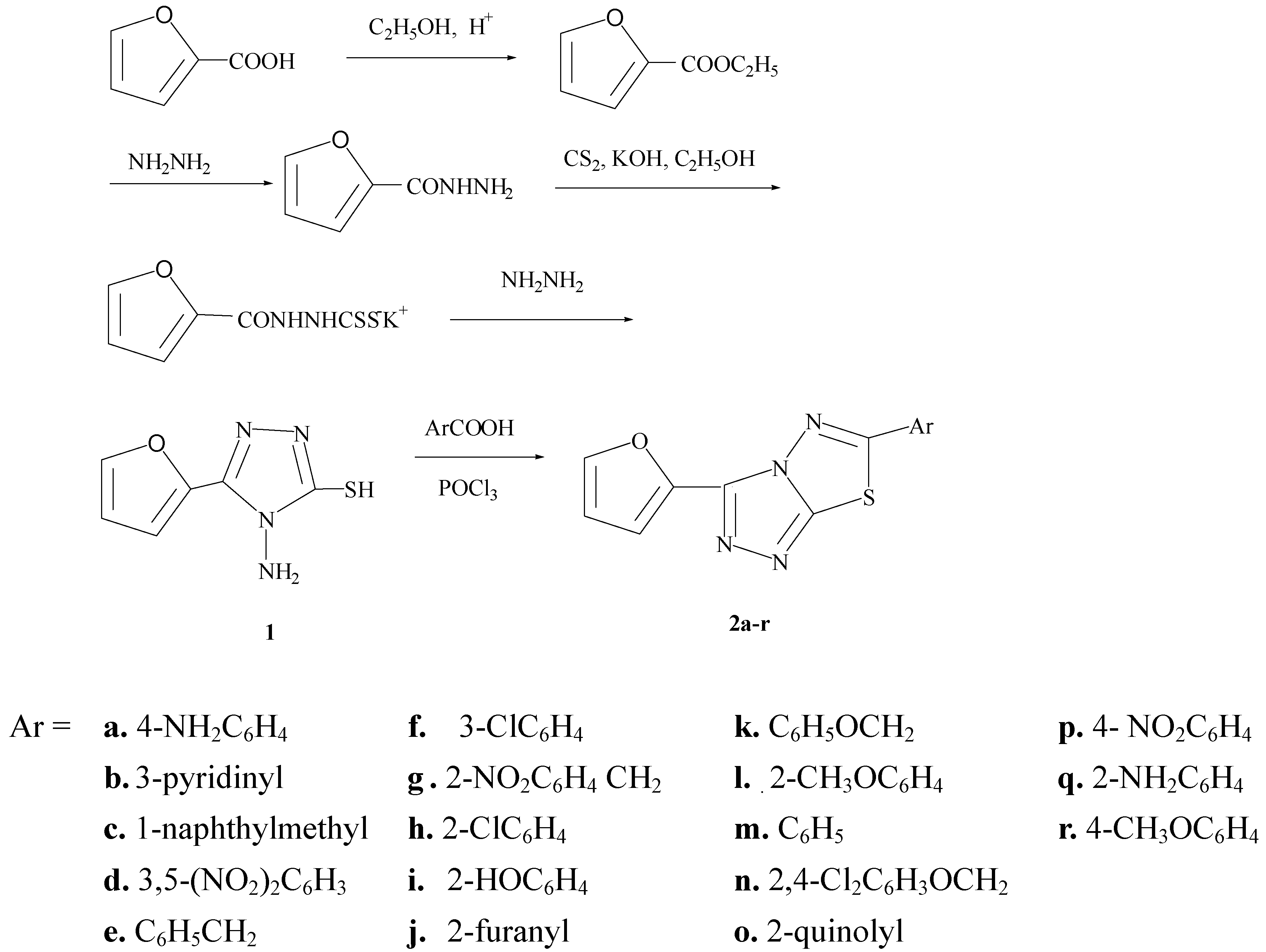
General method for the preparation of 3-(2-furanyl)-6-aryl-1,2,4-triazolo[3,4-b]-1,3,4–thiadiazoles (2a-r).
