General
Melting points were determined on a Reichert hot stage microscope and are uncorrected. IR spectra were measured with a Nicolet Magna 520 instrument, using potassium bromide and results are given in cm-1. 1H-, 13C NMR and 2D H-H, C-H, Cosy NMR spectra were recorded in DMSO-d6 on either a JEOL JNM-GX270, (1H-NMR at 270 MHz), (13C-NMR at 52.89MHz) or a Varian Gemini (1H-NMR at 200 MHz), (13C-NMR at 90.56 MHz ) spectrometer. The chemical shifts are reported in part per million (ppm) downfield from internal tetramethylsilane. Electron impact MS spectra were obtained on a JEOL JMS-HX 100 at 70 eV. Elemental microanalysis were done on a CARLO Erba analyzer model 110. The progress of all reactions was monitored by TLC on 2.0 cm x 6.0 cm aluminium sheets precoated with silica gel 60 containing a fluorescent indicator (Alugram SIL G/UV254 fur die DC Macherey-Nagel/ Germany), to a thickness of 0.25 mm. The developed chromatograms were viewed under ultraviolet light. Column chromatography was performed on [Merck] silica gel (70-230 mesh) (elution with 1:1 cyclohexane-ethyl acetate). The X-ray structure was determined using a NONIUS Kappa Diffractomer. Suitable crystals were grown by slow crystallization from ethanol.
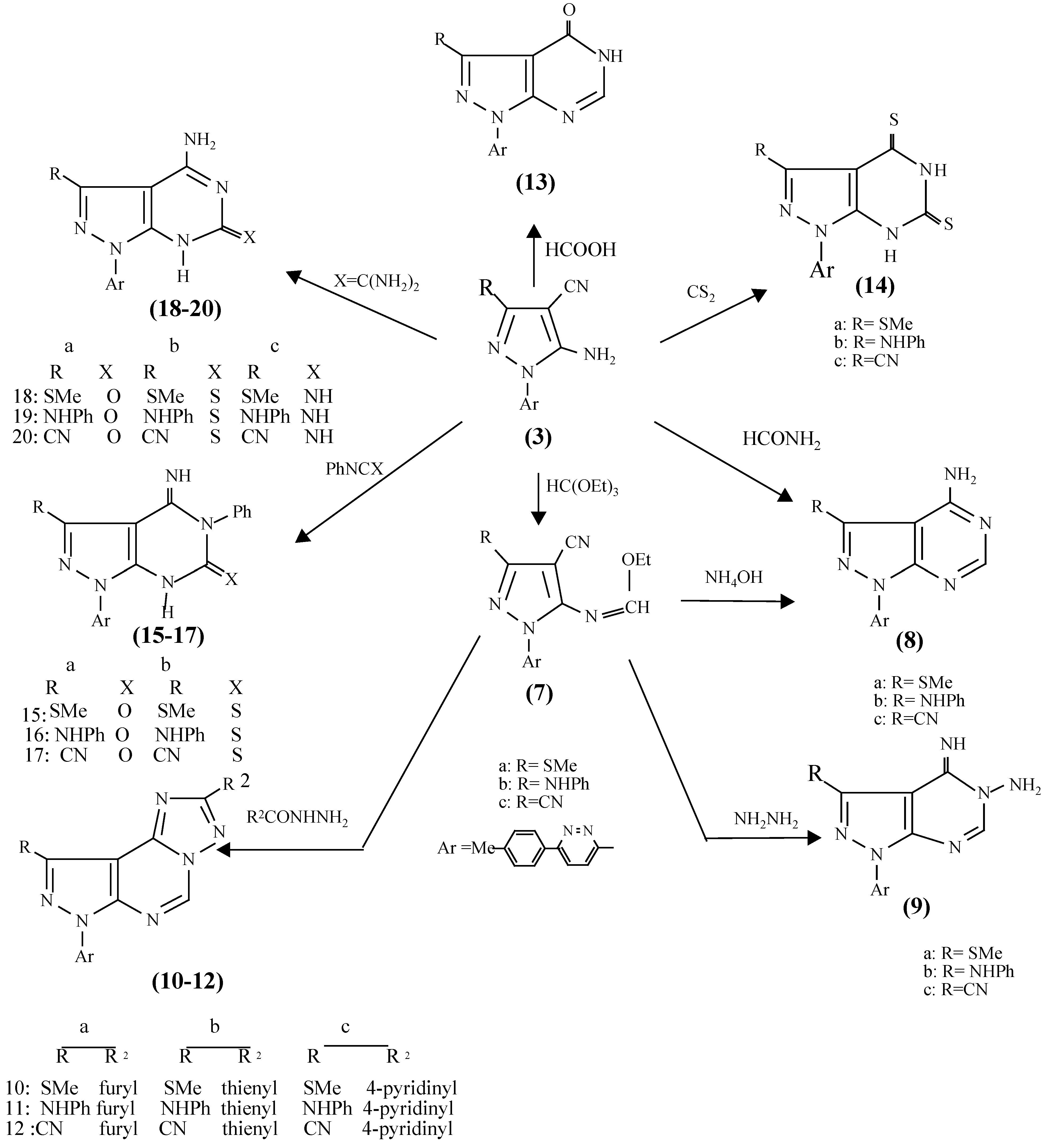
General Procedure for the Preparation of 5-Amino-3-substituted-1-[6-(p-tolyl) pyridazin-3-yl]-pyrazole -4-carbonitriles (3a-c).
To a cold solution of hydrazine 2 (2.0g , 10 mmol) in methanol (100 mL) was added 1 (10 mmol). The reaction mixtures were then stirred at room temperature for 3-6h, left overnight, then the solvent was evaporated and the residue recrystallized from a suitable solvent.
5-Amino-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazole-4-carbonitrile (3a).
Yield: 89 %; colorless needle-like crystals; mp 259-260 °C (from EtOH); IR: ν max cm-1 3379, 3279 (NH2); 2210 (CN); 1H-NMR: δ: 2.41 (s, 3H, CH3-S), 2.60 (s, 3H, p-CH3-tolyl), 7.02 (bs, 2H, NH2, D2O exchangeable), 7.31 (d, 2H, H3, H5 – tolyl, J = 8.0 Hz), 7.90 (d, 2H, H2, H6- tolyl, J = 8.0 Hz), 8.13 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.36 (d, 1H, H4-pyridazine, J = 9.1 Hz); 13C-NMR: δ 15 (CH3-S), 20 (p-CH3-tolyl), 79 (CN), 134 (C-CN), 140 (C-SCH3), 113, 127, 129, 153 (C4,C3,C2 and C1 of tolyl); 118, 126, 154, 156 (pyridazine C5, C4, C6 and C3); 157 (C-NH2); MS m/z (%): M+ 322 (100), 275 (77), 250 (69), 115 (54), 78 (7.7); Anal. Calc. for C16H14N6S (322.39): C 59.61, H 4.38, N 26.07, S 9.94; Found: C 59.25, H 4.26, N 26.10, S 9.95.
