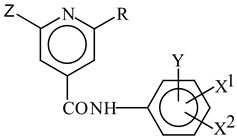
Abstract
Many compounds containing a -CONH- group display photosynthesis inhibiting activity. Based on this structural feature, a group of anilides of 2-alkylthio- (1b-4f) or 2-chloro-6-alkylthio-4-pyridinecarboxylic acids (5a-6c) was synthesised. The prepared compounds were tested for their inhibition of the oxygen evolution rate (OER) in spinach chloroplasts. A quasi-parabolic dependence between photosynthesis-inhibiting activity and the lipophilicity of the compounds was determined for 1b-4f as well as for 5a-6c. The inhibitory activity of compounds 1b-4f was higher than that of 5a-6c for comparable lipophilicity values.
Introduction
Many herbicides acting as photosynthesis inhibitors possess in their molecules an >N-C(=X)- group (X=O or N, not S) and a hydrophobic residue in close vicinity to this group. Shipman concluded that the hydrophilic part of a herbicide binds electrostatically to the terminus of an α-helix at a highly charged amino acid, whereas the hydrophobic part of the inhibitors extends into the hydrophobic part of the membrane [1]. Recently, pronounced photosynthesis-inhibiting activity has been found for alkoxy substituted phenylcarbamates [2,3] as well as for the local anesthetic of the anilide type – trimecaine [4,5,6], i.e., for compounds with -CONH- groups in their molecules.
In order to bring together the structural features of the abovementioned compounds and our previous results with 2-alkyl-4-pyridinecaboxylic acids anilides [7], some anilides of 2-alkylthio-4-pyridinecarboxylic (1b-4f) and 2-chloro-6-alkylthio-4-pyridinecarboxylic acids (5a-6c) were synthesised.
Results and Discussion
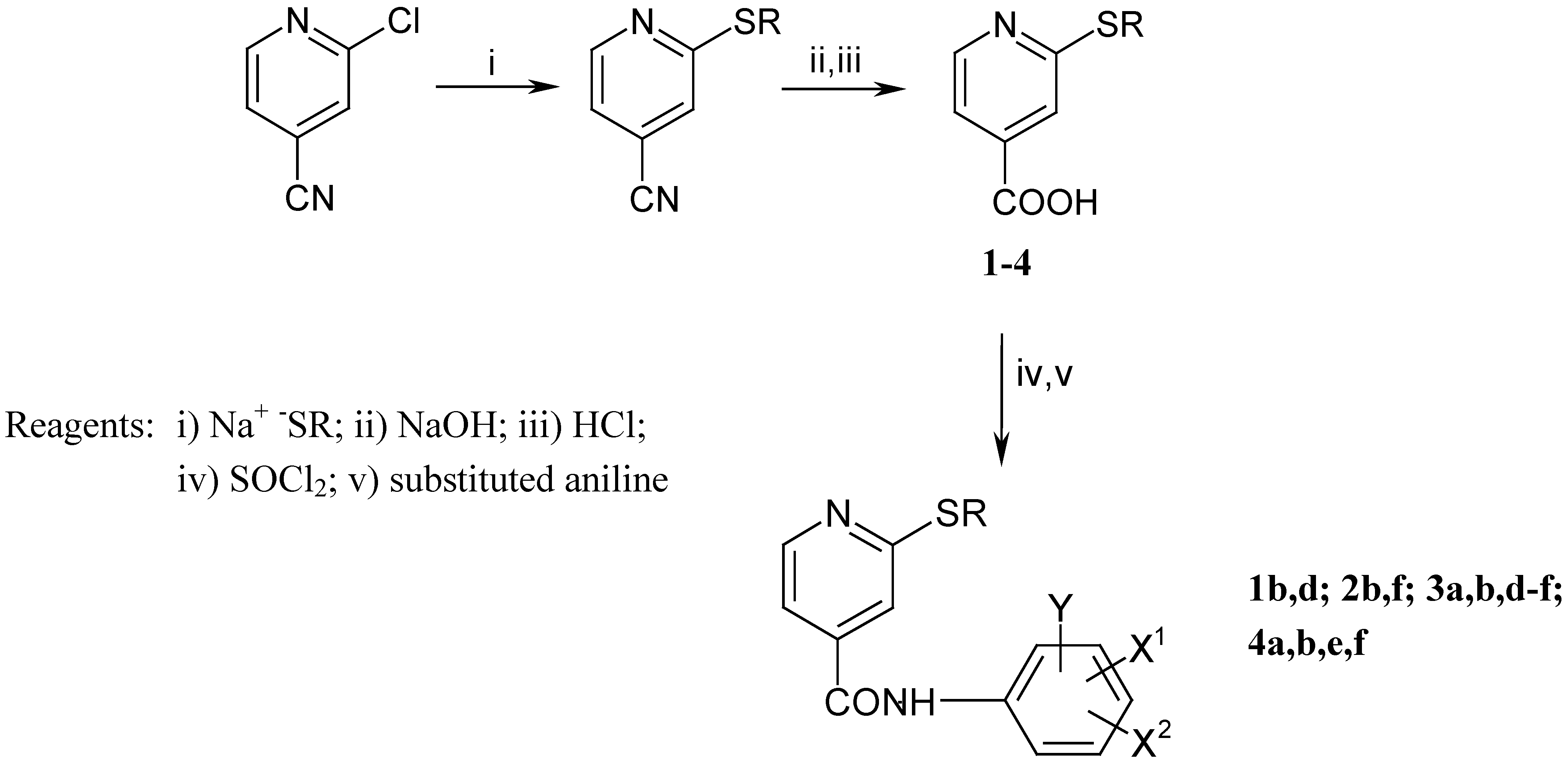
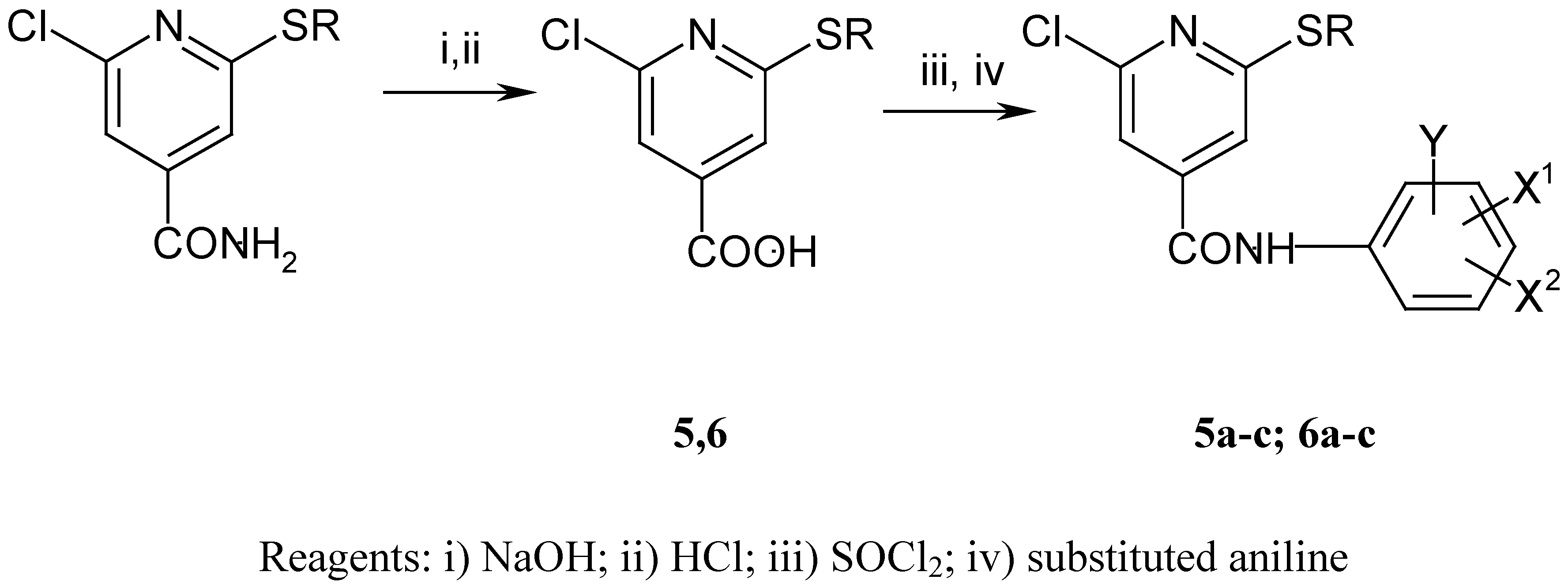