A mixture of 1 (5.0mmol) and an aromatic acid (5.5mmol) in POCl3 (20mL) was refluxed for 7hr. The reaction mixture was gradually poured onto crushed ice with stirring. Some solid K2CO3 was added to the mixture with stirring, then an appropriate amount of solid KOH was added till the pH value was 8. The solid which separated after standing overnight was filtered, washed with cold water, dried, and recrystallized from absolute alcohol to afford the title compounds 2a-r. The physical and spectral data of the title compounds are as follows:
3-(2-furanyl)-6-(4-aminophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2a): Yield 48 %; M.p. 229-231°C; IR ν/cm-1: 3422, 3220 (N-H), 1603 (C=N), 1518, 1496, 1470 (aromatic skeleton), 1226 (N-N=C), 988, 952, 903, 841,739 (Ar-H bending vibration), 699 (C-S-C); 1H-NMR δ: 8.00, 6.78, 7.34 (3H, furan H), 6.69, 7.68 (4H,dd, J=8.7Hz, Ar-H); 13C-NMR δ: 167.80, 153.72, 145.25, 140.39, 139.10, 129.04, 128.04, 115.12, 113.72, 112.18, 111.56; MS (m/z, %): 283 (M+, 72.23), 136 (100), 118 (54.09), 109 (37.86), 93 (31.77), 91 (22.78), 83 (15.41), 79 (11.59), 77 (6.80), 51 (19.23), 39 (22.78). Calcd. for C13H9N5OS (%): C 55.11, H 3.20, N 24.73; Found (%): C 54.87, H 3.22 , N 24.51.
3-(2-furanyl)-6-(3-pyridyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2b): Yield 57 %; Mp. 189-191°C; IR (KBr) ν/cm-1: 3128, 3063 (Ar-H), 1624 (C=N), 1588, 1574, 1517, 1473, 1459 (aromatic skeleton) 1249 (N-N=C), 1224 (furan C-O-C), 989, 960, 904, 885 ,809, 739 (Ar-H bending vibration), 700 (C-S-C); 1H-NMR δ: 8.02, 6.80, 7.44 (3H, furan H), 9.23, 8.83, 8.43, 7.68 (4H, pyridine H); 13C-NMR δ: 164.99, 153.62, 153.43, 147.88, 145.57, 140.00, 139.45, 135.17, 125.54, 124.59, 112.31, 112.13; MS (m/z, %): 269 (M+, 100), 137 (11.09), 122 (66.52), 109 (70.65), 93 (52.69), 78 (12.90), 77 (17.61), 65 (20.96), 51 (35.28), 39 (18.84). Calcd. for C12H7N5OS (%): C 53.52, H 2.62, N 26.01; Found (%): C 53.33, H 2.59, N 26.24.
3-(2-furanyl)-6-(1-naphthylmethyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2c): Yield 64 %; Mp. 146-148°C; IR (KBr) ν/cm-1: 3128, 3063 (Ar-H), 1626 (C=N), 1598, 1517, 1473, 1427 (aromatic skeleton), 1262 (N-N=C), 1226 (furan C-O-C), 696 (C-S-C); 1H-NMR δ: 8.13, 6.77, 7.54 (3H, furan H), 7.18-8.00 (7H, m, naphthalene H), 4.98 (2H, s, CH2); 13C-NMR δ : 171.50, 153.96, 145.43, 140.21, 138.95, 133.69, 131.41, 128.89, 128.54, 128.34, 126.96, 126.33, 125.87, 125.63, 123.76, 112.21, 111.51, 34.95; MS (m/z, %): 332 (M+, 98.94), 185 (3.65), 167 (40.18), 166 (53.24), 152 (30.31), 141 (78.44), 137 (20.09), 115 (32.29), 109 (100), 93 (46.53), 83 (13.49), 65 (14.72), 63 (24.02), 51 (18.48), 39 (15.87). Calcd. for C18H12N4OS (%): C 65.04, H 3.64, N 16.86; Found (%): C 64.81, H 3.60, N 16.99.
3-(2-furanyl)-6-(3,5-dinitrophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2d): Yield 55 %; Mp. 290-292°C; IR (KBr) ν/cm-1: 3136, 3059 (Ar-H), 1628 (C=N), 1595, 1551, 1519, 1477, 1456, 1425 (aromatic skeleton), 1252 (N-N=C), 1224 (furan C-O-C), 981, 916, 903, 886 ,799, 756, 737 (Ar-H bending vibration), 696 (C-S-C); 1H-NMR δ: 8.06, 6.83, 7.47 (3H, furan H), 9.06 (2H, s, Ar-H), 9.02 (1H, s, Ar-H); 13C-NMR δ: 163.86, 154.00, 148.87, 145.76, 139.86, 139.62, 131.73, 127.57, 121.84, 112.41, 112.39; MS (m/z, %): 358 (M+, 64.41), 266 (11.32), 211 (13.03), 165 (16.64), 137 (18.72), 119 (23.84), 111 (15.60), 109 (100), 93 (21.46), 75 (24.95), 57 (50.33), 43 (38.35). Calcd. for C13H6N6O5S (%): C 43.58, H 1.69, N 23.46; Found (%): C 43.23, H 1.60, N 23.66.
