Results and Discussion
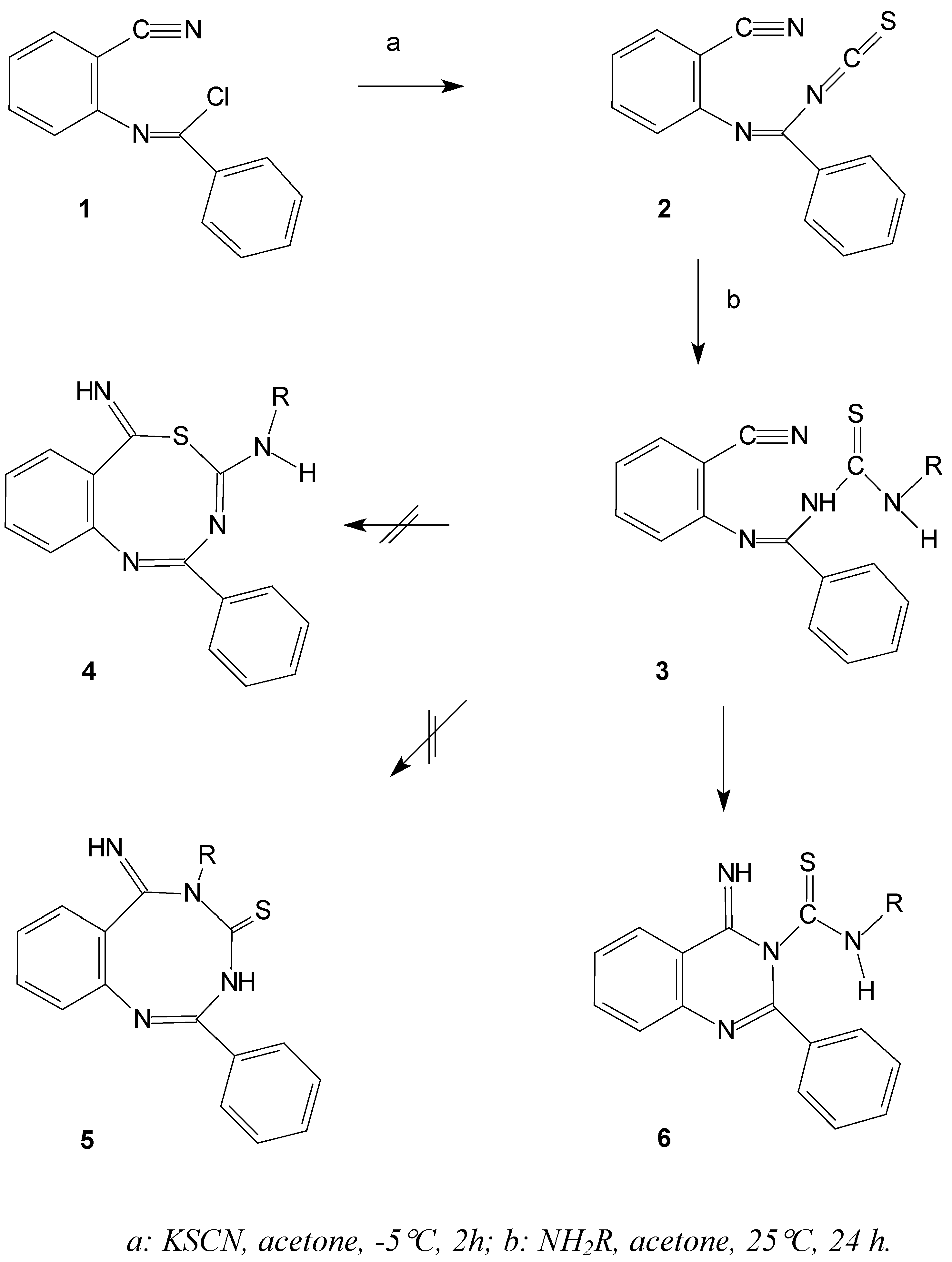
The extension of such a reaction to include primary amines, anilines, heterocyclic amines, and adamantanamine derivatives is now reported in this paper. The reaction proceeds by the addition of primary amines to
N-(2-cyanophenyl)benzimidoyl isothiocyanate (
2) to predictably give the thiourea derivatives
3 [
3] (
Scheme 1).
Scheme 1.
Reaction of the 2 with primary amines and possible reaction pathways
Scheme 1.
