Synthesis, Crystal Structure, and Conformation of Methyl 5-Oacetyl-5-cyano-6-deoxy-2,3-O-isopropylidene-β-L-gulofuranoside
Abstract
:Introduction
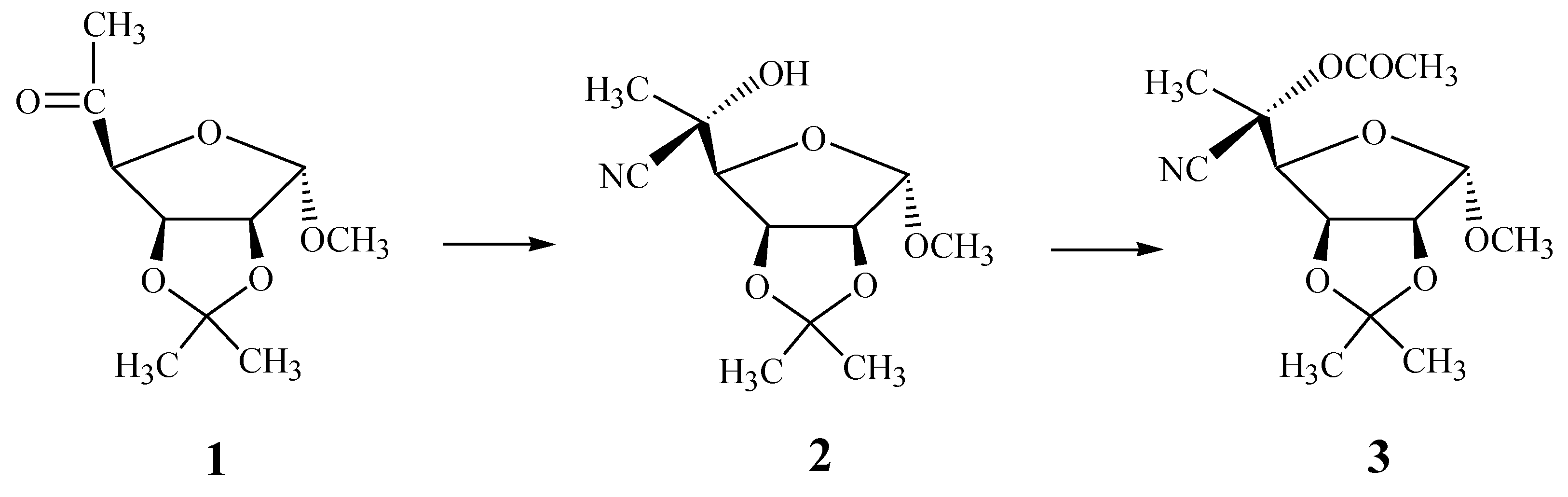
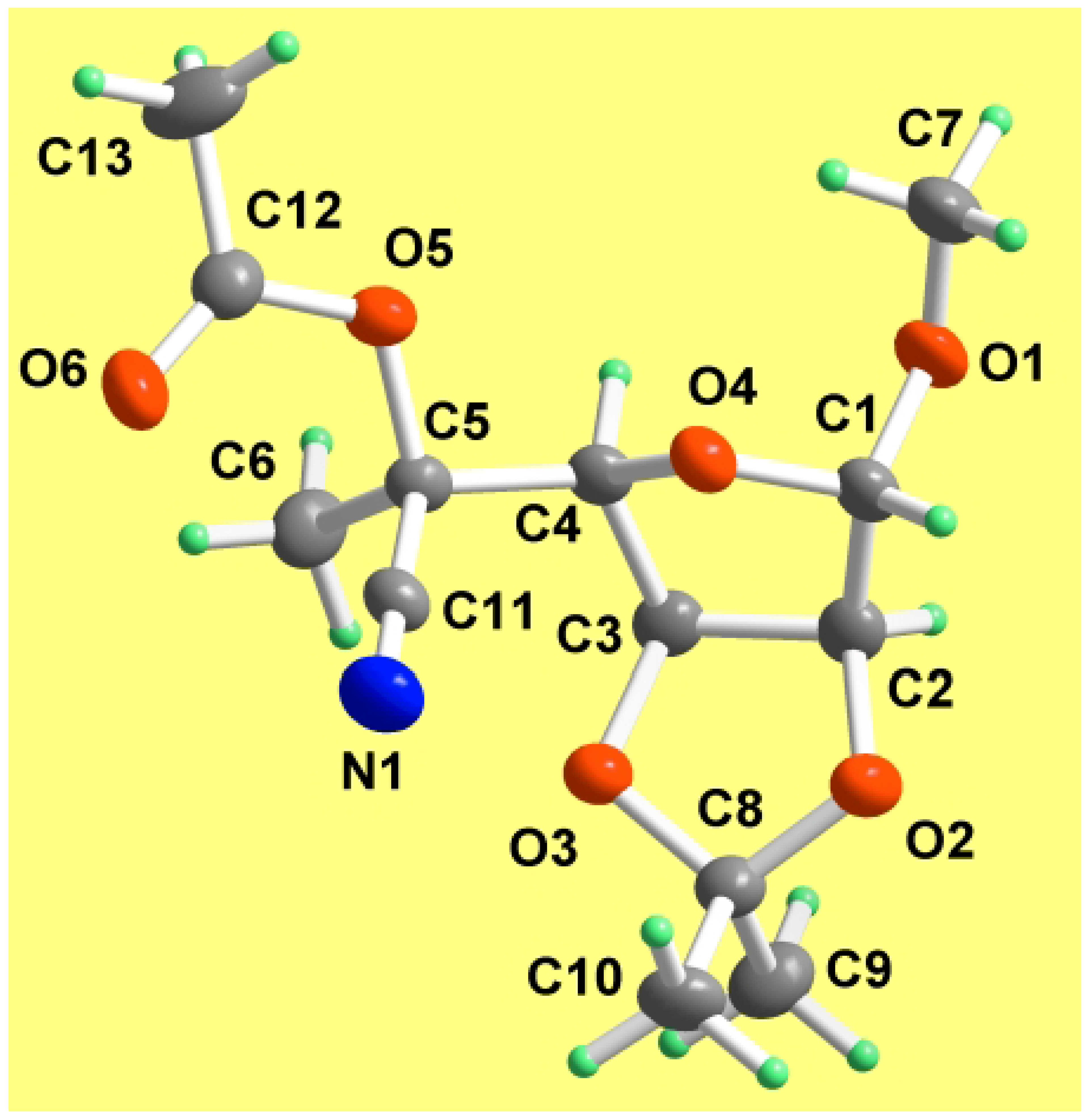
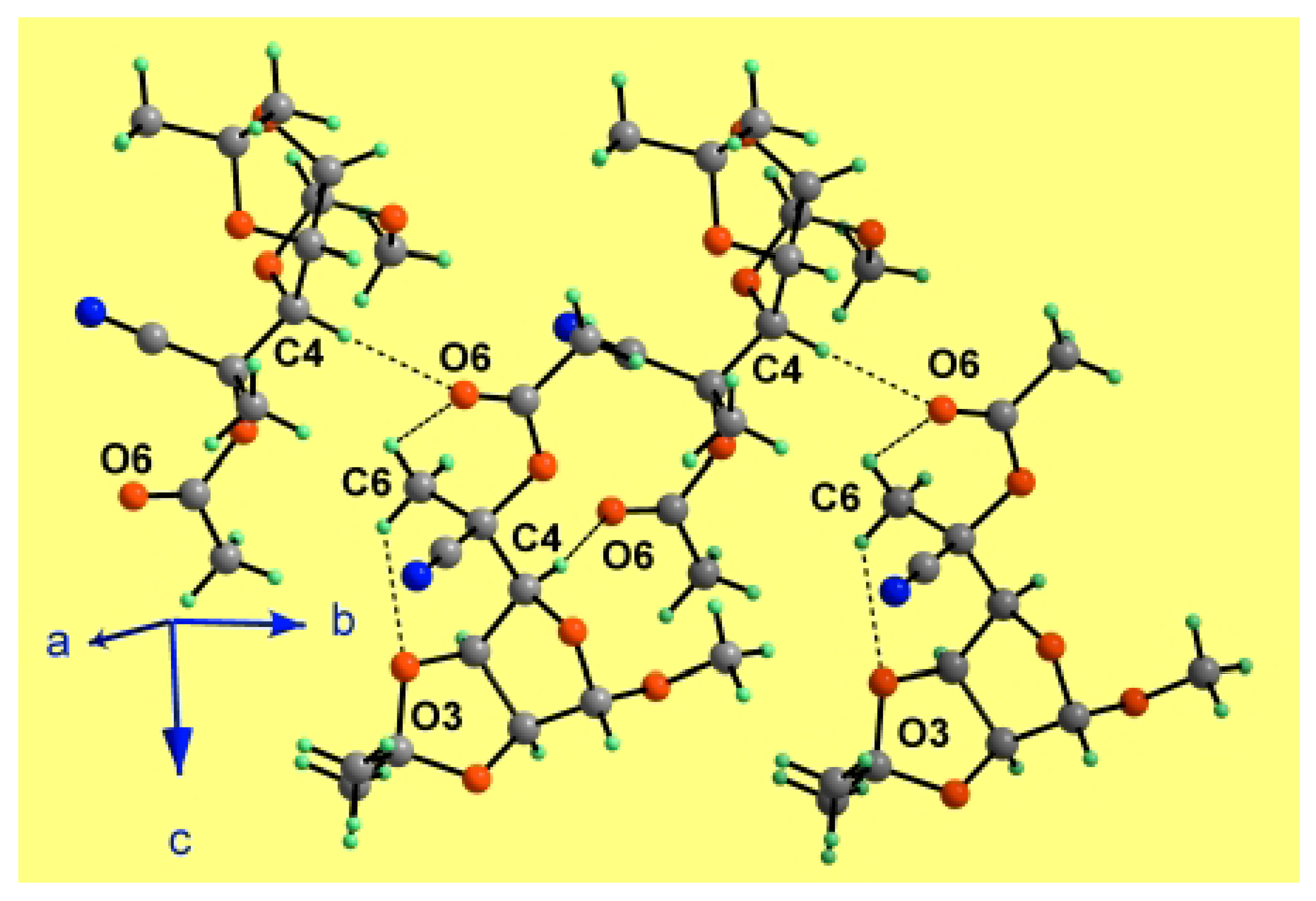
Results and Discussion
Experimental
General
X-ray techniques
Synthetic methods
Acknowledgments
References and Notes
- Steiner, B.; Koos, M.; Langer, V.; Gyepesova, D.; Smrcok, L. 4-Amino-4-cyano-4,6-dideoxy sugar derivatives from methyl 6-deoxy-2,3-O-isopropylidene-α-l-lyxo-hexopyranosid-4-ulose via Strecker-type reaction. Carbohydr. Res. 1998, 311, 1–9. [Google Scholar] [CrossRef]
- Koos, M.; Steiner, B.; Gajdos, J.; Langer, V.; Gyepesova, D.; Smrcok, L.; Durik, M. Crystal Structure of Methyl 4-acetamido-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-β-d-allopyranoside. Molecules 2000, 5, 219–226. [Google Scholar] [CrossRef]
- Koos, M.; Steiner, B.; Langer, V.; Gyepesova, D.; Durik, M. Preparation and structure determination of two sugar amino acids via corresponding hydantoin derivatives. Carbohydr. Res. 2000, 328, 115–126. [Google Scholar] [CrossRef]
- Koos, M.; Steiner, B.; Micova, J.; Langer, V.; Durik, M.; Gyepesova, D.; Smrcok, L. Crystal Structure of Methyl 4-amino-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-α-l-talopyranoside. Molecules 2000, 5, 1113–1120. [Google Scholar] [CrossRef]
