-C6 | 164.263 | 214.907 | 478.821 | 5.128 | 5.402 | 4.127 | 0.424 | 0.725 | 0.764 |
| 224MMM-C6 | 164.000 | 216.617 | 471.400 | 5.085 | 5.392 | 4.104 | 0.421 | 0.718 | 0.757 |
| 225MMM-C6 | 164.103 | 219.201 | 495.366 | 5.365 | 5.269 | 4.184 | 0.419 | 0.766 | 0.749 |
| 2244MMMM-C5 | 164.104 | 214.571 | 408.276 | 4.639 | 5.042 | 4.168 | 0.455 | 0.761 | 0.765 |
Intercorrelation of Topological Distance Indices and van Der Waals Molecular Descriptors
| W | P | F | J | VAD1 | VAD2 | VAD3 | VED1 | VED2 | VED3 | VRD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W | 1.000 | 0.719 | 0.716 | 0.523 | 0.945 | 0.860 | 0.862 | 0.915 | -0.810 | 0.874 | 0.952 |
| P | 1.000 | 0.842 | 0.850 | 0.825 | 0.766 | 0.784 | 0.821 | -0.744 | 0.793 | 0.838 | |
| F | 1.000 | 0.933 | 0.757 | 0.666 | 0.802 | 0.861 | -0.805 | 0.842 | 0.873 | ||
| J | 1.000 | 0.113 | 0.094 | 0.278 | 0.281 | -0.344 | 0.312 | 0.241 | |||
| VAD1 | 1.000 | 0.968 | 0.918 | 0.934 | -0.854 | 0.905 | 0.933 | ||||
| VAD2 | 1.000 | 0.927 | 0.897 | -0.866 | 0.890 | 0.849 | |||||
| VAD3 | 1.000 | 0.982 | -0.989 | 0.993 | 0.927 | ||||||
| VED1 | 1.000 | -0.967 | 0.993 | 0.978 | |||||||
| VED2 | 1.000 | -0.990 | -0.901 | ||||||||
| VED3 | 1.000 | 0.950 | |||||||||
| VRD | 1.000 |
- 1)
- The intercorrelation matrix of the selected topological indices presented in Table 3a reveals that these indices are not strongly intercorrelated, that is their information content about topological structure of the 72 alkanes from table 1 is somewhat independent. Only the indices derived from eigenvalues and eigenvectors are better intercorrelated. The TDIs belonging to this group are very poorly correlated with Balaban’s J-index. Besides, J is also independent when compared to W, and very weakly linked to P. On the other hand it seems to correlate very well with F (r = 0.933). From this point of view it is necessary to avoid the simultaneous use of these indices for studying physical properties in QSPR relations.
- 2)
- The majority of GTDIs, kδλ (k = 1,2,3,4; λ = 0,1,2,3) counterparts are strongly intercorrelated. Taking as criterion for strong correlations r ≥ 0.980 one notices that there exists a strong correlation inside each class λ, which slightly decreases along with the increase in k. This fact is entirely explainable, if we take into consideration the way in which LOVIs are constructed; the more the dimensionality of the space is increased, the interaction between atoms that are separated by the same topological distance decreases, and the influence gets smaller as the distance and the dimensionality of the space get larger. The degree of correlation between indices kδλ of different classes are generally smaller, except those corresponding to λ = 1 and λ = 2, which are greater than r = 0.960.Table 3b. Intercorrelation Matrix of Generalized Topological Distance Indices for Alkanes with up to 9 Carbon Atoms
Table 3b. Intercorrelation Matrix of Generalized Topological Distance Indices for Alkanes with up to 9 Carbon Atoms 1δο 2δο 3δο 4δο 1δ1 2δ1 3δ1 4δ1 1δ2 2δ2 3δ2 4δ2 1δ3 2δ3 3δ3 4δ3 1δο 1.000 0.999 0.996 0.993 0.965 0.978 0.990 0.994 0.809 0.859 0.899 0.922 0.837 0.939 0.967 0.972 2δο 1.000 0.999 0.996 0.967 0.980 0.992 0.997 0.810 0.858 0.898 0.921 0.855 0.948 0.973 0.979 3δο 1.000 0.999 0.978 0.988 0.997 1.000 0.835 0.879 0.915 0.936 0.868 0.959 0.979 0.981 4δο 1.000 0.986 0.994 0.999 1.000 0.856 0.897 0.930 0.948 0.872 0.965 0.981 0.979 1δ1 1.000 0.998 0.991 0.982 0.931 0.958 0.976 0.985 0.861 0.967 0.967 0.952 2δ1 1.000 0.997 0.991 0.908 0.941 0.964 0.977 0.862 0.967 0.973 0.962 3δ1 1.000 0.999 0.873 0.912 0.942 0.959 0.867 0.965 0.979 0.974 4δ1 1.000 