Reaction of the 2 with primary amines and possible reaction pathways
These thioureas
3 contain three active nucleophilic sites capable of attacking the available nitrile group
via an intramolecular cycloaddition reaction. The expected products might conceivably be one or all of the following cyclic products: benzothiadiazocine derivatives (
4) arising
via sulfur attack [
4,
5,
6], quinazoline derivatives (
6)
via nitrogen attack [
3] or finally the benzotriazocines (
5)
via nitrogen attack The reaction could be further extended to involve the Dimroth rearrangement product or could involve tautomerisation to the more stable tautomer [
3].
The intramolecular addition reaction of thioureido derivatives containing functionalized carboxyl groups under neutral and basic conditions takes place on the nitrogen atom [
7,
8]. Consequently, the expected products formed were either the quinazoline
6 or the benzotriazocine
5 derivatives. Of these two options we might expect the quinazoline derivative
6 to be more favored due to the fact that their formation involves creation of a thermodynamically more stable six membered ring. The expected quinazoline derivative
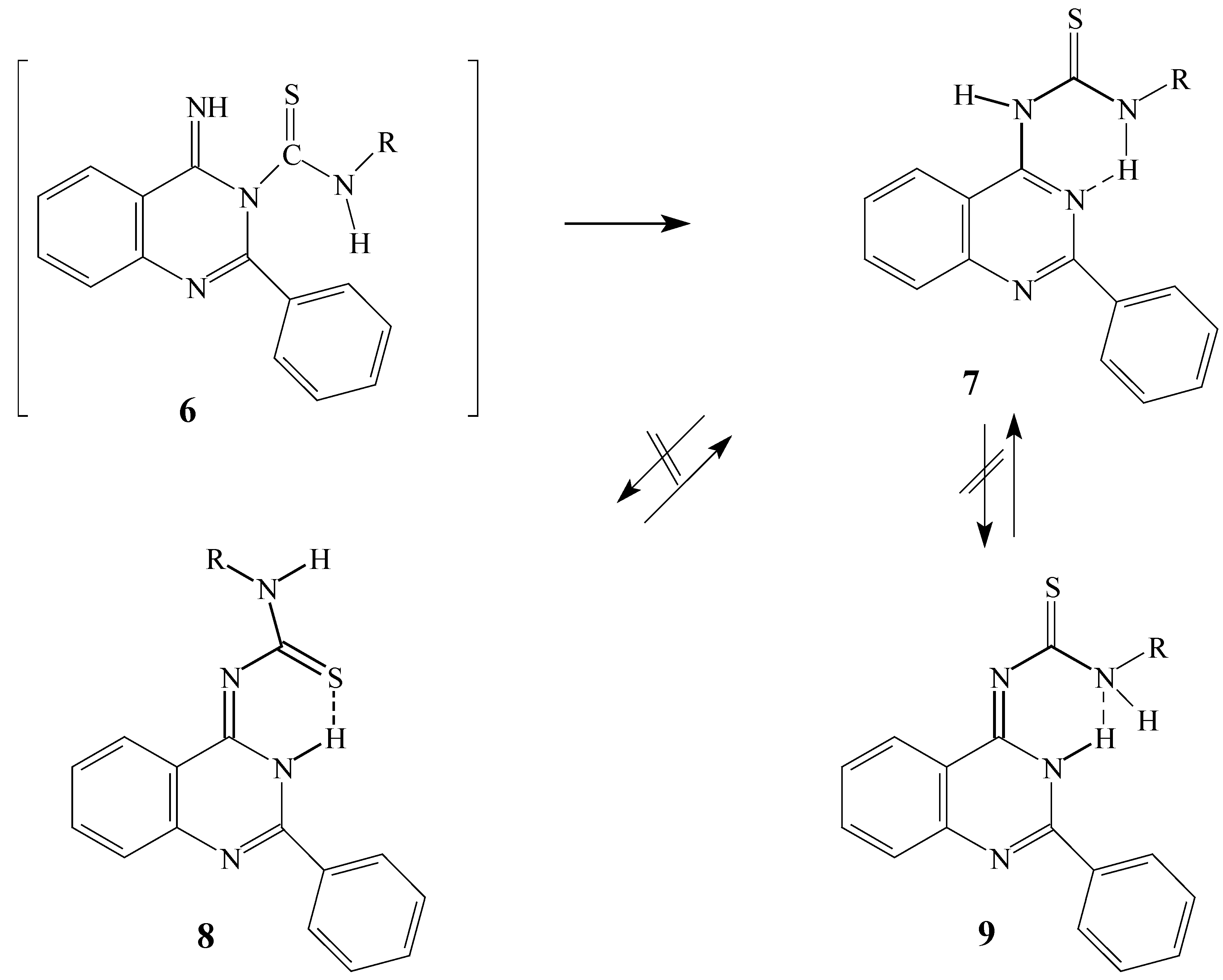
6 might then undergo a Dimroth rearrangement to finally afford the 1-(2-phenylquinazolin-4-yl)-3-substituted thioureas
7. The quinazolines
7 can be stabilized by hydrogen bond interactions to afford an extra six membered ring as shown in
Scheme 2. These compounds
7 could undergo a tautomerisation reaction by the transition of the proton of the N(1) of the substituted thioureas to N(3) of the quinazoline ring to give either of the conformations
8 or
9 that might also be stabilized by hydrogen bond interactions (
Scheme 2).