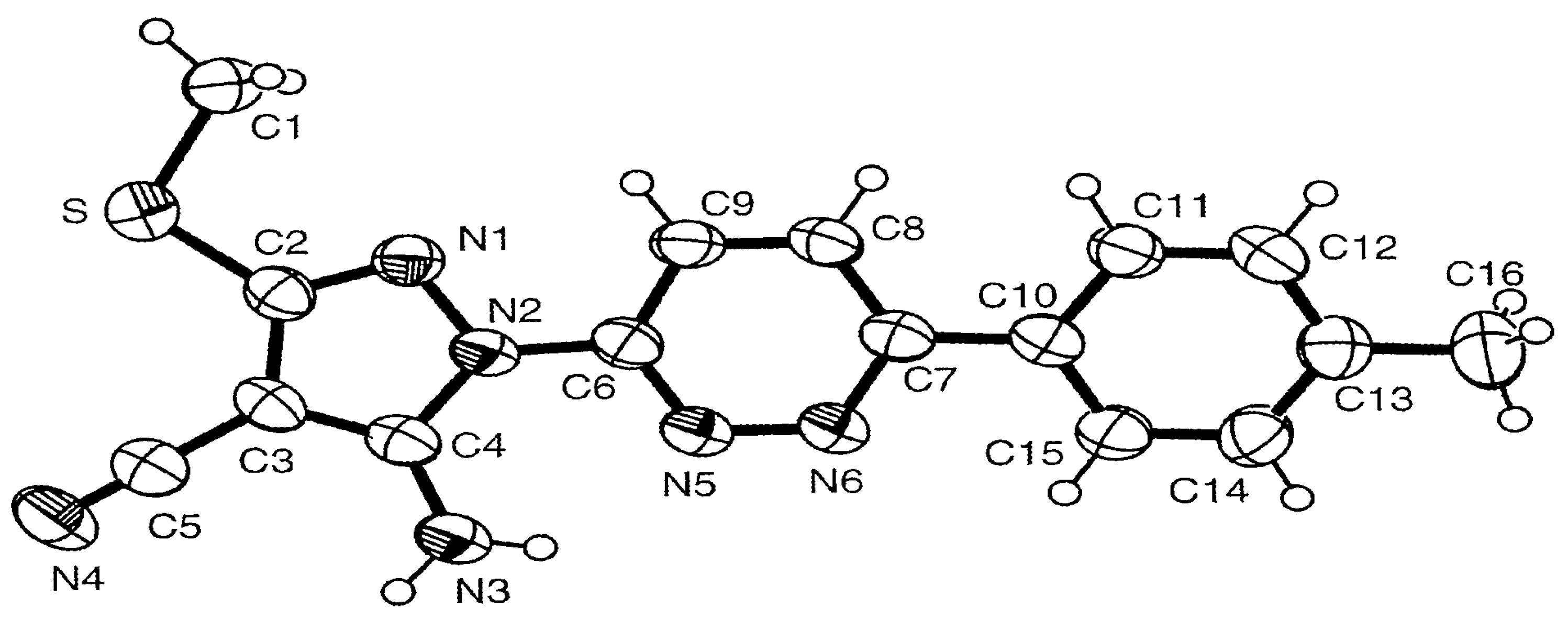
Single-Crystal X-ray Structure Analysis of 3a: Crystallographic Data and Refinement.
Crystal Orthorhombic, space group P212121, a= 4.97330(10)Å, b= 11.1882(3)Å, c= 28.0997(8) Å.
CELL 4.97330 11.18820 28.09970 90.0000 90.0000 90.0000.
ZERR 4 0.00010 0.00029 0.00080 0.000 0.000 0.000.
LATT –1.
SYMM 0.5-X, -Y, 0.5+Z. SYMM 0.5+X, 0.5-Y, -Z. SYMM –X, 0.5+Y, 0.5-Z.
Volume 1563.53(7) Å3 .
Z, calculated density 4, 1.370 /m3.
Absorption coefficient 0.215 mm-1.
F(000) 672.
Theta range for data collection 1.96 to 27.49 deg.
Limiting indices –6<=h<=6, -14<=k<=14, -35<=1<=35.
Reflections collected/unique 3468/3468 [R(int) = 0.0000].
Refinement method full-matrix least-squares on F2.
Goodness-of-fit on F2 0.978.
Final R indices [I>2sigma(I)] R1 = 0.0498, wR2 = 0.1134.
R indices (all data) R1 = 0.1182, wR2 = 0.1374.
5-Amino-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazole-4-carbonitrile (3b).
Yield: 70 %; white solid, mp 297 °C (from EtOH); IR: ν max cm-1 3461-3311 (NH2/NH); 2213 (CN); 1H-NMR δ: 2.45 (s, 3H, p-CH3-tolyl), 6.82 (bs, 2H, NH2, D2O exchangeable), 7.18 (t, 1H, H4-Ph, J = 7.4 Hz), 7.34 (t, 2H, H3, H5-Ph, J = 7.6 Hz), 8.21 (d, 2H, H2, H6-Ph, J = 7.9 Hz), 7.61 (d, 2H, H3, H5- tolyl, J = 8.0 Hz), 8.04 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.26 (d, 1H, H5–pyridazine, J = 9.1 Hz ), 8.42 (d, 1H, H4-pyridazine, J = 9.1 Hz), 9.22 (bs, 1H, NH, D2O exchangeable); MS: m/z (%); M+ 367 (75), 270 (100 ), 115 (42), 78 (8.1); Anal. Calc. for C21H17N7 (367.41): C 68.65, H 4.66, N 26.69; Found: C 68.49, H 4.68, N 26.70.
5-Amino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazole-3,4-dicarbonitrile (3c).
Yield: 86 %; pale brown solid; mp 289-292°C (from n-BuOH); IR: ν max cm-1 3369, 3282 (NH2); 2239, 3231(2CN); 1H-NMR δ: 2.43 (s, 3H, p-CH3-tolyl), 7.42 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 8.04 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.17 (d, 2H, H5-pyridazine, J = 9.1 Hz), 8.51 (d, 2H, H4-pyridazine, J = 9.1 Hz), 8.61 (bs, 2H, NH2, D2O exchangeable); MS: m/z (%); M+ 301(88), 275 (100 ), 78 (52), 65 (65); Anal. Calc. for C16 H11N7 (301.31): C 63.78, H 3.68, N 32.54; Found: C 64.02, H 3.68, N 32.39.