3-(2-furanyl)-6-benzyl-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2e): Yield 62 %; Mp.111-113°C; IR (KBr) ν/cm-1: 3133, 3061, 3029 (Ar-H), 1625 (C=N), 1536, 1518, 1495, 1474, 1456, 1425 (aromatic skeleton), 1265 (N-N=C), 1224 (furan C-O-C), 705 (C-S-C); 1H-NMR δ: 8.00, 6.76, 7.21 (3H, furan H), 7.31-7.44 (5H, m, Ar-H), 4.50 (2H, s, CH2); 13C-NMRδ: 171.16, 154.15, 145.40, 142.23, 138.95, 135.41, 129.36, 129.08, 127.83, 112.20, 111.51, 37.30; MS (m/z, %): 282 (M+, 55.07), 137 (15.15), 135 (4.07), 109 (100), 103 (18.82), 93 (46.41), 91 (47.94), 77 (22.82), 65 (39.07), 51 (42.84), 39 (45.75). Calcd. for C14H10N4OS (%): C 59.56, H 3.57, N 19.85; Found (%): C 59.28, H 3.65, N 20.07.
3-(2-furanyl)-6-(3-chlorophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2f): Yield 66 %; M.p.207-209°C; IR (KBr) ν/cm-1: 3108, 3066 (Ar-H), 1626 (C=N), 1573, 1518, 1478, 1474, 1459, 1423 (aromatic skeleton), 1266 (N-N=C), 1226 (furan C-O-C), 694 (C-S-C); 1H-NMRδ: 8.13, 6.80, 7.45 (3H, furan H), 7.65-8.03 (4H, m, Ar-H); 13C-NMR δ: 165.89, 153.56, 145.56, 140.01, 139.48, 134.52, 132.84, 131.69, 130.95, 126.75, 126.34, 112.31, 112.17; MS (m/z, %): 304 (M++2, 24.00), 303 (M++1, 10.76), 302 (M+, 65.31), 160 (1.43), 158 (3.76), 157 (22.49), 155 (63.07), 139 (11.46), 137 (32.33), 109 (100), 93 (66.16), 75 (29.66), 65 (27.80), 51 (39.10), 39 (29.85); Calcd. for C13H7ClN4OS (%): C 51.57, H 2.33, N 18.51; Found (%): C 51.84, H 2.40, N 18.27.
3-(2-furanyl)-6-(2-nitrophenylmethyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2g): Yield 59 %; M.p.187-189°C; IR (KBr) ν/cm-1: 3128 (Ar-H), 1620 (C=N), 1578, 1524, 1461, 1428 (aromatic skeleton), 1262 (N- N=C), 1225 (furan C-O-C), 695 (C-S-C); 1H-NMR δ: 8.09, 6.74, 7.34 (3H, furan H), 6.84-8.15 (4H, m, Ar-H), 4.82 (2H, s, CH2); MS (m/z, %): 327 (M+, 34.73), 282 (41.91), 137 (21.79), 135 (33.08), 119 (10.19), 109 (100), 77 (25.59), 64 (84.11), 51 (44.52), 39 (49.63); Calcd. for C14H9N5O3S (%): C 51.37, H 2.77, N 21.40; Found (%): C 51.66, H 2.74, N 21.16.
3-2-furanyl-6-(2-chlorophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2h): Yield 54 %; M.p.169-171°C; IR (KBr) ν/cm-1: 3130, 3073, 3013 (Ar-H), 1628 (C=N), 1592, 1517, 1477, 1454, 1427 (aromatic skeleton), 1277 (N-N=C), 1227 (furan C-O-C), 695 (C-S-C); 1H-NMR δ: 8.14, 6.79, 7.34 (3H, furan H), 7.71-8.02 (4H, m, Ar-H); MS (m/z, %): 304 (M++2, 23.52), 303 (M++1, 10.71), 302 (M+, 60.98), 160 (1.17), 158 (3.13), 157 (18.19), 155 (49.15), 137 (27.81), 109 (100), 93 (59.72), 75 (22.51), 64 (41.34), 51 (30.21), 39 (20.59); Calcd. for C13H7ClN4OS (%): C 51.57, H 2.33, N 18.51; Found (%): C 51.33, H 2.40, N 18.23.