Scheme 2.
Possible interconversions from 6 to 9 via Dimroth rearrangement and tautomerisation processes.
Scheme 2.
Possible interconversions from 6 to 9 via Dimroth rearrangement and tautomerisation processes.
The spectral data confirmed that the structures of the isolated products were indeed
7. The secondary amine examples behaved differently, undergoing tautomerisation to give the more stable tautomer to thus afford 1,1-disubstituted-3-(2-phenyl-3
H-quinazolin-4-ylidene)thioureas [
3]. Discrimination between the possible structures of compounds
4-
9 was possible using their spectral data as follows: the infrared spectra show both the disappearance of the ν(NCS) and ν(CN) bands present in case of the isothiocyanate
2 and the presence of νNH and ν(C=N) bands at 3220 cm
-1 and 1613 cm
-1, respectively, for the cyclic forms
7.
The most important peak shown by the 13C-NMR, which plays an important role in the structure identification of the cyclic products, is the one corresponding to the C=S group with a chemical shift of ca 183-184 ppm. Naturally, it is observed in the case of the quinazoline structures 6-9 and the benzotriazocines 5 but not the thiadiazocines 4.
The
1H-NMR spectra show great similarity to the simulated computer spectra; they display an important peak at ca 14 ppm (in the case of the substituted anilines) corresponding to the NH group, which together with X-ray analysis, confirms the hydrogen bond interactions between the quinazoline N(3) and the hydrogen atom of the arylamino group (
Scheme 2). The spectra in the case of the aliphatic analogues displayed a peak at ca 12 ppm corresponding to the hydrogen of the alkylamino group. We might expect that the peaks corresponding to NH groups that have interactions with the thiocarbonyl group as in
8 will appear at ca 16 ppm as in the case of secondary amine analogues [
3]. The
1H-NMR spectrum of product
7f (R= Ph) was measured in CDCl
3 at temperature ranging from -30 °C to 30 °C in order to check whether these three conformers
7-
9 interconvert or not, but no changes were observed in the resultant spectra. The
1H-NMR spectrum of
7b gave a doublet peak at ca 4.98 ppm due to the interaction between the CH
2 group and the neighboring NH group. Measurement of the
1H-NMR spectrum in CDCl
3/D
2O, to give a singlet peak at ca 4.97 ppm, proved this.
The mass spectra for all examined products
7 showed the molecular ion, followed by a molecular fragment at m/e 33 which represents the SH moiety and the tricyclic residue of the isolated product at m/e 337 of compound
7g (R= p-tolyl). This last result confirms that the sulfur atom is exocyclic unlike the secondary amine examples where such a fragment is not formed indicating that the sulfur atom is endocyclic. Other fragmentations are represented in
Figure 1
Figure 1.
Mass spectral fragmentations of 7g.
Figure 1.
Mass spectral fragmentations of 7g.