0.845 0.887 0.922 0.941 0.872 0.963 0.981 0.981 1δ2 1.000 0.994 0.980 0.967 0.745 0.872 0.837 0.795 2δ2 1.000 0.996 0.988 0.754 0.891 0.867 0.831 3δ2 1.000 0.998 0.769 0.907 0.893 0.864 4δ2 1.000 0.780 0.918 0.910 0.885 1δ3 1.000 0.959 0.944 0.937 2δ3 1.000 0.994 0.982 3δ3 1.000 0.997 4δ3 1.000 Table 3c. Intercorrelation Matrix of van der Waals Molecular Descriptors for Alkanes with up to 9 Carbon AtomsTable 3c. Intercorrelation Matrix of van der Waals Molecular Descriptors for Alkanes with up to 9 Carbon Atoms VW SW VEL EX EY EZ GLOB GLEL RWV VW 1.000 0.994 0.924 0.852 0.751 0.566 -0.661 -0.220 0.839 SW 1.000 0.944 0.891 0.806 0.511 -0.729 -0.294 0.782 VEL 1.000 0.906 0.822 0.538 -0.764 -0.288 0.652 EX 1.000 0.850 0.270 -0.825 -0.413 0.528 EY 1.000 0.018 -0.978 -0.761 0.351 EZ 1.000 0.056 0.564 0.757 GLOB 1.000 0.812 -0.240 GLEL 1.000 0.178 RWV 1.000 Table 3d. Intercorrelation Matrix of Generalized Topological Distance Indices (GTDIs) against Topological Distance Indices (TDIs) for Alkanes with up to 9 Carbon AtomsTable 3d. Intercorrelation Matrix of Generalized Topological Distance Indices (GTDIs) against Topological Distance Indices (TDIs) for Alkanes with up to 9 Carbon Atoms W P F J VAD1 VAD2 VAD3 VED1 VED2 VED3 VRD 1δο 0.923 0.885 0.914 0.801 0.937 0.857 0.925 0.975 -0.896 0.947 0.991 2δο 0.912 0.881 0.920 0.817 0.932 0.861 0.939 0.982 -0.916 0.960 0.989 3δο 0.921 0.866 0.905 0.799 0.938 0.874 0.952 0.990 -0.930 0.971 0.991 4δο 0.931 0.852 0.888 0.778 0.944 0.884 0.959 0.994 -0.936 0.975 0.992 1δ1 0.959 0.789 0.802 0.672 0.955 0.910 0.965 0.989 -0.936 0.973 0.981 2δ1 0.954 0.817 0.832 0.710 0.955 0.903 0.964 0.993 -0.937 0.975 0.988 3δ1 0.939 0.844 0.871 0.758 0.948 0.890 0.961 0.995 -0.937 0.976 0.993 4δ1 0.925 0.857 0.897 0.789 0.939 0.876 0.956 0.992 -0.934 0.974 0.992 1δ2 0.924 0.582 0.534 0.375 0.887 0.882 0.882 0.880 -0.842 0.870 0.858 2δ2 0.947 0.660 0.598 0.452 0.922 0.904 0.907 0.913 -0.865 0.898 0.899 3δ2 0.958 0.724 0.659 0.526 0.945 0.918 0.929 0.940 -0.887 0.924 0.930 4δ2 0.960 0.760 0.698 0.573 0.955 0.923 0.941 0.956 -0.900 0.938 0.947 1δ3 0.780 0.555 0.824 0.696 0.752 0.719 0.880 0.891 -0.905 0.904 0.850 2δ3 0.916 0.694 0.844 0.694 0.888 0.837 0.945 0.972 -0.939 0.965 0.956 3δ3 0.912 0.755 0.894 0.756 0.897 0.833 0.942 0.979 -0.934 0.966 0.973 4δ3 0.892 0.780 0.927 0.797 0.885 0.813 0.930 0.972 -0.923 0.958 0.970 - 3)
- Van der Waals molecular descriptors, vdWMDs, are much more independent relative to each other than the GTDIs and TDIs. A strong correlation was observed only between the volume (VW) and the corresponding vdW surface (SW) of the 72 alkanes having 2 – 9 carbon atoms (r = 0.994). This significant correlation was obtained between the vdW volume and surface, but also between them and the molecular vdW volume of alkanes treated as molecules with a more or less ellipsoidal shape. The shift of alkanes to an extended, intercalated conformation greatly influences the volume of the ellipsoid and progressively smaller the vdW surface area and the vdW volume. On the other hand, conformational variations on orthogonal directions are affecting these descriptors on a much smaller measure. Our intercorrelation results suggest the possibility of simultaneously using these indices in QSAR and QSPR relations for global testing of vdW space occupied by molecules (space-filling), along with bulk steric parameters (VW, SW, VEL, GLOB, GLEL, RWV), or certain directions within them (EX, EY, EZ). The simple and fast calculus for any molecular structure and the possibility of immediately testing the degree of orthogonality ensures their large applicability for any series of compounds.