3-(2-furanyl)-6-(2-hydroxyphenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2i): Yield 49 %; M.p. 273-275°C; IR (KBr) ν/cm-1: 3423 (O-H), 3126, 3070 (Ar-H), 1602 (C=N), 1519, 1477, 1460, 1425 (aromatic skeleton), 1254 (N-N=C), 1224 (furan C-O-C), 699 (C-S-C); 1H-NMR δ: 11.70 (1H, s, OH), 8.19, 6.79, 7.39 (3H, furan H), 7.01-8.00 (4H, m, Ar-H); 13C-NMR δ: 163.44, 156.50, 155.16, 145.23, 140.49, 138.72, 127.69, 120.43, 117.03, 115.55, 112.23, 112.07, 111.53; MS (m/z, %): 284 (M+, 93.85), 137 (100), 119 (15.97), 109 (94.61), 93 (61.71), 77 (14.55), 64 (89.09), 51 (34.40), 39 (44.46); Calcd. for C13H8N4O2S (%): C 54.92, H 2.84, N 19.71; Found (%): C 54.68, H 2.91, N 19.98.
3,6-di(2-furanyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2j): Yield 55 %; M.p. 204-206°C; IR (KBr) ν/cm-1: 3120, 3092 (Ar-H), 1623 (C=N), 1593, 1520, 1508, 1481, 1445, 1429 (aromatic skeleton), 1254 (N-N=C), 1224 (furan C-O-C), 990, 960, 904, 886, 818, 754 (Ar-H bending vibration), 699 (C-S-C). 1H-NMR δ: 8.12, 8.01, 7.58, 7.28, 6.85, 6.79 (6H, m, furan H); 13C-NMR δ: 157.05, 153.03, 147.94, 145.50, 143.07, 140.12, 139.45, 115.38, 113.54, 112.24, 111.78; Calcd. for C11H6N4O2S (%): C 51.15, H 2.34, N 21.70; Found (%): C 51.26, H 2.44, N 21.51.
3-(2-furanyl)-6-phenyloxymethyl-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2k): Yield 62 %; M.p.115-117°C; IR (KBr) ν/cm-1: 3115, 3070 (Ar-H), 1624 (C=N), 1589, 1519, 1496, 1475, 1452, 1427 (aromatic skeleton), 1243 (N-N=C), 692 (C-S-C); 1H-NMR δ: 8.00, 6.77, 7.37 (3H, furan H), 7.00-7.34 (5H, m, Ar-H), 5.59 (2H, s, OCH2); MS (m/z, %): 298 (M+, 46.92), 205 (30.84), 109 (24.13), 84 (100), 77 (27.61), 65 (66.98), 57 (72.70), 51 (25.10), 39 (51.15); Calcd. for C14H10N4O2S (%): C 56.36, H 3.38, N 18.79; Found (%): C 56.09, H 3.36, N 19.04.
3-(2-furanyl)-6-(2-methoxyphenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2l): Yield 58 %; M.p. 162-164°C; IR (KBr) ν/cm-1: 3111, 3012 (Ar-H), 1622 (C=N), 1584, 1506, 1462, 1435, 1422 (aromatic skeleton), 1255 (N-N=C), 1223 (furan C-O-C), 699 (C-S-C); 1H-NMR δ: 8.01, 6.80, 7.40 (3H, furan H), 7.21-8.33 (4H, m, Ar-H), 4.04 (3H, s, OCH3); MS (m/z, %): 298 (M+, 100), 149 (33.42), 109 (84.92), 93 (59.71), 77 (23.86), 64 (68.84), 51 (35.38), 39 (48.20); Calcd. for C14H10N4O2S (%): C 56.36, H 3.38, N 18.79; Found (%): C 56.10, H 3.41, N 18.51.
3-(2-furanyl)-6-phenyl-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2m): Yield 53 %; M.p. 203-205°C; IR (KBr) ν/cm-1: 3131, 3078, 3024 (Ar-H), 1624 (C=N), 1518, 1469, 1444, 1426 (aromatic skeleton), 1240 (N-N=C), 1224 (furan C-O-C), 691 (C-S-C); 1H-NMR δ: 8.07, 6.78, 7.40 (3H, furan H), 7.65-8.04 (5H, m, Ar-H); MS (m/z, %): 268 (M+, 100), 137 (15.84), 121 (88.23), 109 (77.76), 93 (52.23), 51 (41.26), 39 (26.39); Calcd. for C13H8N4OS (%): C 58.19, H 3.00, N 20.89; Found (%): C 58.44, H 2.98, N 20.67.