General Procedure for Preparation of 5-Methoxymethyleneamino-3-substituted-1-[6-(p-tolyl)-pyridazin-3-yl]-pyrazole-4-carbonitrilse (7a-c).
A mixture of pyrazole-o-aminonitriles 3a-c (10 mmol), triethylorthoformate (3mL) and acetic anhydride (3 mL) was refluxed for 6h. The solvent was removed under reduced pressure and the resulting solid was recrystalized from ethanol.
5-Methoxymethyleneamino-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazole-4-carbonitrile (7a).
Yield: 75 %; white solid, mp 132 °C ; IR ν max cm-1: 3380 (NH), 2920, 2827 (CH), 2218 (CN).
5-Methoxymethyleneamino-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazole-4-yl-carbonitrile (7b).
Yield: 61 %; white solid, mp 230 °C IR: ν max cm-1: 3326 (NH), 2917, 2849 (CH), 2225 (CN)
5-Methoxymethyleneamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazole-3,4-yl-dicarbonitrile (7c).
Yield: 66% yield; white solid, mp 209-210 °C; IR ν max cm-1: 3416 (NH), 2933, 2860 (CH), 2229, 2225 (2CN).
General procedure for the Preparation of 3-Substituted-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-4-amines (8a-c).
Methanimidates 7a-c (3 mmol) were added to methanol (20 mL) saturated with ammonia at 0°C for 1h, warmed to room temperature and the reaction mixture stirred for 6h. The solid which precipitated was collected and recrystallized from an appropriate solvent.
3-Methylthio-1-[6-(p-tolyl)pyridazin-3-yl]pyrazolo[3,4-d]pyrimidine-4-amine (8a).
Yield: 81 %; pale yellow solid, mp 275 °C (from n-BuOH); IR ν max cm-1: 3410, 3362 (NH2); 1H-NMR δ: 2.46 (s, 3H, S-CH3), 2.60 (s, 3H, p-CH3-tolyl), 7.43 (d, 2H, H3,H5-tolyl, J = 8.0 Hz), 7.71 (bs, 2H, NH2, D2O exchangeable), 8.00 (d, 2H, H2,H6-tolyl, J = 8.0 Hz), 8.20 (d, 2H, H5-pyridazine, J = 9.1 Hz), 8.42 (d, 2H, H4-pyridazine, J = 9.1 Hz); 13C-NMR δ: 18 (S—CH3-tolyl), 20 (p-CH3-tolyl), 112, 118, 124, 128, 131, 133, 138, 151, 155, 156, 158, 159, 160 (aromatic); MS: m/z (%); M+ 349(100), 334(27), 274(94), 244 (49). Anal. Calc. for C17 H15N7S (349.41); C 58.44, H 4.33, N 28.06, S 9.18; Found : C 58.29, H 4.34, N 27.98, S 9.20.
3-N-Phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]pyrazolo[3,4-d]pyrimidine-4-amine (8b).
Yield 34 %; light brown solid, mp 285-287 °C (from dioxane); IR ν max cm-1: 3370-3180 (NH2/NH); 1H-NMR δ: 2.52 (s, 3H, p-CH3-tolyl), 7.04 (t, 1H, H4-Ph, J = 7.4 Hz), 7.30 (t, 2H,H3,H5- Ph, J = 7.6 Hz), 7.41 (d, 2H, H3,H5- tolyl, J = 8.0 Hz), 7.85 (bs, 2H, NH2, D2O exchangeable), 7.90 (d, 2H, H2-Ph, H6-Ph, J = 7.9 Hz), 8.16 (d, 2H, H4. H6-tolyl, J = 8.0 Hz), 8.20 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.33 ( bs , 1H, NH, D2O exchangeable), 8.40 (d, 1H, H4-pyridazine, J = 9.1Hz), 8.64 (s, 1H, pyrimidine); 13C-NMR δ: 20 (p-CH3-tolyl), 114, 121, 122, 123, 124, 128, 130, 131, 133, 135, 138, 151, 155, 156, 158, 159, 160 (aromatic); MS: m/z (%); M+ 394(86), 378(52), 288(100), 244 (73), 77(15); Anal. Calc. for C22 H18N8 (394.44); C 66.99, H 4.60, N 28.41; Found : C 67.05, H 4.60, N 28.39.
Amino-1-[6-(p-tolyl)pyridazin-3-yl]pyrazolo[3,4-d]pyrimidine-3-carbonitrile (8c).
Yield: 65 %; yellow crystals, mp >320 °C (from EtOH); IR ν max cm-1: 3397, 3276 (NH2), 2240 (CN); 1H-NMR δ: 2.45 (s, 3H, p-CH3-tolyl), 7.40 (d, 2H, H3, H5- tolyl, J = 8.0 Hz), 7.93 (bs, 2H, NH2, D2O exchangeable), 8.00 (d, 2H, , H2, H6-tolyl, J = 8.0 Hz), 8.12 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.51 (d, 1H, H4-pyridazine, J = 9.1Hz); MS m/z (%): M+ 328(95), 312(100), 302(44); Anal. Calc. for C17 H12N8 (328.34); C 62.19, H 3.68, N 34.13; Found: C 62.16, H 3.71, N 34.18.
General Procedure for the Preparation of 4-imino-3-substituted-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d]- pyrimidine-5-amines (9a-c).
To a well stirred cold solution of methanimidate 7a-c (20 mmol) in ethanol (10 mL), 99 % hydrazine hydrate (3 mL) was added over 2h, then the mixture was stirred at room temperature for 6h and left overnight. The solid that precipitated was filtered off and purified by passage though a column of silica gel with cyclohexane -ethyl acetate (1:1) as eluent.
4-Imino-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d] pyrimidine-5-amine (9a).
Yield: 96 %; white solid, mp 238 °C; IR ν max cm-1: 3352-3204 (NH2/NH); 1H-NMR δ: 2.37 (s, 3H, S-CH3), 2.66 ( s, 3H, p-CH3-tolyl), 6.35 (bs, 2H, NH2, D2O exchangeable), 7.41 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 8.06 (d, 2H, H3, H5- tolyl, J = 8.0 Hz), 8.22 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.30 (bs, 1H, NH, D2O exchangeable), 8.41 (d, 1H, , H4-pyridazine, J = 9.1Hz) , 8.54 (s, 1H, pyrimidine); MS: m/z (%); M+ 364(95), 348(100), 317(33), 301 (58); Anal. Calc. for C17 H16N8S (364.43): C 56.03, H 4.43, N 30.75, S 8.80; Found: C 55.96, H 4.46, N 30.70, S 8.74.
4-Imino-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d]- pyrimidine-5-amine (9b).