- 4)
- Generalized topological distance indices derived from the reciprocical distance matrix, GTDIs, present significant correlations with topological indices derived from eigenvalues and eigenvectors of the distance matrix, D. Repeatedly, the strongest are those between kδλ (k = 1,2,3,4, λ = 0,1) and VRD. In this case a more rigorous statistical analysis is imposed on the relation between distance indices, kδλ, and the VADi and VEDi parameters, i = 1,2,3. The intercorrelation between GTDIs and the first indices defined on the distance matrix is decreasing in the following order: W, F, P. Although, generally speaking, the Wiener index, W, correlates well with GTDIs, there are two surprising exceptions for indices 1δ3 and 4δ3. Investigating the physical meaning of GTDIs could emerge interesting information on other topological indices. The work is in progress.
- 5)
- Are the topological indices steric measures of molecular van der Waals space? Although some reported that they correlate well with molecular volume [7] or surface area, extensive studies on this subject have not yet been performed. In Table 4 we present the intercorrelation matrix of molecular vdW descriptors and of topological indices described in this work. The best results were obtained for the correlations with the van der Waals molecular volume (VW) and surface (SW) against Wiener indices (W), derived from eigenvectors and eigenvalues of the distance matrix, and GTDIs, kδλ, except for indices with λ = 2 and k = 1 (r = 0.886), and λ = 3 and k = 1 (r = 0.869). Except for P, F and J indices, the others should be viewed as bulk steric parameters, as measured by vdW volume and surface of tested alkanes. The steric component of most topological indices is poorly explained by vdW volumes of ellipsoid-assimilated alkanes (revolving around r = 0.900). Weak correlations were also obtained for P, F and J. The results suggest the impossibility of testing the vector nature of steric effects by means of topological distance indices, which is rather important for modeling biological interactions. This is a possible explanation for the lesser utility of topological indices for QSAR studies.
