Synthesis of Novel Quinazoline Derivatives via Pyrimidine ortho-Quinodimethane
Abstract
:Introduction
Results and Discussion
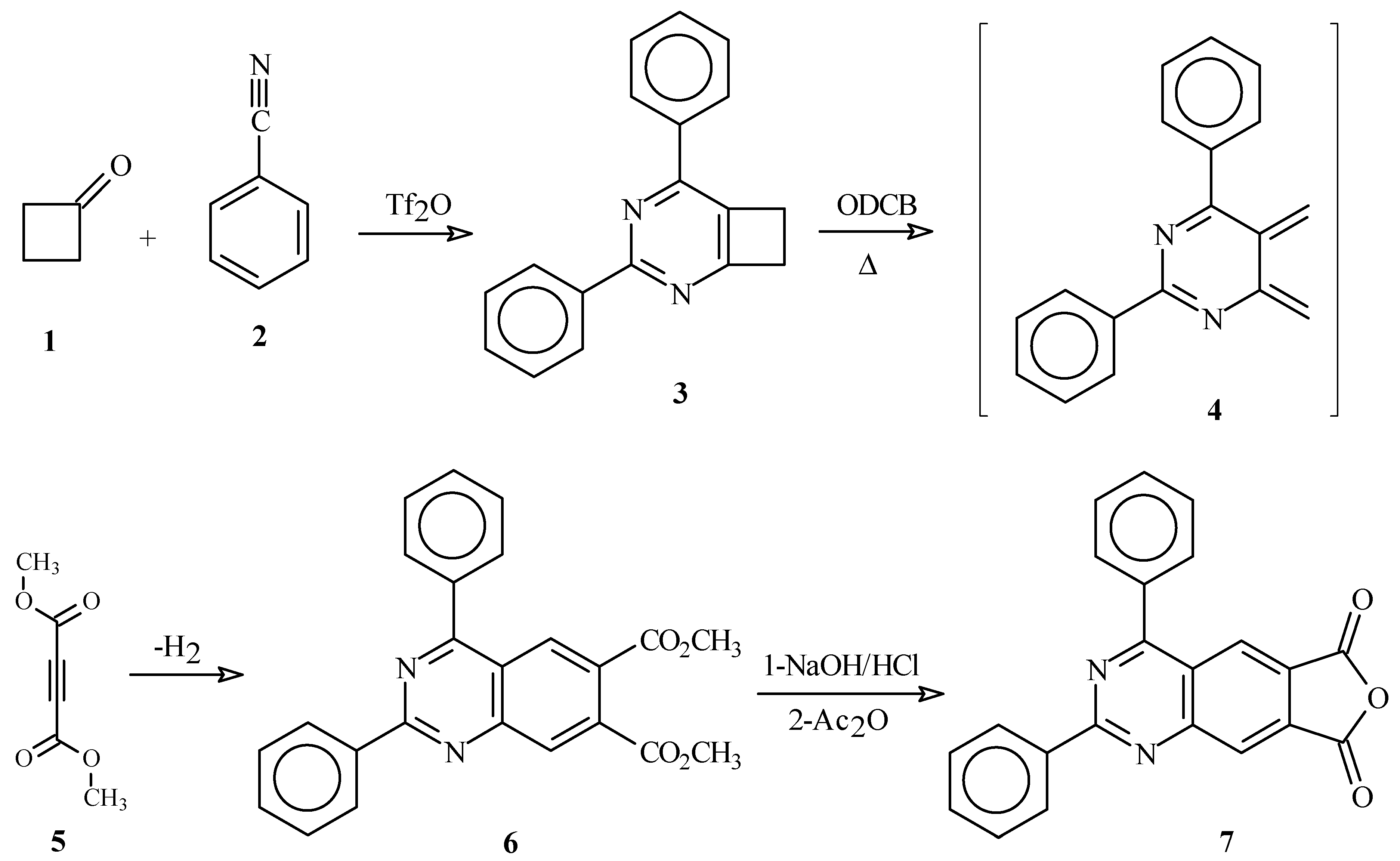
Conclusions
Acknowledgements
Experimental
General
Synthesis of dimethyl 2,4-diphenylquinazoline-6,7- dicarboxylate (6).
Synthesis of 2,4-diphenylfuro[3,4-g]quinazoline-6,8-dione (7).
References
- Szarics, E; Nyikos, L; Barabas, P; Kovacs, I; Skuban, N; Temesvarine-Major, E; Egyed, O; Nagy, P.I.; Kokosi, J; Takacs-Novak, K; Kardos, J. Mol. Pharmacol. 2001, 59, 920–928. [PubMed]
- Sielecki, T.M.; Johnson, T.L.; Liu, J; Muckelbauer, J.K.; Grafstrom, R.H.; Cox, S.; Boylan, J.; Burton, C.R.; Chen, H.Y.; Smallwood, A.; Chang, C.H.; Boisclair, M.; Benfield, P.A.; Trainor, G.L.; Seitz, S.P. Bioorg. Med. Chem. Lett. 2001, 11, 1157–1160. [PubMed]
- Hanusek, J; Hejtmankova, L; Kubicova, L; Sedlak, M. Molecules 2001, 6, 323–337.
- Shibuya, I; Gama, Y; Shimizu, M. Heterocycles 2001, 55, 381–386.
- Herrera, A; Martinez, R; Gonzalez, B; Illescas, B; Martin, N; Seoane, C. Tetrahedron Lett. 1997, 38, 4873–4876.
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Chioua, R.; Benabdelouahab, F.; Chioua, M.; Martínez-Alvarez, R.; Fernández, A.H. Synthesis of Novel Quinazoline Derivatives via Pyrimidine ortho-Quinodimethane. Molecules 2002, 7, 507-510. https://doi.org/10.3390/70700507
Chioua R, Benabdelouahab F, Chioua M, Martínez-Alvarez R, Fernández AH. Synthesis of Novel Quinazoline Derivatives via Pyrimidine ortho-Quinodimethane. Molecules. 2002; 7(7):507-510. https://doi.org/10.3390/70700507
Chicago/Turabian StyleChioua, R., F. Benabdelouahab, M. Chioua, R. Martínez-Alvarez, and A. Herrera Fernández. 2002. "Synthesis of Novel Quinazoline Derivatives via Pyrimidine ortho-Quinodimethane" Molecules 7, no. 7: 507-510. https://doi.org/10.3390/70700507
APA StyleChioua, R., Benabdelouahab, F., Chioua, M., Martínez-Alvarez, R., & Fernández, A. H. (2002). Synthesis of Novel Quinazoline Derivatives via Pyrimidine ortho-Quinodimethane. Molecules, 7(7), 507-510. https://doi.org/10.3390/70700507