Single crystals of
7f,
7a and
7b (R= Ph, n-butyl and benzyl, respectively) were used for X-ray analysis [
9]. These substituents cover a range of different types to ensure that the structure of the final product is not dependent on the substituent, or the basicity of the amines compared to the anilines [
3]. The structural data presented in
Table 1,
Table 2 and
Table 3 correspond to a great extent with those calculated by the
ab initio DFT quantum chemistry methods for
7f (R= Ph) [
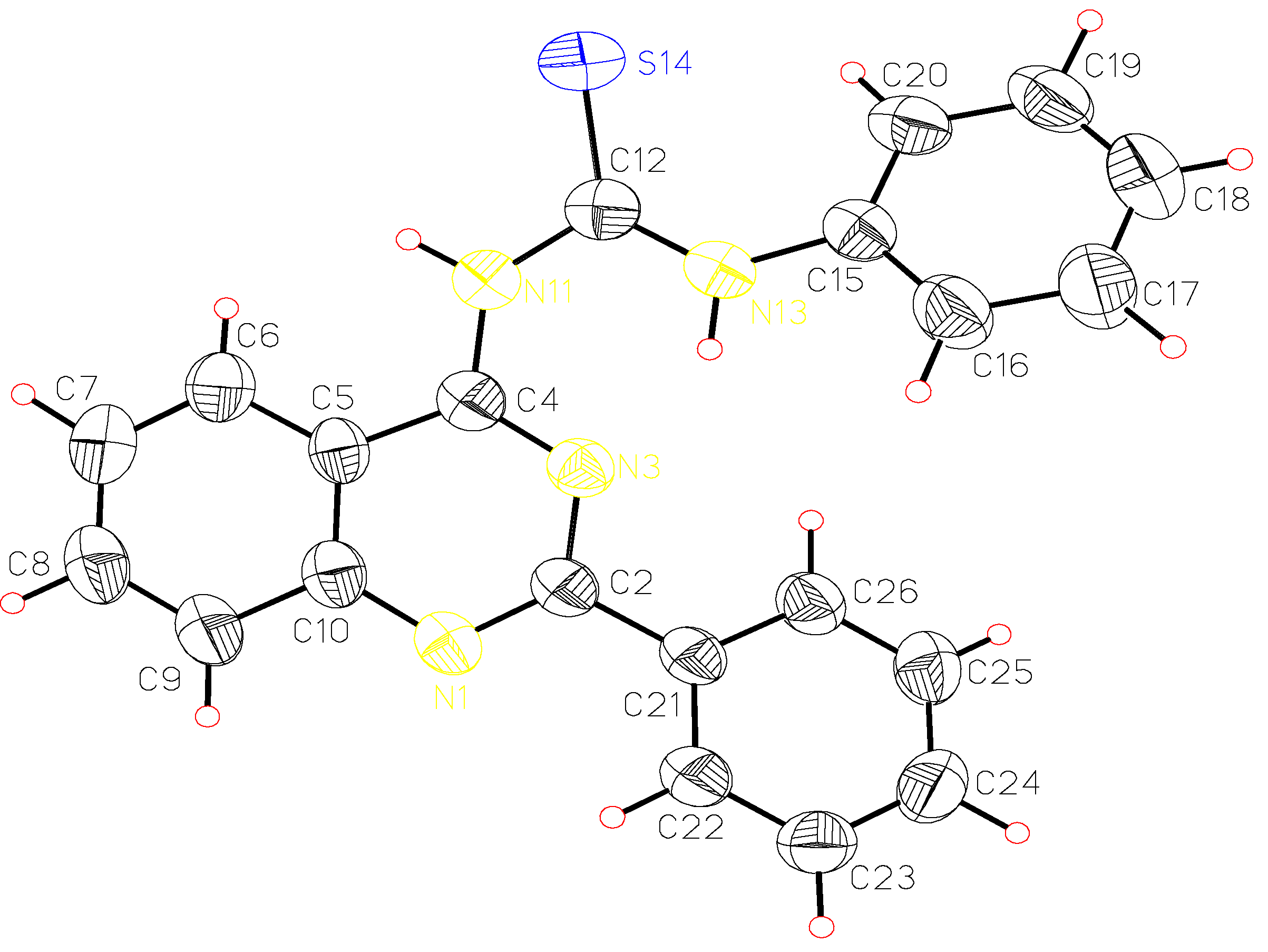
9]. The ORTEP diagram and the calculated model of compound
7f are shown in
Figure 2 and
Figure 3, respectively.