| VW | SW | VEL | EX | EY | EZ | GLOB | GLEL | RWV | |
|---|---|---|---|---|---|---|---|---|---|
| W | 0.944 | 0.958 | 0.930 | 0.873 | 0.830 | 0.386 | -0.741 | -0.373 | 0.642 |
| P | 0.834 | 0.778 | 0.666 | 0.519 | 0.416 | 0.636 | -0.274 | 0.074 | 0.913 |
| F | 0.859 | 0.809 | 0.687 | 0.559 | 0.354 | 0.751 | -0.237 | 0.191 | 0.937 |
| J | 0.743 | 0.677 | 0.537 | 0.392 | 0.179 | 0.820 | -0.073 | 0.343 | 0.970 |
| VAD1 | 0.951 | 0.950 | 0.895 | 0.831 | 0.779 | 0.455 | -0.680 | -0.295 | 0.747 |
| VAD2 | 0.896 | 0.902 | 0.847 | 0.826 | 0.795 | 0.386 | -0.722 | -0.358 | 0.711 |
| VAD3 | 0.965 | 0.965 | 0.890 | 0.857 | 0.761 | 0.540 | -0.702 | -0.258 | 0.840 |
| VED1 | 0.996 | 0.990 | 0.916 | 0.854 | 0.744 | 0.581 | -0.664 | -0.211 | 0.856 |
| VED2 | -0.940 | -0.939 | -0.860 | -0.832 | -0.717 | -0.570 | 0.670 | 0.215 | -0.849 |
| VED3 | 0.978 | 0.975 | 0.898 | 0.851 | 0.738 | 0.581 | -0.672 | -0.213 | 0.860 |
| VRD | 0.991 | 0.981 | 0.910 | 0.820 | 0.718 | 0.572 | -0.618 | -0.191 | 0.829 |
| 1δο | 0.986 | 0.965 | 0.880 | 0.776 | 0.656 | 0.621 | -0.546 | -0.112 | 0.878 |
| 2δο | 0.989 | 0.968 | 0.881 | 0.782 | 0.656 | 0.632 | -0.550 | -0.107 | 0.892 |
| 3δο | 0.995 | 0.979 | 0.897 | 0.808 | 0.687 | 0.616 | -0.587 | -0.142 | 0.880 |
| 4δο | 0.998 | 0.986 | 0.909 | 0.827 | 0.714 | 0.597 | -0.618 | -0.173 | 0.865 |
| 1δ1 | 0.994 | 0.999 | 0.944 | 0.890 | 0.812 | 0.504 | -0.733 | -0.303 | 0.783 |
| 2δ1 | 0.999 | 0.998 | 0.936 | 0.869 | 0.779 | 0.541 | -0.694 | -0.256 | 0.813 |
| 3δ1 | 1.000 | 0.991 | 0.920 | 0.841 | 0.732 | 0.585 | -0.640 | -0.194 | 0.850 |
| 4δ1 | 0.997 | 0.983 | 0.905 | 0.820 | 0.698 | 0.612 | -0.601 | -0.151 | 0.873 |
| 1δ2 | 0.886 | 0.926 | 0.916 | 0.932 | 0.951 | 0.229 | -0.913 | -0.574 | 0.532 |
| 2δ2 | 0.922 | 0.953 | 0.934 | 0.919 | 0.925 | 0.305 | -0.872 | -0.510 | 0.600 |
| 3δ2 | 0.950 | 0.971 | 0.941 | 0.905 | 0.893 | 0.373 | -0.828 | -0.445 | 0.665 |
| 4δ2 | 0.965 | 0.980 | 0.943 | 0.895 | 0.869 | 0.416 | -0.797 | -0.402 | 0.706 |
| 1δ3 | 0.869 | 0.876 | 0.809 | 0.808 | 0.633 | 0.542 | -0.598 | -0.151 | 0.738 |
| 2δ3 | 0.968 | 0.975 | 0.913 | 0.881 | 0.752 | 0.533 | -0.689 | -0.239 | 0.775 |
| 3δ3 | 0.978 | 0.974 | 0.900 | 0.843 | 0.699 | 0.589 | -0.621 | -0.165 | 0.827 |
| 4δ3 | 0.972 | 0.959 | 0.875 | 0.804 | 0.647 | 0.626 | -0.561 | -0.103 | 0.858 |
Correlations with Boiling Points of Alkanes
| Eq. | Xi | r | α | ±Δα | β | ±Δβ | s | F | EV | t |
|---|---|---|---|---|---|---|---|---|---|---|
| 36 | W | 0.916 | 4.31 | 5.70 | 1.42 | 0.07 | 18.715 | 374.5 | 0.837 | 19.4 |
| 37 | F | 0.834 | 19.13 | 7.43 | 13.18 | 1.03 | 25.728 | 164.3 | 0.691 | 12.8 |
| 38 | P | 0.803 | -8.29 | 10.53 | 6.99 | 0.61 | 27.783 | 130.6 | 0.640 | 11.4 |
| 39 | J | 0.722 | -85.26 | 21.97 | 60.77 | 6.87 | 32.260 | 78.3 | 0.514 | 8.8 |
| 40 | VAD1 | 0.947 | -29.08 | 5.66 | 7.57 | 0.30 | 14.910 | 631.5 | 0.896 | 25.1 |
| 41 | VAD2 | 0.925 | -103.00 | 10.34 | 95.04 | 4.60 | 17.714 | 426.4 | 0.854 | 20.6 |