Yield: 70 %; yellow solid, mp 279-281 °C; IRν max cm-1: 3458-3120, (NH2/2NH); 1H-NMR δ: 2.51 (s, 3H, p-CH3-tolyl), 6.91 (t, 1H, H4-Ph, J = 7.4 Hz), 7.23 (t, 2H, H3, H5-Ph, J = 7.6 Hz), 7.36 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.42 (bs, 2H, NH2, D2O exchangeable), 7.64 (d, 2H, H2, H6-Ph, J= 7.9 Hz), 8.00 (d, 1H, H2, H6-tolyl, J = 8.0 Hz), 8.10 (d, 1H, , H5-pyridazine, J = 9.1 Hz), 8.41 (d, 1H, H4-pyridazine, J = 9.1Hz), 8.55 (s, 1H, pyrimidine), 9.03 (bs, 1H, NH, D2O exchangeable); MS: m/z (%); M+ 409 (100), 394 (51), 288 (84). Anal. Calc. for C22 H19N9 (409.45): C 64.54, H 4.68, N 30.79; Found: C 64.18, H 4.67, N 31.01.
5-amino-4-imino-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d]pyrimidine-3-carbonitrile (9c).
Yield: 89 %; beige solid, mp 271-273 °C; IR ν max cm-1 3420-3230 (NH2, 2NH); 2236 (CN); 1H-NMR δ 2.42 (3H, s, p-CH3-tolyl), 7.33 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.65 (2H, bs, NH2, D2O exchangeable), 8.00 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.21 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.42 (d, 1H, H4-pyridazine, J = 9.1Hz), 8.77 (1H, bs, NH, D2O exchangeable); MS m/z (%): M+ 343 (100), 237 (66), 211 (87).Anal. Calc. for C17H13N9 (343.35): C 59.47; H 3.82 ; N 36.71; Found: C 59.47, H 3.84, N 36.70.
General procedure for the Preparation of 2-Aryl-9-substituted-7-[6-(p-tolyl) pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo[5,1-f] pyrimidines (10-12).
Methanimidates 7a-c (20 mmol) were dissolved in dioxane (20 mL), and 2-furancarboxylic acid hydrazide, 2-thiophenecarboxylic acid hydrazide or 4-pyridinecarboxylic acid hydrazide (22 mmol) was added. The mixture was refluxed for 6-14 h, cooled, the solvent removed under reduced pressure and the resulting precipitate was purified by recrystallization or column chromatography.
2-(2-Furyl)-9-methylthio-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo[5,1-f]-pyrimidine (10a).
Yield: 78 %; pale yellow solid, mp 215-216 °C (from DMF/H2O); IR ν max cm-1: 3096, 2919, 1628; 1H-NMR δ: 2.54 (s, 3H, S-CH3), 2.66 (s, 3H, p-CH3-tolyl), 6.81 (m, 1H, furyl), 7.42 (m, 2H + 1H, H-tolyl+ 1H-furyl), 7.65 (d, 2H, H3, H5- tolyl, J = 8.0 Hz), 7.87 (m, 1H, furyl), 8.01 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.32 (d, 1H, , H4-pyridazine, J = 9.1 Hz), 8.65 (s, 1H, pyrimidine); MS m/z (%): M+ 440 (95), 393 (100), 326 (39); Anal. Calc. for C22 H16N8SO (440.48): C 59.99, H 3.66, N 25.44, S 7.28; Found : C 59.85, H 3.61, N 25.40, S 7.29.
2-(2-Thienyl)-9-methylthio-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo[5,1-f] pyrimidine (10b).
Yield: 47 %; beige solid, mp 247-249 °C (from DMF/ H2O); IR ν max cm-1: 3101, 2918, 1625; 1H-NMR δ: 2.53 (s, 3H, S-CH3), 2.64 ( s, 3H, p-CH3-tolyl), 7.21 (m, 1H, thienyl), 7.55 (m, 2H + 1H, H-tolyl+1H-thienyl), 7.76 (d, 2H, H3, H5- tolyl, J = 8.0 Hz), 7.87 (m, 1H, thienyl), 8.21 (d, 1H, H5- pyridazine, J = 9.1Hz), 8.43 (d,1H, H4-pyridazine, J = 9.1 Hz), 8.71 (s, 1H, pyrimidine); MS m/z (%):M+ 456 (95), 409 (100), 326 (57); Anal. Calc. for C22 H16N8S2 (456.55): C 57.88, H 3.53, N 24.54, S 14.04; Found : C 57.87, H 3.56, N 24.51, S 13.99.
2-(4-pyridyl)-9-methylthio-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo[5,1-f]pyrimidine (10c).
Yield: 71 %; white solid, mp 240-243 °C [column chromatography, (5:1) cyclohexane/ethyl acetate]; IR ν max cm-1: 3084, 2926, 1627; 1H-NMR δ: 2.42 (s, 3H, S-CH3), 2.60 ( s, 3H, p-CH3-tolyl), 7.43 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.81 (d, 2H, pyridyl, J = 8.0 Hz), 8.10 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.46 (d, 1H, H5- pyridazine, J = 9.1 Hz ), 8.55 (d, 1H, H4-pyridazine, J = 9.1, Hz), 8.67 (s, 1H, pyrimidine), 8.71 (d, 2H, pyridyl J = 8.0 Hz); MS m/z (%): M+ 451 (68), 404 (100), 326 (51); Anal. Calc. for C23 H17N9S (451.51): C 61.18, H 3.79, N 27.92, S 7.10; Found : C 61.16, H 3.77, N 27.94, S 6.99.
2-(2-Furyl)-9-N-phenylamino-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo-[5,1-f] pyrimidine (11a).
Yield: 64 %; white solid, mp 285 °C [column chromatography, (5:1) cyclohexane/ethyl acetate]; IR ν max cm-1: 3415, 3086, 2921, 1616; 1H-NMR δ: 2.50 ( s, 3H, p-CH3-tolyl), 6.82 (m, 1H, furyl), 6.90 (t, 1H, H4-Ph, J = 7.4 Hz), 7.27 (m, 2H + 2H+ 1H, H-tolyl + H-phenyl + H-furyl), 7.65 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.00 (m, 1H+ 2H, H-furyl, H-phenyl), 8.19 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.34 (d, 1H, H4-pyridazine, J = 9.1 Hz), 8.52 (s, 1H, pyrimidine); MS m/z (%): M+ 485 (93), 393 (100), 326 (69); Anal. Calc. for C27 H19N9O (485.50): C 66.80, H 3.94, N 25.96; Found : C 66.80, H 3.97, N 25.98.
2-(2-Thienyl)-9-N-phenylamino-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo- [5,1-f] pyrimidine (11b).