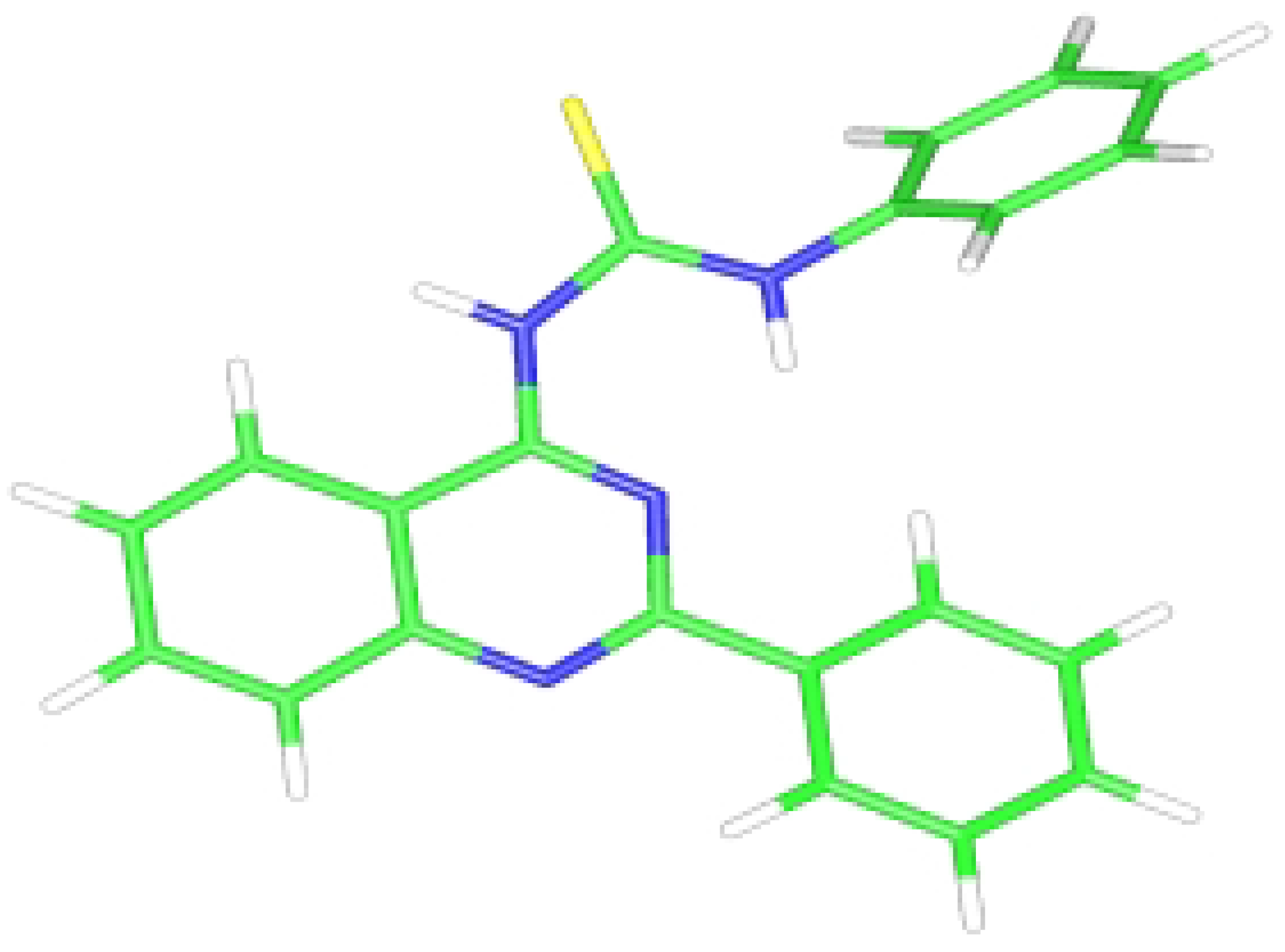
Figure 2.
The ORTEP diagram of compound 7f.
Figure 2.
The ORTEP diagram of compound 7f.
Figure 3.
The calculated model of compound 7f.
Figure 3.
The calculated model of compound 7f.
The X-ray structural analysis shows that the isolated products correspond to the Dimroth rearrangement products
7 and not to the two possible tautomeric conformations
8 and
9. The
ab initio computational studies together with the X-ray analysis show that the compound
7f (R= Ph) is almost planar having the phenyl ring at position 2 slightly out of plane. The C(15) carbon of the phenylthiourea together with the thiourea moiety and the quinazoline cycle are almost in one plane. The π-electrons are in good conjugation as depicted in
Figure 3. The hydrogen bond interactions between the NH group and the N(3) of the quinazoline ring add extra stability to the isolated product
7f (
Scheme 2). These hydrogen bond interactions were identified from the X-ray analysis and the computational studies, which show a bond distance of about 1.86 Å and 1.94 Å, respectively.