| 42 | VAD3 | 0.984 | -36.40 | 3.18 | 56.68 | 1.20 | 8.259 | 2221.0 | 0.968 | 47.1 |
| 43 | VED1 | 0.989 | -294.47 | 6.95 | 146.42 | 2.52 | 6.744 | 3366.1 | 0.979 | 58.0 |
| 44 | VED2 | 0.960 | 370.10 | 9.15 | -731.86 | 25.02 | 12.985 | 855.6 | 0.921 | 29.3 |
| 45 | VED3 | 0.984 | 24.68 | 1.96 | 112.36 | 2.36 | 8.177 | 2267.0 | 0.969 | 47.6 |
| 46 | VRD | 0.962 | -44.30 | 5.28 | 6.82 | 0.23 | 12.802 | 882.2 | 0.924 | 29.7 |
| 47 | 1δο | 0.956 | -43.28 | 5.66 | 5.12 | 0.19 | 13.716 | 759.3 | 0.912 | 27.6 |
| 48 | 2δο | 0.963 | -63.35 | 5.82 | 8.51 | 0.28 | 12.635 | 907.7 | 0.926 | 30.1 |
| 49 | 3δο | 0.973 | -77.71 | 5.31 | 11.24 | 0.32 | 10.786 | 1272.2 | 0.946 | 35.7 |
| 50 | 4δο | 0.979 | -85.16 | 4.79 | 12.85 | 0.31 | 9.439 | 1683.2 | 0.958 | 41.0 |
| 51 | 1δ1 | 0.988 | -218.36 | 6.16 | 78.24 | 1.47 | 7.341 | 2830.3 | 0.975 | 53.2 |
| 52 | 2δ1 | 0.987 | -166.85 | 5.25 | 53.23 | 1.01 | 7.403 | 2781.4 | 0.974 | 52.7 |
| 53 | 3δ1 | 0.983 | -146.99 | 5.74 | 43.90 | 0.98 | 8.675 | 2006.0 | 0.965 | 44.8 |
| 54 | 4δ1 | 0.975 | -137.52 | 6.59 | 39.85 | 1.06 | 10.260 | 1413.6 | 0.951 | 37.6 |
| 55 | 1δ2 | 0.911 | -235.00 | 18.32 | 97.53 | 5.20 | 19.205 | 352.0 | 0.828 | 18.8 |
| 56 | 2δ2 | 0.941 | -140.75 | 10.66 | 49.69 | 2.11 | 15.817 | 553.1 | 0.883 | 23.5 |
| 57 | 3δ2 | 0.963 | -122.07 | 7.70 | 38.08 | 1.26 | 12.603 | 912.7 | 0.926 | 30.2 |
| 58 | 4δ2 | 0.974 | -117.21 | 6.23 | 34.00 | 0.93 | 10.534 | 1337.3 | 0.948 | 36.6 |
| 59 | 1δ3 | 0.832 | -299.41 | 32.01 | 52.84 | 4.15 | 25.849 | 162.1 | 0.688 | 12.7 |
| 60 | 2δ3 | 0.941 | -158.93 | 11.41 | 20.34 | 0.86 | 15.800 | 554.5 | 0.884 | 23.5 |
| 61 | 3δ3 | 0.945 | -118.22 | 9.37 | 12.88 | 0.53 | 15.304 | 595.7 | 0.891 | 24.4 |
| 62 | 4δ3 | 0.932 | -100.17 | 9.65 | 10.23 | 0.47 | 16.877 | 477.0 | 0.867 | 21.8 |
| 63 | VW | 0.986 | -140.62 | 5.06 | 1.71 | 0.03 | 7.852 | 2464.6 | 0.971 | 49.6 |
| 64 | SW | 0.984 | -165.00 | 5.89 | 1.40 | 0.03 | 8.339 | 2177.0 | 0.968 | 46.7 |
| 65 | VEL | 0.911 | -83.11 | 10.35 | 0.45 | 0.02 | 19.229 | 351.0 | 0.827 | 18.7 |
| 66 | EX | 0.849 | -291.59 | 29.28 | 82.60 | 6.05 | 24.600 | 186.4 | 0.718 | 13.7 |
| 67 | EY | 0.790 | -178.72 | 26.28 | 54.54 | 4.99 | 28.575 | 119.5 | 0.619 | 10.9 |
| 68 | EZ | 0.523 | -112.71 | 42.28 | 55.91 | 10.73 | 39.718 | 27.1 | 0.264 | 5.2 |
| 69 | GLOB | 0.710 | 383.22 | 32.62 | -642.13 | 75.10 | 32.829 | 73.1 | 0.497 | 8.6 |
| 70 | GLEL | 0.285 | 176.94 | 28.50 | -101.46 | 40.22 | 44.673 | 6.4 | 0.069 | 2.5 |
| 71 | RWV | 0.833 | -1060.80 | 91.34 | 1568.38 | 122.68 | 25.773 | 163.4 | 0.690 | 12.8 |
Concluding remarks
Acknowledgements
References
- Balaban, A.T. QSPR/QSAR Studies by Molecular Descriptors; Diudea, M.V., Ed.; NOVA Science: Huntington, 2001; pp. 1–30. [Google Scholar]
- Devillers, J.; Balaban, A.T. (Eds.) Topological Indices and Related Descriptors in QSAR and QSPR; Gordon and Breach: Reading, UK, 1999.