The synthesis of the anilides is shown in Scheme 1 and Scheme 2. The 2-alkylthio-4-cyanopyridines were synthesised as described previously [8]. Subsequent treatment with ethanolic sodium hydroxide solution afforded the corresponding acids 1-4. 2-chloro-6-alkylthio-4-pyridinecarboxylic acids 5 and 6 were obtained by a similar procedure from 2-chloro-6-alkylthio-4-carbamoylpyridines [9]. The anilides were prepared from the acids by reaction of the corresponding acyl chlorides with substituted anilines and aminophenols (Scheme 3). The melting points, yields, and elemental analyses for compounds are given in Table 2 and Table 4, and IR and 1H-NMR spectroscopic data in Table 3 and Table 5.
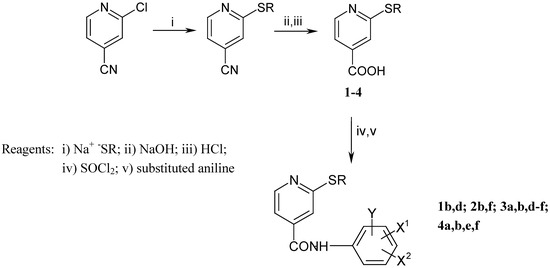
Scheme 1.
Preparation of 2-alkylthio-4-pyridinecarboxylic acids and derived anilides.
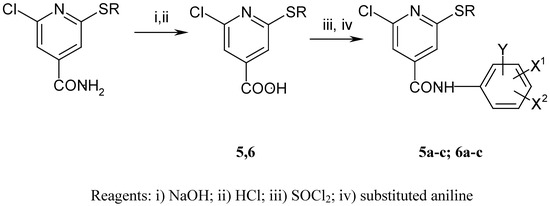
Scheme 2.
Preparation of 2-chloro 6-alkylthio-4-pyridinecarboxylic acids and derived anilides.