Yield: 83 %; pale brown solid, mp >320 °C (from dioxane); IR ν max cm-1: 3413, 3110, 2921, 1607; 1H-NMR δ: 2.52 (s, 3H, p-CH3-tolyl), 6.91 (t, 1H, H4-Ph, J = 7.4 Hz), 7.15 (m, 1H, thienyl), 7.40 (m, 2H , H-tolyl+ H-phenyl+ 1H-thienyl), 7.69 (d, 2H, H2, H6- tolyl, J = 8.4 Hz), 7.82 (m, 1H, thienyl), 8.02 (m, 2H-phenyl,1H-pyridazine), 8.36 (d, 1H, H4-pyridazine), 8.50 (s, 1H, pyrimidine); MS m/z (%): M+ 501 (94), 418 (100), 326 (90); Anal. Calc. for C27 H19N9S (501.57): C 64.66, H 3.82, N 25.13, S 6.39; Found : C 64.66, H 3.80, N 25.09, S 6.36.
2-(4-Pyridyl)-9-N-phenylamino-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo-[5,1-f] pyrimidine (11c).
Yield: 61 %; yellow flakes, mp >320 °C (from dioxane); IR ν max cm-1: 3375, 3095, 2921, 1599; 1H-NMR δ: 2.53 (d, 3H, p-CH3-tolyl), 7.08 (t, 1H, H4-Ph, J = 7.4 Hz), 7.30 (m, 2H+2H, H-toly+H-phenyl), 7.50 (d, 2H, pyridyl, J = 8.0 Hz), 7.82 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.06 (d, 2H, H2, H6- Ph, J = 7.9 Hz), 8.25 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.39 (d, 1H, H4-pyridazine, J = 9.1 Hz), 8.47 (s, 1H, pyrimidine), 8.70 (d, 2H, pyridyl J = 8.0Hz): MS m/z (%): M+ 496 (100), 404 (70), 418 (61); Anal. Calc. for C28 H20N10 (496.53); C 67.73, H 4.00, N 28.21; Found : C 67.79, H 4.06, N 28.26.
2-(2-Furyl)-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo[5,1-f]pyrimidine-9-carbonitrile (12a).
Yield: 46 %; beige solid, mp 230 °C (from dioxane); IR ν max cm-1: 3089, 2918, 2229, 1633. 1H-NMR δ: 2.35 ( s, 3H, p-CH3-tolyl), 7.07 (m, 1H, furyl), 7.31 (m, 2H-tolyl+1H-furyl), 8.09 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.15 (m, 1H, furyl), 8.28 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.45 (d, 1H, H4-pyridazine, J = 9.1 Hz), 8.70 (s, 1H, pyrimidine); MS m/z (%): M+ 419 (100), 393 (60), 352 (45); Anal. Calc. for C22 H13N9O (419.41); C 63.00, H 3.12, N 30.06; Found : C 62.99, H 3.15, N 30.02.
2-(2-Thienyl)-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo[5,1-f]pyrimidine-9-carbonitrile (12b).
Yield: 40 %; beige solid, mp 262 °C (from dioxane); IR ν max cm-1:3104, 2917, 2224, 1630; 1H-NMR δ: 2.35 ( s, 3H, p-CH3-tolyl), 7.38 (m, 1H, thienyl), 7.44 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.80 (m, 1H, H-thienyl), 7.92 (m, 1H, thienyl), 8.05 (d, 2H, H2, H6- tolyl, J = 8.0 Hz ), 8.15 (d, 1H, H5- pyridazine, J = 9.1 Hz), 8.45 (d, 2H, H4-pyridazine, J = 9.1 Hz), 8.70 (s, 1H, pyrimidine); MS m/z (%): M+ 435 (91), 409 (100), 352 (70); Anal. Calc. for C22H13N9S (435.47): C 60.68, H 3.01, N 28.95, S 7.36; Found : C 60.59, H 3.08, N 28.91, S 7.33.
2-(4-Pyridyl)-7-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-1,2,4-triazolo[5,1-f]pyrimidine-9-carbonitrile (12c).
Yield: 70 %; pale brown crystals, mp 320 °C (from EtOH); IR ν max cm-1: 3090, 2933, 2228, 1635; 1H-NMR δ: 2.44 (d, 3H, p-CH3-tolyl), 7.31 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.72 (d, 2H, pyridyl, J = 8.0 Hz), 8.06 (d, 2H, H2, H6-tolyl, J = 8.4 Hz), 8.13(d, 1H, H5-pyridazine, , J = 9.1 Hz), 8.52 (d, 1H, H4-pyridazine, J = 9.1 Hz), 8.62 (s, 1H, pyrimidine), 8.75 (d, 2H, pyridyl J = 8.0 Hz); MS m/z (%): M+ 430 (100), 404 (78), 352 (60); Anal. Calc. for C23 H14N10 (430.43): C 64.18, H 3.28, N 32.54; Found : C 64.22, H 3.20, N 32.55.
General Procedure for the Preparation of 3-Substituted-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-4(5H)-ones (13a-c).
Compound 3a-c (5 mmol) was added to formic acid (5 mL, 85%) and the mixture was refluxed for 6h. The solid that precipitated was collected and recrystallized.
3-Methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-4(5H)-one (13a).
Yield: 62 %; colorless needle-like crystals, mp 244 °C (from EtOH); IR ν max cm-1: 3381 (NH), 1658 (CO); 1H-NMR δ: 2.44 (s, 3H, S-CH3), 2.60 (s, 3H, p-CH3-tolyl), 7.42 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 8.00 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.14 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.35 (d, 1H, H4-pyridazine, J = 9.1 Hz), 8.53 (s, 1H, pyrimidine), 11.16 (s, 1H, NH, D2O exchangable); 13C-NMR δ: 14 (S—CH3), 20 (p-CH3-tolyl), 114–158 (aromatic), 131 (C-S-CH3), 163 (CO, pyrimidine); MS m/z (%): M+ 350 (72), 323 (100), 275 (96), 129 (84); Anal. Calc. for C17 H14N6OS (350.39): C 58.27, H 4.03, N 23.98, S 9.15; Found: C 57.95, H 4.14, N 24.02, S 9.20.
3-N-Phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-4(5H)-one (13b).