- Ciubotariu, D.; Gogonea, V.; Medeleanu, M. QSPR/QSAR Studies by Molecular Descriptors; Diudea, M.V., Ed.; NOVA Science: Huntington, 2001; pp. 281–315. [Google Scholar]
- Szabo, A.; Ostlund, N.S. Modern Quantum Chemistry, Introduction to Advanced Electronic Theory; McGraw-Hill: New York, 1989. [Google Scholar]
- Ciubotariu, D. Structure-Reactivity Relations in the Series of Carbonic Acide Derivatives. PhD Thesis, Polytechnical Institute of Bucharest, 1987. [Google Scholar]
- Niculescu-Duvaz, I.; Ciubotariu, D.A.; Simon, Z.; Voiculetz, N. Modeling of Cancer Genesis and Prevention; Voicultez, N., Balaban, A.T., Niculescu-Duvaz, I., Simon, Z., Eds.; CRC Press: Boca Raton, 1991; pp. 157–214. [Google Scholar]
- Balaban, A.T.; Motoc, I.; Boncher, D.; Mekenyan, O. Topological Indices for Structure-Activity Correlations. Top. Curr. Chem. 1983, 114, 21–55. [Google Scholar]
- de Brujin, J; Heruvens, J. Relationship between Octanol/Water Partition Coefficients and Total Molecular Surface Area and Total Molecular Volume of Hydrophobic Organic Chemicals. Quant. Struct.-Act. Relat. 1990, 9, 11–21. [Google Scholar]
- Heiden, W; Moeckel, G.; Brickmann, K. A New Approach to Analysis and Display Lipophilicity/Hydrophilicity Mapped on Molecular Surfaces. J. Comput.-Aided Mol. Design 1993, 7, 503–514. [Google Scholar]
- Hermann, R.B. Modeling Hydrophobic Solvation of Non-Spherical Systems: Comparison of Useof Molecular Surface Area with Accessible Surface Area. J. Comput. Chem. 1997, 18, 115–125. [Google Scholar]
- Todeschini, R.; Gramatica, P. 3D-Modelling and Prediction by WHIM Descriptors. Part 5. Theory Development and Chemical Meaning of WHIM Descriptors. Quant. Struct.-Act. Relat. 1997, 16, 113–119. [Google Scholar]
- Medeleanu, M. Structure - Properties Correlations by Topological Methods. PhD Thesis, Politechnical University of Bucharest, 1997. [Google Scholar]
- Balaban, A.T.; Ciubotariu, D.; Medeleanu, M. Topological Indices and Real Vertex Invariants Based on Graph Eigenvalues or Eigenvectors. J. Chem. Inf. Comput. Sci. 1991, 31, 517–523. [Google Scholar]
- Medeleanu, M.; Balaban, A.T. Real-Number Vertex Invariants and Schultz-Type Indices Based on Eigenvectors of Adjacency and Distance Matrices. J. Chem. Inf. Comput. Sci. 1998, 38, 1038–1047. [Google Scholar]
- Ciubotariu, D.; Medeleanu, M.; Gogonea, V. Quantitative Treatment of Organic Molecules. I. Distance Connectivity Index d as Similarity Measure and Correlation Parameter for Alkanes. Chem. Bull. Univ. Tech. (Timisoara) 1995, 40, 21–36. [Google Scholar]
- Ciubotariu, D.; Medeleanu, M.; Gogonea, V. Quantitative Treatment of Organic Molecules. II. A New Topological Index Based on Distance Matrix. Chem. Bull. “Politehnica” Univ. (Timisoara) 1996, 41, 19–24. [Google Scholar]
- Balaban, A.T.; Ciubotariu, D.; Invanciuc, O. Design of Topolofical Indices. Part 2. Distance Measure Connectivity Indices. Math. Chem.(MATCH) 1990, 25, 41–70. [Google Scholar]
- Gogonea, V.; Ciubotariu, D.; Deretey, E.; Popescu, M.; Iorga, I.; Medeleanu, M. Surface Area of Organic Molecules: a New Methodof Computation. Rev. Roum. Chim. 1991, 36, 465–471. [Google Scholar]