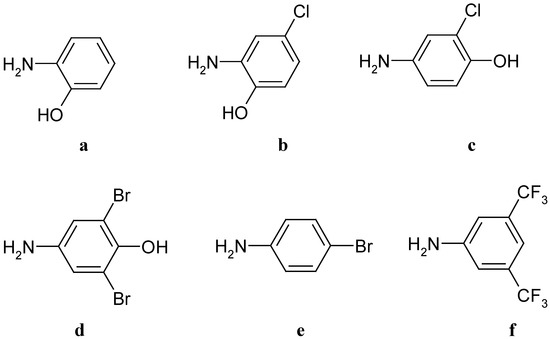
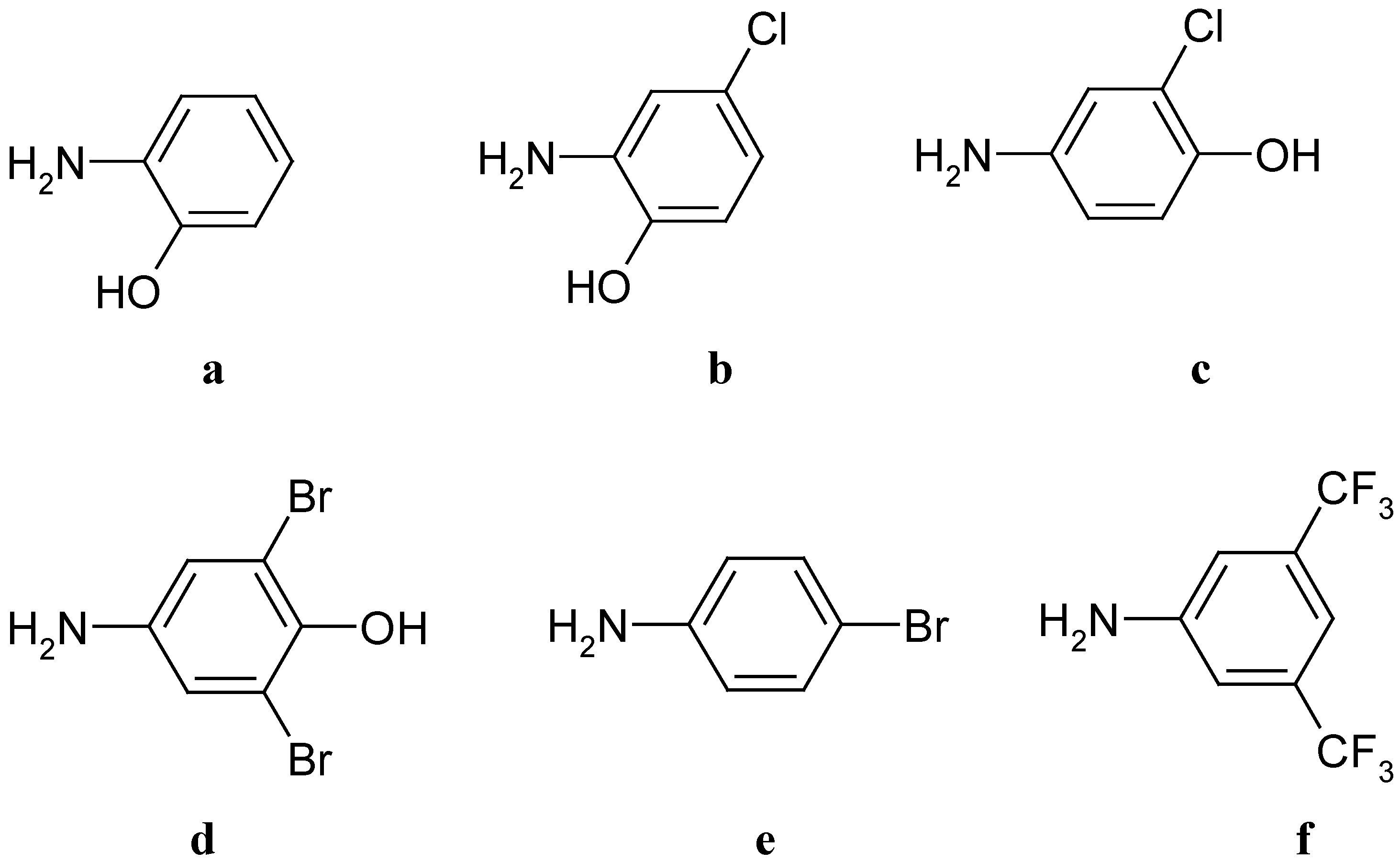
Scheme 3.
Aminophenols and anilines used.
The biological activity of anilides of 2-alkylthio-4-pyridinecarboxylic acids (1b-4f) and 2-chloro-6- alkylthio-4-pyridinecarboxylic acids (5a-6c) with regards to inhibition of oxygen evolution rate in spinach chloroplasts was investigated. The inhibitory activity of the compounds has been expressed by IC50 values (Table 1). The IC50 values, i.e. molar concentrations of the compounds causing 50 % activity decrease with respect to the untreated control, varied in the range of 6.0 - 69.1 μmol.dm-3 for 1b-4f and 14.2 - 32.5 μmol.dm-3, respectively, for 5a-6c.