Yield: 34 %; colorless needle-like crystals, mp>320 °C (from EtOH); IR ν max cm-1: 3337, 3265 (2NH), 1660 (CO); 1H-NMR δ: 2.60 (s, 3H, p-CH3-tolyl), 6.88 (t, 1H, H4-Ph, J = 7.4 Hz), 7.22 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.51 (t, 2H, H3, H5-Ph, J = 7.6 Hz) 8.01 (m, 2H+ 2H, H-tolyl + H-phenyl), 8.24 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.33 (s, 1H, NH, D2O exchangable) 8.48 (d, 1H, H4 – pyridazine, J = 9.1 Hz), 8.60 (s, 1H, pyrimidine), 11.09 (s, 1H, NH, D2O exchangable); 13C-NMR (DMSO) δ: 15 (p-CH3-tolyl), 117-158 (aromatic), 131 (C-S-CH3), 169 (CO pyrimidine); MS m/z (%): M+ 395 (100), 323 (85), 292 (75); Anal. Calc. for C22 H17N7O (395.42): C 66.83, H 4.33, N 24.80; Found: C 66.81, H 4.34, N 24.78.
4(5H)-Oxo-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (13c).
Yield: 55 %; colorless needle-like crystals, mp>320 °C (from dioxane); IR ν max cm-1: 3367 (NH), 1645 (CO); 1H-NMR δ: 2.63 (s, 3H, p-CH3-tolyl), 7.38 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 8.16 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.22 (d, 1H, H5- pyridazine, J = 9.1 Hz), 8.40 (d, 1H, H4-pyridazine, J = 8.0 Hz), 8.65 (s, 1H, pyrimidine), 12.50 (s, 1H, NH, D2O exchangable); 13C-NMR (DMSO) δ: 13 (p-CH3-tolyl), 84 (CN), (115–157 aromatic), (168, CO), pyrimidine]; MS m/z (%): M+ 329(90), 303(100), 271(81); Anal. Calc. for C17 H11N7O (329.39): C 62.00, H 3.37, N 29.77; Found: C 62.01, H 3.40, N 29.74.
General procedure for the Preparation of 3-Substituted-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo-[3,4-d]pyrimidine-4,6(5H,7H)-dithiones (14a-c).
To a solution of 3a-c (10 mmol) in DMF (20 mL), carbon disulfide (10 mL,15 mmol) and 10 mL sodium methoxide (prepared from 0.59g of sodium metal and 30 mL methanol) were added. The mixture was refluxed for 15h, and then poured into ice cold water. A solution of sodium hydroxide (10 mL, 1M) was added to it and left overnight. The solution was filtered and acidified with dilute acetic acid to give a yellow precipitate. It was collected, washed with dilute acetic acid, dried and recrystallized from ethanol.
3-Methythio1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dithione (14a).
Yield: 50 %; yellow needle crystals, mp 269°C; IR ν max cm-1: 3344, 3320 (2NH); 1H-NMR δ: 2.45 (s, 3H, S-CH3), 2.62 (s, 3H, p-CH3-tolyl), 7.50 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 8.14 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.21 (d, 1H, H5- pyridazine, J = 9.1 Hz), 8.52 (d, 1H, H4-pyridazine, J = 9.1 Hz), 8.70 (s, 1H, NH, D2O exchangable), 8.90 (s, 1H, NH, D2O exchangable); MS m/z (%): M+ 398 (72), 372 (100), 325 (96); Anal. Calc. for C17H14N6S3 (398.52): C 51.24, H 3.54, N 21.09, S 24.13; Found: 50.97, H 3.29, N 21.10, S 24.12.
3-N-Phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dithione (14b).
Yield: 35 %; yellow solid, mp 260 °C; IR ν max cm-1: 3376 -3265 (3NH); 1H-NMR δ: 2.52 (s, 3H, p-CH3-tolyl), 6.90 (t, 1H, H4-Ph, J = 7.4 Hz), 7.16 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.52 (t, 2H, H3, H5- Ph, J = 7.6 Hz) 8.00 (m, 2H+ 2H, tolyl + H-phenyl), 8.14 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.36 (s, 1H, NH, D2O exchangable) 8.50 (d, 1H, H4-pyridazine, J = 9.1 Hz), 9.04 (s, 1H, NH, D2O exchangable), 9.77 (s, 1H, NH, D2O exchangable); MS m/z (%): M+ 443(100), 340(95), 308(81); Anal. Calc. for C22 H17N7S2 (443.54); C 59.58, H 3.86, N 22.11, S 14.46; Found: C 59.57, H 3.90, N 21.97, S 14.46.
1-[6-(p-tolyl)pyridazin-3-yl]-4,6-Dithioxo-4,5,6,7-tetrahydropyrazolo[3,4-d]pyrimidine-3-carbonitrile (14c).
Yield: 32 %; yellow solid, mp 239 °C; IR ν max cm-1: 3340- 3300 (2NH), 2220 (CN); 1H-NMR δ: 2.62 (s, 3H, p-CH3-tolyl), 7.10 (d, 2H, H3, H5- tolyl, J = 8.0 Hz), 7.81 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.06 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.42 (d, 1H, H4-pyridazine, J = 9.1 Hz), 9.27 (s, 1H, NH, D2O exchangable), 10.00 (s, 1H, NH, D2O exchangable); MS m/z (%): M+ 377 (100), 351 (98), 325 (75); Anal. Calc. for C17 H11N7S2 (377.44); C 54.10, H 2.94, N 25.98, S 16.99; Found: C 53.88, H 3.03, N 26.07, S 17.07.
General Procedure for the Preparation of 4-Imino-5-phenyl-3-substituted-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d]- pyrimidine-6(7H)-thiones/ones (15-17).
A mixture of 3a-c (10 mmol) and phenylisocyanate or phenylisothiocyanate (10 mmol) in pyridine (20 mL) was refluxed for 5h. The reaction mixture was cooled and poured onto ice/water and neutralized with diluted HCl. The solid product so formed was collected by filtration and recrystallized from ethanol.
4-Imino-5-phenyl-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d]-pyrimidine-6(7H)-one (15a).
Yield: 90 %; white solid, mp 229 °C; IR ν max cm-1: 3395, 3326 (2NH), 1649 (CO); 1H-NMR δ: 2.44 (s, 3H, S-CH3), 2.56 (s, 3H, p-CH3-tolyl), 6.65 (t, 1H, phenyl, J = 6.6 Hz), 7.00 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.50 (m, 2H+ 2H, H-phenyl + H-tolyl), 7.81 (m, 2H + 2H, H-tolyl + H-phenyl), 8.06 (d, 1H, H5- pyridazine, J = 9.1Hz), 8.32 (d, 1H, H4-pyridazine, J = 9.1Hz), 8.80 (bs, 1H, NH, D2O exchangeable), 9.05 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 441 (90), 426 (100), 365 (42); Anal. Calc. for C23H19N7OS (441.50): C 62.57, H 4.34, N 22.21, S 7.26; Found: C 62.55, H 4.33, N 22.20, S 7.17.
4-Imino-5-phenyl-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo-[3,4-d]-pyrimidine-6(7H)-thione (15b).