- Koos, M.; Steiner, B.; Micova, J.; Langer, V.; Durik, M.; Gyepesova, D. Synthesis and structure determination of some sugar amino acids related to alanine and 6-deoxymannojirimycin. Carbohydr. Res. 2001, 332, 351–361. [Google Scholar] [CrossRef]
- Gourlain, T.; Wadouachi, A.; Beaupere, D. A new 6-C-alkylation from an alkyl mannofuranoside 5,6-cyclic sulfate. Carbohydr. Res. 2000, 324, 66–73. [Google Scholar] [CrossRef]
- Krajewski, J. W.; Gluzinski, P.; Pakulski, Z.; Zamojski, A.; Mishnev, A.; Kemme, A. Synthesis, crystal structure, and conformation of methyl 6-deoxy-2,3-O-isopropylidene-α-d-manno-heptofuranoside. Carbohydr. Res. 1994, 252, 97–105. [Google Scholar]
- Barton, D. H. R.; Gero, S. D.; Quiclet-Sire, B.; Samadi, M. Stereocontrolled Radical Reactions in Carbohydrate and Nucleoside Chemistry. Tetrahedron: Asymmetry 1994, 5, 2123–2136. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, L.-N. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem., Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J. A. A General Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Patoprsty, V.; Kovacik, V.; Karacsonyi, S. Enhancement of Methylation Analysis of Complex Carbohydrates Using Pyridine as Reagent in Chemical Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 1995, 9, 840–844. [Google Scholar] [CrossRef]
- Bruker AXS Inc. SHELXTL Version 5.10. Madison, WI, USA, 1997. [Google Scholar]