Table 1.
IC50 values for inhibition of oxygen evolution rate in spinach chloroplasts and calculated logP values of the compounds tested.
A quasi-parabolic dependence of photosynthesis-inhibiting activity upon the lipophilicity (log P) of the compounds was determined for 1b-4f as well as for 5a-6c (Table 1). The comparison of the biological activity of compounds 1b-4f and 5a-6c having the same lipophilicity showed that the introduction of a halogen substituent in the 6 position led to a partial decrease of the biological activity. The previous study with anilides of 2-alkyl-4-pyridinecarboxylic acids showed that the site of their inhibitory action is the intermediate Z+/D+ corresponding to the tyrosine radicals TyrZ and TyrD which are situated at 161th position in D1 and D2 proteins located on the donor side of photosystem (PS) 2 [10]. The same site of action in the photosynthetic apparatus of spinach chloroplasts can also be expected for the studied compounds 1b-4f and 5a-6c.
From the quasi-parabolic course of the dependence log (1/IC50) vs. log P it can be assumed that the most active inhibitors are compounds with sufficiently high lipophilicity for securing their passage through the lipidic parts of the biological membranes, but enabling also their sufficiently high concentration in the aqueous phase. This is necessary for their interaction with the intermediates Z+/D+ situated at the lumenal side of photosynthetic membranes in D1 and D2 proteins [11].
Acknowledgements
This study was supported by the Ministry of Education, Youth and Sports of the Czech Republic (Research Plan No. 111600001), by the Grant Agency of Charles University (Grant No. 26/1998- BCH), and by the Scientific Grant Agency VEGA of the Slovak Republic (No. 1/7262/20).
Experimental
General
Column chromatography was performed on silica gel (Silpearl, Kavalier Votice). Melting points were determined on a Kofler block, and are uncorrected. IR spectra were recorded on a Nicolet Impact 400 spectrometer in chloroform. 1H-NMR spectra were determined for solutions in CDCl3 or DMSO (substituted 4-pyridinecarboxylic acids) with a BS 587 (Tesla, Brno) 80 MHz apparatus or a Varian Mercury - Vx BB 300 spectrometer operating at 300 MHz. Chemical shifts were recorded as δ values in parts per million (ppm), and were indirectly referenced to tetramethylsilane via the solvent signal (7.26 for 1H). Multiplicities are given together with the coupling constants (in Hz). Elemental analyses were performed on a EA 1110 CHNS-O CE INSTRUMENTS elemental analyser. Lipophilicity of the compounds was computed using a program ACD/LogP version 1.0 (Advanced Chemistry Development Inc., Toronto).
Synthesis of 2-alkylthio-4-cyanopyridines and 2-phenylmethylthio-4-cyanopyridine.
2-Chloro-4-cyanopyridine (10 mmol) and the appropriate thiol (10 mmol) were dissolved in 10 mL of anhydrous N,N-dimethylformamide. To this solution sodium methoxide (10 mmol) in 5 mL methanol was added dropwise with stirring under a nitrogen atmosphere at 20 °C. The stirring was continued until TLC (6:1 hexane-ethyl acetate) indicated completion of the reaction. The reaction mixture was concentrated in vacuo and after evaporation of the solvents, the 2-alkylthio-4- cyanopyridines or 2-phenylmethylthio-4-cyanopyridine were distilled off from the oily product. The boiling points corresponded with the previously described [8].