There were no great energy differences between the two structures
7f (–891009.73 kcal.mol
-1) and
8f (–891006.05 kcal.mol
-1). This led us to the conclusion that the sulfur atom could be involved in intermolecular hydrogen interactions. However, the probability of this conclusion was decreased after measuring the
1H-NMR at a temperature ranging from –30 °C to 30 °C, which gave the same spectra. The other conclusion, based on the reaction sequence reported in
Scheme 2, is that the major product is the one that is formed first, which identify the formation of the Dimroth rearrangement product as the first step and is the isolated product. On the contrary in the secondary amine application [
3], the Dimroth rearrangement product is not stabilized by hydrogen interactions so it is less stable than the tautomeric form which have hydrogen bond interactions between the N3 hydrogen and the thiocarbonyl group.
Table 1.
Selected interatomic distances in 7f.
Table 1.
Selected interatomic distances in 7f.
| Bond | Bond Length [Å] | Bond | Bond Length [Å] |
|---|
| X-ray | HF/6-31G** | X-ray | HF/6-31G** |
|---|
| N1-C2 | 1.31 | 1.29 | C4-C5 | 1.43 | 1.44 |
| N1-C10 | 1.37 | 1.36 | N11-C12 | 1.39 | 1.38 |
| C2-N3 | 1.36 | 1.36 | C12-N13 | 1.33 | 1.32 |
| C2-C21 | 1.48 | 1.49 | C12-S14 | 1.66 | 1.68 |
| N3-C4 | 1.32 | 1.30 | N13-C15 | 1.42 | 1.43 |
| C4-N11 | 1.38 | 1.38 | C5-C10 | 1.41 | 1.40 |
Table 2.
The most important bond angles in 7f.
Table 2.
The most important bond angles in 7f.
| Angle [°] | X-ray | HF/6-31G** | Angle [°] | X-ray | HF/6-31G** |
|---|
| N1-C2-C21 | 119.2 | 118.4 | N13-C12-N11 | 114.9 | 116.8 |
| C21-C2-N3 | 115.2 | 116.5 | N13-C12-S14 | 127.6 | 126.4 |
| C2-N1-C10 | 116.3 | 116.0 | C12-N13-C15 | 131.5 | 126.8 |
| N1-C2-N3 | 125.6 | 125.1 | C16-C15-N13 | 115.8 | 118.4 |
| C2-N3-C4 | 118.6 | 118.9 | C4-N11-C12 | 130.7 | 132.7 |
| N3-C4-N11 | 118.7 | 120.1 | N1-C10-C5 | 122.8 | 122.3 |
| N3-C4-C5 | 121.4 | 121.5 | C2-C21-C22 | 120.8 | 119.7 |
Table 3.
The most important torsion angles in 7f.
Table 3.
The most important torsion angles in 7f.
| Torsion angle [°] | X-ray | HF/6-31G** | Torsion angle [°] | X-ray | HF/6-31G** |
|---|
| S14-C12-N13-C15 | 1.16 | 0.2640 | C10-N1-C2-C3 | 3.28 | 0.310 |
| N3-C2-C21-C22 | 157.74 | 164.77 | N1-C2- N3-C4 | -1.38 | -0.331 |
| N3-C4-N11-C12 | -4.41 | -3.338 | N1-C2-C21-C22 | -23.62 | -15.107 |
| C4-N11-C12-N13 | 2.59 | 0.0585 | C2-N3-C4-C5 | -2.76 | -0.026 |
| N1-C2-C10-C5 | -1.12 | 0.061 | C2-N3-C4-N11 | 177.88 | 179.752 |
| C2-N1-C10-C9 | 177.17 | 179.922 | C4-N11-C12-S14 | -177.39 | -179.366 |
We confirmed the structures of the compounds 1-(2-phenylquinazolin-4-yl)-3-substituted thioureas
7a-7s on the basis of the above spectral data, reference data from the computer simulated spectra and from knowledge about the chemistry of the isothiocyanate derivative
2 [
3].