Yield: 79 %; beige solid, mp 275-276 °C; IR ν max cm-1: 3389-3279 (2NH); 1H-NMR δ: 2.36 (s, 3H, S-CH3), 2.60 (s, 3H, p-CH3-tolyl), 6.77 (t,1H,phenyl. J = 6.6 Hz), 7.00 (m, 2H, phenyl + tolyl), 7.44 (m, 2H+ 2H, phenyl + tolyl), 8.22 (d, 2H, H5-pyridazine, J = 9.1Hz), 8.42 (bs, 1H, NH, D2O exchangeable), 8.53 (d, 1H, H4-pyridazine, J = 9.1Hz), 9.33 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 457 (100), 365 (76), 318 (39); Anal. Calc. for C23H19N7S2 (457.57): C 60.37, H 4.19, N 21.43, S 14.01; Found: C 60.35, H 4.10,N 21.26, S 13.98.
4-Imino-5-phenyl-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d] pyrimidine-6(7H)-one (16a).
Yield: 44 %; white solid, mp 255 °C; IR ν max cm-1: 3380-3210(3NH), 1648 (CO); 1H-NMR δ: 2.51 (s, 3H, p-CH3-tolyl), 6.90-8.41 (m, 16H, aromatic), 8.55 (bs, 1H, NH, D2O exchangeable), 9.09 (bs, 1H, NH, D2O exchangeable), 11.08 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 486 (100), 394 (82), 317 (54); Anal. Calc. for C28 H22N8O (486.53): C 69.12, H 4.56, N 23.03; Found: C 69.12, H 4.57, N 23.02.
4-Imino-5-phenyl-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo[3,4-d]pyrimidine-6(7H)-thione (16b).
Yield: 46 %; beige solid, mp 306 °C; IR ν max cm-1: 3460-3298 (3NH); 1H-NMR δ: 2.45 (s, 3H, p-CH3-tolyl), 7.00-8.35 (m, 16H, aromatic), 8.60 (bs, 1H, NH, D2O exchangeable), 9.18 (bs, 1H, NH, D2O exchangeable), 11.13 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 502 (98), 410 (100), 333 (56); Anal. Calc. for C28H22N8S (502.60): C 66.91, H 4.41, N 22.29, S 6.38; Found: C 66.75, H 4.40, N 22.25, S 6.35.
4-Imino-5-phenyl-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydro-6(7H)-oxo-pyrazolo[3,4-d]-pyrimidine-3-carbonitrile (17a).
Yield: 43 %; beige solid, mp >320 °C; IR ν max cm-1: 3368-3307 (2NH), 2247 (CN); 1H-NMR δ: 2.42 (s, 3H, p-CH3-tolyl), 6.80 (t, 1H, phenyl, J = 6.6 Hz), 7.33-7.50 (m, 6H, phenyl + tolyl), 7.80 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.06 (d, 1H, H5-pyridazine, J= 9.1 Hz), 8.38 (d, 1H, H4-pyridazine, J = 9.1 Hz), 8.80 (bs, 1H, NH, D2O exchangeable), 11.07 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 420 (100), 394 (68), 333 (66); Anal. Calc. for C23H16N8O (420.43): C 65.71, H 3.84, N 26.65; Found: C 65.71, H 3.97, N 26.61.
4-Imino-5-phenyl-3-carbonitrile-1-[6-(p-tolyl)pyridazin-3-yl]-4,5-dihydropyrazolo-[3,4-d]-pyrimidine-6(7H)-thione (17b).
Yield: 42 %; yellow solid, mp >320 °C; IR ν max cm-1: 3343-3222 (2NH), 2247(CN); 1H-NMR δ: 2.44 (s, 3H, p-CH3-tolyl), 7.10 (t, 1H, phenyl, J = 6.6 Hz), 7.23-7.40 (m, 6H, phenyl + tolyl), 7.85 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.03 (d, 1H, pyridazine, J= 9.1 Hz), 8.36 (d, 1H, pyridazine, J = 9.1 Hz), 8.60 (bs, 1H, NH, D2O exchangeable), 11.13 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 436 (92), 369 (100), 353 (34); Anal. Calc. for C23H16N8S (436.49): C 63.29, H 3.69, N 25.67, S 7.34; Found: C 63.16, H 3.66, N 25.68, S 7.33.
General Procedure for the Preparation of 4-Amino-3-substituted-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-6-ones/thiones/amines (18-20).
Compound 3a-c (5 mmol) and urea (10 mmol) or thiourea (10 mmol) or guanidine carbonate (5 mmol) were mixed in a mortar, The mixture was then heated at 180 °C in an oil bath for 20 minutes, heating was continued 2h, at the melting point of the pyrazole derivatives using reduced pressure. The molten product was boiled 10 minutes with water, cooled and filtered. The product was finally recrystallized from a suitable solvent.
Amino-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-6(7H)-one (18a).
Yield: 54 %; white solid, mp 275 °C (from dioxane); IR ν max cm-1: 3466-3206 (NH2/NH), 1685 (CO); 1H-NMR δ: 2.42 (s, 3H, S-CH3), 2.50 (s, 3H, p-CH3-tolyl), 7.35 (d, 2H, H3, H5-tolyl, J =8.0 Hz), 7.70 (d, 2H, H2,H6-tolyl, J = 8.0 Hz), 8.12 (bs, 2H, NH2, D2O exchangeable), 8.38 (d, 1H,H5-pyridazine, J = 9.1 Hz), 8.55 (d, 1H, H4-pyridazine, J = 9.1 Hz), 11.02, (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 365 (100), 316 (87), 318 (56); Anal. Calc. for C17H15N7OS (365.41): C 55.88, H 4.14, N 26.83, S 8.77; Found: C 55.87, H 4.08,N 26.90, S 8.75.
4-Amino-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-6(7H)-thione (18b).
Yield: 63 %; yellow solid, mp >320 °C (from DMF/H2O); IR ν max cm-1: 3402-3180, (NH2/NH); 1H-NMR δ: 2.54 (s, 3H, S-CH3), 2.62 ( s, 3H, p-CH3-tolyl), 7.22 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.64 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 7.81 (bs, 2H, NH2, D2O exchangeable), 8.08 (d, 2H, H5-pyridazine), 8.54 (d, 1H, H4-pyridazine, J = 9.1Hz), 9.06 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 381 (93), 365 (100), 334 (39); Anal. Calc. for C17H15N7S2 (381.47): C 53.53, H 3.96, N 25.70, S 16.81; Found: C 53.53, H 4.02, N 25.70, S 16.79.
4,6-Diamino-3-methylthio-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine (18c).