- Brandenburg, K. DIAMOND: Visual Crystal Structure Information System, Version 2.1d. Crystal Impact GbR, Bonn, Germany, 2000. [Google Scholar]
- Samples Availability: The product 2 reported in this paper is available from MDPI.
| Empirical formula | C13H19NO6 |
| Formula weight | 285.29 |
| Temperature, T (K) | 193(2) |
| Wavelength, λ (Å) | 0.71073 |
| Crystal system | orthorhombic |
| Space group | P212121 |
| Unit cell dimensions (Å) | a = 6.0926(2) |
| b = 9.6035(3) | |
| c = 25.5321(7) | |
| Unit cell volume, V (Å3) | 1493.89(8) |
| Formula units per unit cell, Z | 4 |
| Dcalcd (g/cm3) | 1.268 |
| Absorption coefficient, µ (mm-1) | 0.101 |
| F(000) | 608 |
| Crystal size (mm) | 0.80 (max) 0.35 (min) |
| Diffractometer | Siemens SMART CCD |
| θ Range (°) | 2.27–32.79 |
| Range of h | – 9→9 |
| Range of k | – 14→14 |
| Range of l | – 38→38 |
| Reflections | 23913 |
| Independent reflections | 5203 (Rint = 0.0365) |
| Completeness to θ = 32.79 (%) | 96.1 |
| Max. and min. transmission | 0.9656 and 0.9238 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 5203 / 0 / 205 |
| Goodness-of-fit (all) | 1.063 |
| Final R indices [I >2σ(I)] | R1 = 0.0454, wR2 = 0.1126 |
| R indices (all data) | R1 = 0.0581, wR2 = 0.1212 |
| Largest difference peak and hole (e/Å3) | 0.277 and –0.193 |
| O1-C1 | 1.4160(16) | O1-C1-C2 | 106.88(13) |
| O1-C7 | 1.440(3) | O4-C1-C2 | 105.44(11) |
| O2-C2 | 1.4336(17) | O2-C2-C1 | 109.70(12) |
| O2-C8 | 1.4438(17) | O2-C2-C3 | 104.54(11) |