Experimental
General
Melting points of all the compounds were measured on a Boetius Rapido PHMK 79/2106 (Wägetechnik) instrument. TLC was carried out on Silufol UV 254 plates (Kavalier, Votice). TLC detection was accomplished with a Fluotes universal (Quarzlampen, Hanau) instrument and iodine vapors. The eluent used was a (20: 80) mixture of acetone and benzene. Purity of compounds
7 was proven by their elemental analysis, measured on an Erba 1102 instrument. FTIR spectra (results reported as
/cm
-1) were taken on a Genesis (Unicam) spectrometer on potassium bromide pellets. NMR spectra were measured on a Bruker Avance DRX-500 spectrometer. Unless stated otherwise the
1H- and
13C-NMR spectra were measured in CDCl
3 solutions and tetramethylsilane was used as an internal standard. Results are reported in ppm on the δ scale. The measured
13C- and
1H-NMR spectra were correlated with those obtained by on-line simulation (Advanced Chemistry Development, Inc., Toronto, Canada). The X-ray crystal data and structure refinement of compound
7f (
Table 4) were collected with a KUMA KM-4 kappa four-circle diffractometer. The structure was solved by direct methods using SHELXS86 [
10] and refined on
F2 for all reflections using SHELXl93 [
11]. Crystals suitable for X-ray determination were obtained in the form of white prisms by recrystallization from CHCl
3/petroleum ether at room temperature. The crystallographic data for
7a,
7b and
7f have been deposited with the Cambridge Crystallographic Data Center as supplementary publications number CCDC 149679 – 149681. Geometry optimization of structures
7a,
7b, and
7f was performed at
ab initio level of quantum chemical calculation, RHF/6-316** and DFT/VWN/6-316**, respectively. Mass spectra were determined (electron impact, 70 eV) with a Fisons TRIO 1000 and GC 8000 series instrument.
Table 4.
Crystal data and structure refinement for 7f.
Table 4.
Crystal data and structure refinement for 7f.
| Empirical formula | C21H16N4S |
| Molecular weight | 356.44 |
| Temperature, k | 293(2) k |
| Wavelength, Å | 0.71073 Å |
| Crystal system, space group | monoclinic, P2 (1) / c |
| Unit cell dimensions | |
| a, Å; α, ° | 9.8405 (8) Å, α= 90 ° |
| b, Å; β, ° | 21.327 (2) Å, β= 95.710 (8)° |
| b, Å; γ, ° | 16.722 (2) Å, γ= 90 ° |
| Volume, Å3 | 3492.0(5) Å |
| Z; density calculated, mg m-3 | 8; 1.356 mg m-3 |
| Absorption coefficient, mm-1 | 0.197 mm-1 |
| F(000) | 1488 |
| Crystal size, mm | 0.65 X 0.25 X 0.10 mm |
| θ Range for data collection, | 3.50- 25.00° |
| Range of
h, k, l | -12< =
h< =13, -27< = k< = 26,
-28< = l< = 21, |
| Reflections collected | 18128 |
| Independent reflections | 6123 [R(INT) = 0.04231] |
| Refinement method | full-matrix least-squares on
F2 |
| Data; restraints; parameters | 5483 / 0 / 597 |
| Goodness-of-fit on F2 | 0.950 |
| Final R indices [I > 2σ(I)] | R1 = 0.0462, wR2 = 0.1192 |
| R indices (all data) | R1 = 0.0818, wR2 = 0.1322 |
| Largest diff. Peak and hole | 0.230 and –0.260 e. Å-3 |
N-(2-cyanophenyl)benzimidoyl isothiocyanate (2) - acetone solution.
A mixture of
N-(2-cyanophenyl)benzamide [
3] (0.5 g, 2.25 mmol), and phosphorous pentachloride (0.5 g, 2.4 mmol) in dry toluene were refluxed for 8 h. The solvent was removed under reduced pressure to give a brownish colored oil of
N-(2-cyanophenyl)benzimidoyl chloride (
1) [
3] which was not further purified. A solution of potassium thiocyanate (0.22 g, 2.25 mmol) in dry acetone was added portionwise over 2 h to a stirred and cooled (-5 °C) solution of crude
1 in dry acetone. The precipitated potassium chloride was filtered off to give the acetone solution of
N-(2-cyanophenyl)benzimidoyl isothiocyanate (
2). The IR spectrum of this solution displayed a strong peak at 2059 cm
-1 corresponding to the νNCS band. The isothiocyanate was used without further isolation and purification (to avoid polymerization and destruction of this very reactive compound).
General procedure for the synthesis of 1-substituted–3-(2-phenylquinazolin-4-yl) thioureas (7).
The appropriate primary amine (2.25 mmol) was added portionwise while stirring at room temperature over a period of 1h to the acetone solution of isothiocyanate 3. An additional amount of triethylamine (0.6 ml, 4.5 mmol) was added in case of the adamantylamine hydrochloride examples 7q-7s. The reaction mixture was then stirred for 24h. The precipitated quinazoline thiourea 7 was filtered off and recrystallized from ethyl alcohol. Spectral data are given below.
1-Butyl-3-(2-phenylquinazolin-4-yl) thiourea (7a).