- Ciubotariu, D.; Deretey, E.; Medeleanu, M.; Gogonea, V.; Iorga, I. New Shape Descriptors for Quantitative Treatment of Steric Effects. 1. The Molecular van der Waals Volume. Chem. Bull. PIT 1990, 35, 83–92. [Google Scholar]
- Ivanciuc, O.; Balaban, T.S.; Balaban, A.T. Design of Topological Indices. Part 4. Reciprocical Distance Matrix, Related Local Vertex Invariants and Topological Indices. J. Math. Chem. 1993, 12, 309–318. [Google Scholar]
- Mihalić, Z.; Nikolić, S.; Trinajstić, N. Comparative Study of Molecular Descriptors Derived from the Distance Matrix. J. Chem. Inf. Comput. Sci. 1992, 32, 28–37. [Google Scholar]
- Harary, F. Graph Theory; 2nd edition; Addison-Wesley: Reading, 1971. [Google Scholar]
- Rouvray, D.H. Predicting Chemistry from Topology. Sci.Am. 1986, 254, 36–43. [Google Scholar]
- Wiener, H. Structural Determination of Paraffin Boiling Points. J. Am. Chem. Soc. 1947, 69, 17–20. [Google Scholar]
- Wiener, H. Correlation of Heats of Isomerization and Differences in Heats of Vaporization of Isomers among the Paraffinic Hydrocarbons. J. Am. Chem. Soc. 1947, 69, 2636–2638. [Google Scholar]
- Platt, J.R. Prediction of Isomeric Differences in Paraffin Properties. J. Phys. Chem. 1952, 56, 328–336. [Google Scholar]
- Balaban, A.T. Highly Discriminating Distance-Based Topological Index. Chem. Phys. Lett. 1982, 80, 399–404. [Google Scholar]
- Balaban, A.T. Topological Indices Based on Topological Distances in Molecular Graphs. Pure Appl. Chem. 1983, 55, 199–206. [Google Scholar]
- Hosoya, H. Topological Index. A Newly Proposed Quantity Characterizing the Topological Nature of Structural Isomers of Saturated Hydrocarbons. Bull. Chem. Soc. Jpn. 1971, 44, 2332–2339. [Google Scholar]
- Gutman, I.; Markovic, S. Benzenoid Graphs with Maximal Eigenvalues. J. Math. Chem. 1993, 13, 213–215. [Google Scholar]
- Ciubotariu, C.; Medeleanu, M.; Ciubotariu, D. IRS – a Computer Program Package for QSAR and QSPR Studies. Chem. Bull. “POLITEHNICA” Univ. of Timisoara 2004, 49. in press. [Google Scholar]
- Todeschini, R.; Consonni, V.; Pavan, M. Dragon Software ver 2.1; Milano Chemometrics and QSAR Research Group, 2002. [Google Scholar]
- Plavšić, D.; Nikolić, S.; Trinajstić, N.; Mihalić, Z. On the 42-Harrary Index for Characterization of Chemical Graphs. J. Math. Chem. 1993, 12, 235–253. [Google Scholar]
- Randić, M. On the Characterization of Molecular Branching. J. Am. Chem. Soc. 1975, 97, 6609–6615. [Google Scholar]
- Kier, L.B.; Hall, L.H.; Murray, W.J.; Randić, M. Molecular Connectivity. Part 1. Relation to Nonspecific Local Anesthetics. J. Pharm. Sci. 1975, 64, 1971–1974. [Google Scholar]
- Ciubotariu, D.; Holban, S.; Motoc, I. Computation of Molecular van der Waals Volume by means of Monte Carlo Method. Preprint, Univ. Timisoara, Fac. St. Nat., Sect. Chimie 1975, 3, 1–8. [Google Scholar]
- Ciubotariu, D.; Gogonea, V.; Iorga, I.; Deretey, E.; Medeleanu, M.; Muresan, S.; Bologa, C. New Shape Descriptors for Quantitative Treatment of Steric Effects. II. The Molecular van der Waals Volume: Two Monte Carlo Algorithms. Chem. Bull. Tech. (Timisoara) 1993, 38, 67–75. [Google Scholar]