| O3-C3 | 1.4330(16) | C1-C2-C3 | 104.34(11) |
| O3-C8 | 1.4367(16) | O3-C3-C4 | 110.70(10) |
| O4-C1 | 1.4295(18) | O3-C3-C2 | 104.40(10) |
| O4-C4 | 1.4468(17) | C4-C3-C2 | 103.10(11) |
| O5-C12 | 1.3721(18) | O4-C4-C5 | 109.71(10) |
| O5-C5 | 1.4659(16) | O4-C4-C3 | 104.95(10) |
| O6-C12 | 1.209(2) | C5-C4-C3 | 116.78(11) |
| N1-C11 | 1.1476(19) | O5-C5-C11 | 110.31(11) |
| C1-C2 | 1.532(2) | O5-C5-C4 | 103.84(10) |
| C2-C3 | 1.560(2) | C11-C5-C4 | 110.95(10) |
| C3-C4 | 1.5411(19) | O5-C5-C6 | 109.02(11) |
| C4-C5 | 1.5349(18) | C11-C5-C6 | 111.87(11) |
| C5-C11 | 1.4961(18) | C4-C5-C6 | 110.54(12) |
| C5-C6 | 1.5375(19) | O3-C8-O2 | 104.43(10) |
| C8-C10 | 1.521(2) | O3-C8-C10 | 108.19(12) |
| C8-C9 | 1.526(2) | O2-C8-C10 | 108.76(12) |
| C12-C13 | 1.501(2) | O3-C8-C9 | 110.44(11) |
| C1-O1-C7 | 112.24(15) | O2-C8-C9 | 111.40(13) |
| C2-O2-C8 | 107.64(10) | C10-C8-C9 | 113.21(13) |
| C3-O3-C8 | 107.66(10) | N1-C11-C5 | 177.77(15) |
| C1-O4-C4 | 105.49(10) | O6-C12-O5 | 123.20(13) |
| C1-O5-C5 | 118.74(11) | O6-C12-C13 | 125.90(15) |
| O1-C1-O4 | 111.53(11) | O5-C12-C13 | 110.90(14) |
| C7-O1-C1-C2 | 177.05(12) | C1-O4-C4-C5 | 167.05(10) |
| C4-O4-C1-O1 | 76.03(14) | C1-O4-C4-C3 | 40.82(12) |
| C4-O4-C1-C2 | –39.62(12) | O3-C3-C4-O4 | 86.09(12) |
| C8-O2-C2-C1 | 130.48(12) | C2-C3-C4-O4 | –25.06(12) |
| C8-O2-C2-C3 | 19.09(14) | O3-C3-C4-C5 | –35.62(15) |
| O1-C1-C2-O2 | 152.07(11) | C2-C3-C4-C5 | –146.77(11) |