Synthesis of 2-chloro-6-alkylthio-4-carbamoylpyridines
2,6-Dichloro-4-carbamoylpyridine [13] (10 mmol) and the appropriate thiol (10 mmol) were dissolved in anhydrous N,N-dimethylformamide (10 mL). To the stirred solution sodium methoxide (10 mmol) in methanol (5 mL) was added dropwise. The reaction mixture was stirred at room temperature until TLC (2:1 hexane-ethyl acetate) indicated the reaction was complete. The mixture was then poured into cold water. The crude product was filtered off, purified by column chromatography (2:1 hexane-ethyl acetate), and recrystallised from aqueous ethanol. The boiling points and spectral data agreed with those previously described [9].
Synthesis of 2-alkylthio, 2-phenylmethylthio and 2-chloro-6-alkylthio-4-pyridinecarboxylic acids 1-6
The 2- or 2,6-substituted 4-cyano or carbamoylpyridine (10 mmol) in 10 mL of ethanol was mixed with 25% aqueous sodium hydroxide (30 mmol) and refluxed until the evolution of the ammonia ceased. The reaction mixture was then diluted with twice its volume of water and acidified with 10% hydrochloric acid to pH 4-5. The crude product was collected, washed with water, and recrystallised from aqueous ethanol. TLC for checking of the purity of final products was performed using hexaneethyl acetate-acetic acid (50:45:5) as the mobile phase. The yields, melting points, and elemental analyses are given in Table 2, IR spectral data and 1H-NMR chemical shifts in Table 3.

Table 2.
Analytical data of the prepared 2-alkylthio- and 2-chloro-6-alkylthio-4-pyridinecarboxylic acids.

Table 3.
IR and 1H-NMR spectroscopic data of the 2-alkylthio and 2-chloro-6-alkylthio-4-pyridinecarboxylic acids (DMSO).
General method for the synthesis of anilides of 2-alkylthio, 2-phenylmethylthio and 2-chloro-6- alkylthio-4-pyridinecarboxylic acids (1b,d; 2b,f; 3a,b,d-f; 4a,b,e,f; 5a-c; 6a-c).
A mixture of the 2- or 2,6 substituted-4-pyridinecarboxylic acid (10 mmol) and thionyl chloride (15 mmol) in 10 mL of dry benzene was refluxed for about 1 h. The excess of thionyl chloride was removed by repeated evaporation of dry benzene solutions in vacuo. The resulting crude acyl chloride dissolved in 10 mL of dry acetone was added dropwise to a stirred solution of substituted aniline or aminophenol (10 mmol) in 10 mL of dry pyridine keeping the temperature at 10 °C. After addition of the aniline or aminophenol was complete, stirring at 10 °C was continued for another 30 min. The low temperature was essential in the case of aminophenols in order to avoid the partial esterification of acyl chloride. The reaction mixture was poured into 40 mL of cold water. Crude anilide was collected and recrystallised from aqueous ethanol. TLC was performed using hexane-ethyl acetate (50:50) as the mobile phase. The yields, melting points and elemental analyses of the anilides are given in Table 4, IR spectral data and 1H-NMR chemical shifts in Table 5.

Table 4.
Analytical data of the prepared anilides.

Table 5.
IR and 1H-NMR spectroscopic data of the prepared anilides.
Biological assays
Chloroplasts were prepared from locally purchased spinach according to the procedure described by Walker [14]. The oxygen evolution rate (OER) in spinach chloroplasts was determined spectrophotometrically (Specord UV VIS Zeiss Jena, Germany) by the Hill reaction. The measurements were carried out in phosphate buffer (20 mmol, pH = 7.2) containing sucrose (0.4 mol.dm-3), MgCl2 (5 mmol.dm-3) and NaCl (15 mmol.dm-3) using 2,6-dichlorophenol-indophenol as electron acceptor.
Chlorophyll content in the samples was 30 mg.dm-3 and the samples were irradiated (~ 100 W.m-2) from 10cm distance with a halogen lamp (250 W) using a water filter to prevent warming of the samples (suspension temperature 22 °C). The compounds were dissolved in dimethyl sulfoxide (DMSO) because of their limited water solubility. The applied DMSO concentration (up to 5 %) did not affect OER.