- Muresan, S.; Sulea, T.; Ciubotariu, D.; Kurunczi, L.; Simon, Z. Van der Waals Intersection Envelope Volumes as a Possible Basis for Steric Interaction in CoMFA. Quant. Struct.-Act. Relat. 1996, 15, 31–32. [Google Scholar]
- Desiraju, G.R. Crystal Engineering the Design of Organic Solids; Elsevier: Amsterdam, 1989; pp. 27–62. [Google Scholar]
- Bondi, A.J. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar]
- Francl, M.M.; Hout, J.R.; Hehre, R.F. Representation of Electron Densities. I. Sphere Fit to Total Electron Density Surfaces. J. Am. Chem. Soc. 1984, 106, 563–570. [Google Scholar]
- Demidovich, B.P.; Maron, I.A. Computation Mathematics; Mir Publishers: Moscow, 1987; pp. 649–674. [Google Scholar]
- Pearlmann, R.S. SAREA Program. QCPE Nr. 413. 1983. [Google Scholar]
- Gogonea, V. An Approach to Solvent Effect Modelling by the Combined Scaled-Particle Theory and Dielectric Continuum-Medium Method. PhD. Thesis, Toyohashi Univ. of Technology, Japan, 1996. [Google Scholar]
- Cohen, C. Computer Assisted Drug Design; Olson, E.C., Christoffersen, R.E., Eds.; ACS Symp. Ser.: Washington, DC, 1979; Volume 112, pp. 371–382. [Google Scholar]
- Vranceanu, Gh.; Hangan, Th.; Teleman, C. Geometrie elementară din punct de vedere modern; Tehnică: Bucureşti, 1967; pp. 56–78. [Google Scholar]
- Mayer, A.Y.; Farin, D.; Avair, D. Cross-Sectional Areas of Alkanoic Acids. A Comparative Study Applying Fractal Theory of Adsorption and Considerations of Molecular Shape. J. Am. Chem. Soc. 1986, 108, 7897–7905. [Google Scholar]
- Motoc, I.; Balaban, A.T. Topological Indices: Intercorrelations, Physical Meaning, Correlational Ability. Rev. Roum. Chim. 1981, 26, 305–306. [Google Scholar]
- Medeleanu, M.; Ciubotariu, C.; Ciubotariu, D. A New Globularity Measure for Quantitative Treatment of Molecular Shape. Chem. Bull. “POLITEHNICA” Univ. of Timisoara 2004, 49. in press. [Google Scholar]
© 2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ciubotariu, D.; Medeleanu, M.; Vlaia, V.; Olariu, T.; Ciubotariu, C.; Dragos, D.; Corina, S. Molecular van der Waals Space and Topological Indices from the Distance Matrix. Molecules 2004, 9, 1053-1078. https://doi.org/10.3390/91201053
Ciubotariu D, Medeleanu M, Vlaia V, Olariu T, Ciubotariu C, Dragos D, Corina S. Molecular van der Waals Space and Topological Indices from the Distance Matrix. Molecules. 2004; 9(12):1053-1078. https://doi.org/10.3390/91201053
Chicago/Turabian StyleCiubotariu, Dan, Mihai Medeleanu, Vicentiu Vlaia, Tudor Olariu, Ciprian Ciubotariu, Dan Dragos, and Seiman Corina. 2004. "Molecular van der Waals Space and Topological Indices from the Distance Matrix" Molecules 9, no. 12: 1053-1078. https://doi.org/10.3390/91201053
APA StyleCiubotariu, D., Medeleanu, M., Vlaia, V., Olariu, T., Ciubotariu, C., Dragos, D., & Corina, S. (2004). Molecular van der Waals Space and Topological Indices from the Distance Matrix. Molecules, 9(12), 1053-1078. https://doi.org/10.3390/91201053