| O4-C1-C2-O2 | –89.14(13) | O4-C4-C5-O5 | 72.73(12) |
| O1-C1-C2-C3 | –96.41(12) | C3-C4-C5-O5 | –168.08(10) |
| O4-C1-C2-C3 | 22.38(13) | O4-C4-C5-C11 | –45.77(14) |
| C8-O3-C3-C4 | –131.55(11) | C3-C4-C5-C11 | 73.42(14) |
| C8-O3-C3-C2 | –21.24(14) | O4-C4-C5-C6 | –170.48(11) |
| O2-C2-C3-O3 | 1.24(14) | C3-C4-C5-C6 | –51.29(15) |
| C1-C2-C3-O3 | –113.97(11) | C3-O3-C8-O2 | 33.46(14) |
| O2-C2-C3-C4 | 116.99(11) | C2-O2-C8-O3 | –32.57(14) |
| C1-C2-C3-C4 | 1.79(13) | C4-C5-C11-N1 | –64(4) |
| Atom | x | y | z | U(eq) |
| O1 | 4992(2) | 9235(1) | 9060(1) | 46(1) |
| O2 | 6664(2) | 6043(1) | 9659(1) | 38(1) |
| O3 | 7554(2) | 5008(1) | 8886(1) | 33(1) |
| O4 | 3751(2) | 7059(1) | 8757(1) | 33(1) |
| O5 | 3436(2) | 6486(1) | 7648(1) | 35(1) |
| O6 | 2411(2) | 4514(1) | 7222(1) | 47(1) |
| N1 | 3034(2) | 3574(1) | 8438(1) | 44(1) |
| C1 | 4630(3) | 7820(1) | 9191(1) | 35(1) |
| C2 | 6885(2) | 7175(1) | 9297(1) | 35(1) |
| C3 | 7528(2) | 6467(1) | 8769(1) | 32(1) |
| C4 | 5584(2) | 6829(1) | 8408(1) | 30(1) |
| C5 | 4956(2) | 5741(1) | 7994(1) | 29(1) |
| C6 | 6985(2) | 5298(2) | 7676(1) | 40(1) |
| C7 | 2989(4) | 9944(2) | 8924(1) | 60(1) |
| C8 | 7812(2) | 4861(1) | 9442(1) | 32(1) |
| C9 | 10240(3) | 4881(2) | 9590(1) | 44(1) |
| C10 | 6643(3) | 3536(2) | 9612(1) | 44(1) |
| C11 | 3833(2) | 4520(1) | 8240(1) | 31(1) |
| C12 | 2457(2) | 5772(2) | 7246(1) | 36(1) |