3-(2-furanyl)-6-(2,4-dichlorophenoxymethyl ) -1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2n): Yield 51 %; M.p.193-195°C; IR (KBr) ν/cm-1: 3096, 3030 (Ar-H), 1625 (C=N), 1586, 1520, 1481, 1428 (aromatic skeleton), 1249 (N-N=C), 1224 (furan C-O-C), 687 (C-S-C); 1H-NMR δ: 8.01, 6.78, 7.46 (3H, furan H), 7.20-7.66 (3H, m, Ar-H), 5.71 (2H, s, OCH2); MS (m/z, %): 370 (M++4, 1.82), 368 (M++2, 8.86), 366 (M+, 12.83), 205 (58.79), 133 (12.64), 109 (22.09), 94 (45.70), 84 (100), 75 (6.40), 64 (19.66), 39 (9.28); Calcd. for C14H8Cl2N4O2S (%): C 45.79, H 2.19, N 15.26; Found (%): C 45.83, H 2.17, N 15.50.
3-(2-furanyl)-6-(2-quinolyl ) -1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2o):Yield 48 %; M.p. 267-269°C; IR (KBr) ν/cm-1: 3128, 3069 (Ar-H), 1622 (C=N), 1585, 1561, 1519, 1474, 1445, 1427 (aromatic skeleton), 1288 (N-N=C), 1225 (furan C-O-C), 695 (C-S-C); 1H-NMR δ: 7.98, 6.71, 7.54 (3H, furan H), 6.22-9.05 (6H, m, quinoline H); Calcd. for C16H9 N5OS (%): C 60.17, H 2.84, N 21.94; Found (%): C 60.43, H 2.88, N 21.81.
3-(2-furanyl)-6-(4-nitrophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2p): Yield 59 %; M.p. 304-306°C; IR (KBr) ν/cm-1: 3099, 3061 (Ar-H), 1622 (C=N), 1527, 1486, 1469, 1428 (aromatic skeleton), 1283 (N-N=C), 1228 (furan C-O-C), 688 (C-S-C); 1H-NMR δ: 8.05, 6.83, 7.39 (3H, furan H), 8.34-8.43 (4H, dd, J=9.0Hz, Ar-H); MS (m/z, %): 313 (M+, 78.32), 166 (29.75), 137 (19.97), 120 (34.58), 109 (100), 93 (71.97), 76 (22.26), 67 (14.30), 64 (72.07), 57 (39.21); Calcd. for C13H7 N5O3S (%): C 49.83, H 2.25, N 22.36; Found(%): C 50.10, H 2.27, N 22.12.
3-(2-furanyl)-6-(2-aminophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2q): Yield 46 %; M.p. 144-146°C; R (KBr) ν/cm-1: 3448, 3346 (N-H), 3123, 3030 (Ar-H), 1621 (C=N), 1563, 1510, 1475, 1440, 1424 (aromatic skeleton), 1273 (N-N=C), 1232 (furan C-O-C), 697 (C-S-C); 1H-NMR δ: 8.01, 6.79, 7.50 (3H, furan H), 6.81-7.27 (4H, m, Ar-H), 6.70 (2H, s, NH2 ); 13C-NMR δ: 168.01, 152.43, 147.50, 145.45, 140.34, 139.49, 133.41, 129.53, 127.08, 116.52, 112.29, 111.53, 109.95; MS (m/z, %): 283(M+, 100), 136 (91.75), 118 (64.48), 109 (50.94), 93 (43.32), 91 (37.30), 77 (16.05), 64 (71.60), 51 (31.91), 39 (34.01); Calcd. for C13H9N5OS (%): C 55.11, H 3.20, N 24.73; Found (%): C 54.85, H 3.22, N 24.96.
3-(2-furanyl)-6-(4-methoxyphenyl )-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (2r): Yield 61 %; M.p. 177-179°C; IR (KBr) ν/cm-1: 3102, 3003 (Ar-H), 1607 (C=N), 1576, 1518, 1496, 1473, 1420 (aromatic skeleton), 1264 (N-N=C), 1225 (furan C-O-C), 702 (C-S-C); 1H-NMR δ: 8.01, 6.79, 7.38 (3H, furan H), 7.15-8.00 (4H, dd, J=9.0Hz, Ar-H), 3.86 (3H, s, OCH3); 13C-NMR δ: 167.05, 163.07, 153.40, 145.44, 140.22, 139.34, 129.27, 121.31, 115.21,112.28, 111.86, 55.85; MS (m/z, %): 298 (M+,100), 177 (8.45), 151 (98.97), 137 (28.18), 133 (21.33), 93 (35.40); Calcd. for C14H10N4O2S (%): C 56.36, H 3.38, N 18.79; Found (%): C 56.64, H 3.48, N 18.57.