Yield: 46 % yield; brown solid, mp 266 °C (from DMF/H2O); IR ν max cm-1: 3410-3254 (2NH2); 1H NMR δ: 2.53 (s, 3H, S-CH3), 2.60 (s, 3H, p-CH3-tolyl), 7.04 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.44 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 7.70 (bs, 2H, NH2, D2O exchangeable), 8.22 (d, 2H, H5-pyridazine, J = 9.1 Hz), 8.41 (bs, 1H, NH, D2O exchangeable), 8.66 (d, 1H, H4-pyridazine, J = 9.1 Hz),. MS: m/z (%); M+ 364(100), 348(56), 301(48); Anal. Calc. for C17 H16N8S (364.43): C 56.03, H 4.43, N 30.75, S 8.80; Found: C 56.00, H 4.41, N 30.72, S 8.79.
4-Amino-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]yrimidine-6(7H)-one (19a).
Yield: 43 %; white solid, mp 270 °C (from DMF/H2O); IR ν max cm-1: 3411-3160 (NH2/2NH), 1675 (CO); 1H-NMR δ: 2.53 (s, 3H, p-CH3-tolyl), 6.74(t, 1H,H4-phenyl, J = 7.4 Hz), 7.22 (m, 4H, H3, H5-phenyl+H3, H5-tolyl), 7.77 (t, 2H, H2, H6-phenyl, J = 7.9 Hz), 8.00(d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.15 (bs, 2H, NH2, D2O exchangeable), 8.33 (d, 1H, H5-pyridazine, J = 9.1 Hz), 8.54 (d, 2H, H4-pyridazine, J = 9.1 Hz), 12.36 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 410 (95), 394 (100), 318 (74); Anal. Calc. for C22 H18N8O (410.43): C 64.38, H 4.42, N 27.30; Found: C 64.36, H 4.46, N 27.30.
4-Amino-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-6(7H)-thione (19b).
Yield: 44 % yield; yellow solid, mp 276 °C (from EtOH); IR ν max cm-1: 3411-3160 (NH2/2NH); 1H-NMR δ: 2.54 (s, 3H, p-CH3-tolyl), 6.90(t, 1H, H4- phenyl, J = 7.4 Hz), 7.20 (m, 4H, H3, H5-phenyl+ H3, H5-tolyl), 7.66 (m, 4H, H2, H6-phenyl +H2, H6-tolyl), 8.09 (bs, 2H, NH2, D2O exchangeable), 8.26 (d, 1H, H5-pyridazine, J = 9.1Hz), 8.40 (d, 1H, H4-pyridazine, J = 9.1 Hz), 11.45 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 426 (95), 334 (100), 318 (64); Anal. Calc. for C22H18N8S (426.50); C 61.96, H 4.25, N 26.27, S 7.52; Found: C 62.01, H 4.33, N 26.25, S 7.50.
4,6-Diamino-3-N-phenylamino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-pyrimidine (19c).
Yield: 52 %; yellow solid, mp>320 °C (from dioxane); IR ν max cm-1: 3397 -3110 (2NH2/NH); 1H-NMR δ: 2.61 (s, 3H, p-CH3-tolyl), 6.80 (t, 1H, H4-phenyl, J = 7.4 Hz), 7.22 (m, 4H, H3, H5-phenyl + H3, H5-tolyl), 7.45 (bs, 2H, NH2, D2O exchangeable) 7.60 (m, 4H, H2, H6 -phenyl + H2, H6-tolyl), 7.90 (bs, 2H, NH2, D2O exchangeable), 8.26 (d, 2H, H5-pyridazine, J = 9.1Hz), 8.50 (bs, 1H, NH, D2O exchangeable), 8.63 (d, 1H, H4”-pyridazine, J = 9.1Hz); MS m/z (%): M+ 409 (100), 317 (80), 301 (91); Anal. Calc. for C22H19N9 (409.45); C 64.54, H 4.68, N 30.79; Found: C 64.54, H 4.70, N 30.91.
4-Amino-3-carbonitrile-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]pyrimidine-6(7H)-one (20a).
Yield: 35 % yield; beige solid, mp >320 °C (from EtOH); IR ν max cm-1: 3421-3368 (NH2/NH), 2221 (CN), 1669 (CO); 1H-NMR δ: 2.50 (s, 3H, S-CH3), 2.63 (s, 3H, p-CH3-tolyl), 7.24 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.56 (d, 2H,H2, H6- tolyl, J = 8.0 Hz), 8.03 (bs, 2H, NH2, D2O exchangeable), 8.28 (d, 1H, H5-pyridazine, J = 9.1Hz), 8.40 (d, 1H, H4-pyridazine, J = 9.1Hz), 12.00 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 344 (95), 328 (100), 318 (62); Anal. Calc. for C17H12N8O (344.33): C 59.30, H 3.51, N 32.51; Found: C 59.29, H 3.46, N 32.50.
4-Amino-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d]-6(7H)-thioxopyrimidine-3-carbonitrile (20b).
Yield: 38 %; yellow solid, mp 280 °C (from EtOH); IR ν max cm-1: 3399-3260 (NH2/NH), 2234 (CN); 1H-NMR δ: 2.45 (s, 3H, S-CH3), 2.61 (s, 3H, p-CH3-tolyl), 7.06 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.47 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 7.55 (bs, 2H, NH2, D2O exchangeable), 8.20 (d, 2H, H5-pyridazine, J = 9.1 Hz), 8.43 (d, 1H, H4-pyridazine, J = 9.1 Hz), 9.06 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 360 (92), 344 (100), 318 (39); Anal. Calc. for C17H12N8S (360.40): C 56.66, H 3.36, N 31.09, S 8.90; Found: C 56.90, H 3.42, N 30.99, S 8.79.
4,6-Diamino-3-carbonitrile-1-[6-(p-tolyl)pyridazin-3-yl]-pyrazolo[3,4-d] pyrimidine (20c).
Yield: 42 %; beige solid, mp >320 °C (from DMF/H2O); IR ν max cm-1: 3411-3270 (2 x NH2), 2218 (CN); 1H-NMR δ: 2.44 (s, 3H, S-CH3), 2.61 (s, 3H, p-CH3-tolyl), 7.38 (d, 2H, H3, H5-tolyl, J = 8.0 Hz), 7.53 (d, 2H, H2, H6-tolyl, J = 8.0 Hz), 8.05 (bs, 2H, NH2, D2O exchangeable), 8.22 (d, 1H, H5-pyridazine, J = 9.1Hz), 8.53 (d, 1H, H4-pyridazine, J = 9.1Hz), 8.64 (bs, 1H, NH, D2O exchangeable); MS m/z (%): M+ 343 (87), 311 (100), 285 (55); Anal. Calc. for C17H13N9 (343.35): C 59.47, H 3.82, N 36.71; Found: C 59.49, H 3.80, N 36.71.