| C13 | 1479(3) | 6757(2) | 6853(1) | 55(1) |
| X-H...Y | Symmetry code | X-H (Å) | H...Y (Å) | X...Y (Å) | X-H...Y (°) |
| C4-H4...O6 | –x+1, y+1/2, –z+3/2 | 1.00 | 2.30 | 3.2752(17) | 164.9 |
| C6-H6A...O6 | 0.98 | 2.59 | 3.111(2) | 113.6 | |
| C6-H6B...O3 | 0.98 | 2.50 | 3.1202(18) | 120.7 |
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mičová, J.; Steiner, B.; Koóš, M.; Gajdoš, J.; Langer, V.; Gyepesová, D. Synthesis, Crystal Structure, and Conformation of Methyl 5-Oacetyl-5-cyano-6-deoxy-2,3-O-isopropylidene-β-L-gulofuranoside. Molecules 2002, 7, 437-446. https://doi.org/10.3390/70500437
Mičová J, Steiner B, Koóš M, Gajdoš J, Langer V, Gyepesová D. Synthesis, Crystal Structure, and Conformation of Methyl 5-Oacetyl-5-cyano-6-deoxy-2,3-O-isopropylidene-β-L-gulofuranoside. Molecules. 2002; 7(5):437-446. https://doi.org/10.3390/70500437
Chicago/Turabian StyleMičová, Júlia, Bohumil Steiner, Miroslav Koóš, Ján Gajdoš, Vratislav Langer, and Dalma Gyepesová. 2002. "Synthesis, Crystal Structure, and Conformation of Methyl 5-Oacetyl-5-cyano-6-deoxy-2,3-O-isopropylidene-β-L-gulofuranoside" Molecules 7, no. 5: 437-446. https://doi.org/10.3390/70500437
APA StyleMičová, J., Steiner, B., Koóš, M., Gajdoš, J., Langer, V., & Gyepesová, D. (2002). Synthesis, Crystal Structure, and Conformation of Methyl 5-Oacetyl-5-cyano-6-deoxy-2,3-O-isopropylidene-β-L-gulofuranoside. Molecules, 7(5), 437-446. https://doi.org/10.3390/70500437