References
- Shipman, L. L. Biochemical Responses Induced by Herbicides; Moreland, D. E., St. John, J. B., Hess, F.D., Eds.; ACS Symposium Series 181; Washington, 1982; p. 23. [Google Scholar]
- Kráľová, K.; Šeršeň, F.; Čižmárik, J. Inhibitory effect of piperidinoethylesters of alkoxyphenyl- carbamic acids on photosynthesis. Gen. Physiol. Biophys. 1992, 11, 261. [Google Scholar]
- Kráľová, K.; Bujdáková, H.; Čižmárik, J. Antifungal and antialgal activity of piperidinopropyl esters of alkoxy substituted phenylcarbamic acids. Pharmazie 1995, 50, 440. [Google Scholar]
- Šeršeň, F.; Kráľová, K. Mechanism of inhibitory action of the local anaesthetic trimecaine on the growth of Chlorella vulgaris algae. Gen. Physiol. Biophys. 1994, 13, 329. [Google Scholar]
- Semin, B. K.; Chudinovskikh, M. N.; Ivanov, I. I. Study of mechanism of inhibition by local anesthetic of electron transport at the donor side of photosystem II. Biokhimiya 1987, 52, 1279. [Google Scholar]
- Semin, B. K.; Chudinovskikh, M. N.; Ivanov, I. I. Local anesthetic-induced inhibition of chloroplast electron transport. Gen. Physiol. Biophys. 1989, 9, 233. [Google Scholar]
- Miletín, M.; Hartl, J.; Macháček, M. Synthesis of some anilides of 2-alkyl-4-pyridinecarboxylic acids and their photosynthesis-nhibiting activity. Collect. Czech. Chem. Commun. 1997, 62, 672. [Google Scholar]
- Klimešová, V.; Otčenášek, M.; Waisser, K. Potential antifungal agents. Synthesis and activity of 2-alkylthiopyrididine-4-carbothioamides. Eur. J. Med. Chem. 1996, 31, 389. [Google Scholar]
- Miletín, M.; Hartl, J.; Doležal, M.; Odlerová, Ž.; Kráľová, K.; Macháček, M. Synthesis of Some 2, 6-Disubstituted 4-Amidopyridines and -Thioamidopyridines, and Their Antimycobacterial and Photosynthesis-Inhibiting activity. Molecules 2000, 5, 208–218. [Google Scholar]
- Kráľová, K.; Šeršeň, F.; Miletín, M.; Hartl, J. Inhibition of photosynthetic electron transport by some anilides of 2-alkylpyridine-4-carboxylic acids in spinach chloroplasts. Chem. Papers 1998, 52, 52. [Google Scholar]
- Svensson, B.; Vass, I.; Styring, S. Sequence analysis of the D1 and D2 reaction center proteins of photosystem II. Z. Naturforsch. 1991, 46c, 765. [Google Scholar] [CrossRef]
- Itai, Sekiima: α-Thioalkyl and α-alkylsulfonyl derivatives of isonicotinic acids and their hydrazides. Bl. Nation. Hyg. Labor. Tokyo 1956, 74, 119, in Chem. Abstr. 1957, 8740.
- Levelt, W.H.; Wibaut, J.P. 2,6-Dibromopyridine-4-carboxylic acid, 2,6-dichloropyridine-4-carboxylic acid and some their derivatives. Rec. Trav. Chim. Pays-Bas 1929, 48, 466. [Google Scholar] [CrossRef]
- Walker, D. A. Preparation of higher plants. In Methods in Enzymology; Colowick, S. P., Kaplan, N. O., Eds.; Academic Press: New York-London-Toronto-Sydney-San Francisco, 1980; Vol. 69, p. 94. [Google Scholar]
Sample availability: Supporting samples of the following compounds are available at MDPI: 5, 1b, 2f, 3b, 3e, 3f, 5a-c, 6a-c. |
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.