(NH2R= butylamine); Yield: 0.21 g (29%); M.p. 165-166 °C; Calc. for C19H20N4S (336.45): 67.83% C, 5.99% H, 16.65% N, 9.53% S; Found: 67.64% C, 5.81% H, 16.48% N, 9.43% S; FTIR: 3427.6 (NH), 2952.8, 2928.0, 2869.2 (CH), 1618.2 (C=N); 1H-NMR: 12.16 (1H, s, NH), 8.79 (1H, s, NH), 8.24-7.48 (9H, m, ArH), 3.79-3.75 (2H, m, NHCH2), 1.83-1.76 (2H, m, NCH2CH2), 1.49-1.42 (2H, m, HNCH2CH2CH2), 0.94 (3H, t, CH2CH3) (JA,B= 7.31 Hz); 13C-NMR: 180.11 (C=S), 158.90 (Cq), 156.16 (Cq), 151.57 (Cq), 137.70 (Cq), 134.57 (CHAr), 131.18 (CHAr), 129.89 (CHAr), 128.93 (CHAr), 128.38 (CHAr), 127.81 (CHAr), 120.67 (CHAr), 112.70 (Cq), 46.55 (NCH2), 31.05 (NCH2CH2), 20.60 (HNCH2CH2CH2), 13.99 (CH3); MS, m/z (Ir/%): 337 (24), 336 (71), 304 (15), 303 (82), 264 (58), 247 (28), 222 (59), 221 (100), 206 (37), 205 (89), 130 (15), 118 (38), 104 (25), 102 (36), 77 (50), 72 (18), 41 (17).
1-Benzyl-3-(2-phenylquinazolin-4-yl) thiourea (7b).
(NH2R= benzylamine); Yield: 0.3 g (36%); M.p. 189-190 °C; Calc. for C22H18N4S (370.47): 71.33% C, 4.90% H, 15.12% N, 8.65% S; Found: 71.24% C, 4.88% H, 15.03% N, 8.59% S; FTIR: 3292.1 (NH), 3024.58 (CH), 1619.81 (C=N); 1H-NMR: 12.31 (1H, s, NHCH2), 8.88 (1H, s, NH), 8.03-7.18 (14H, m, ArH), 4.98 (2H, d, NHCH2) (JA,B= 4.66 Hz); 13C-NMR: 179.82 (C=S), 158.90 (Cq), 156.00 (Cq), 151.36 (Cq), 136.89 (Cq), 136.49 (Cq), 134.62 (CHAr), 130.94 (CHAr), 129.77 (CHAr), 129.37 (CHAr), 129.20 (CHAr), 128.85 (CHAr), 128.59 (CHAr), 128.15 (CHAr), 127.83 (CHAr), 120.59 (CHAr), 112.57 (Cq), 51.31 (NCH2); MS, m/z (Ir/%): 371 (12), 370 (28), 339 (9), 337 (48), 264 (22), 263 (41), 239 (11), 222 (64), 221 (100), 206 (27), 205 (94), 149 (32), 118 (38), 104 (25), 102 (34), 91 (100), 77 (53), 65 (45), 51 (25).
1-Allyl-3-(2-phenylquinazolin-4-yl) thiourea (7c).
(NH2R= allylamine); Yield: 0.18 g (25%); M.p. 189-190 °C; Calc. for C18H16N4S (320.41): 67.48% C, 5.03% H, 17.49% N, 10.01% S; found: 67.32% C, 4.98% H, 17.37% N, 9.83% S; FTIR: 3441.6 (NH), 3021.8 (CH), 1620.6 (C=N); 1H-NMR: 12.30 (1H, s, NH), 8.83 (1H, s, NH), 8.30-7.49 (9H, m, ArH), 6.19-6.06 (1H, m, CH=CH2), 5.47 (1H, d, CH=CH2) (JA,B= 17.2 Hz), 5.38 (1H, d, CH=CH2) (JA,B= 9.9 Hz), 4.49 (2H, m, HNCH2); 13C-NMR: 180.16 (C=S), 158.65 (Cq), 156.04 (Cq), 151.53 (Cq), 137.27 (Cq), 134.61 (CHAr), 132.73(1H, m, CH=CH2), 131.21 (CHAr), 129.85 (CHAr), 128.90 (CHAr), 128.42 (CHAr), 127.83 (CHAr), 120.63 (CHAr),118.98 (1H, m, CH=CH2), 112.59 (Cq), 49.16 (NCH2).
1-Isobutyl-3-(2-phenylquinazolin-4-yl) thiourea (7d).
(NH2R= isobutylamine); Yield: 0.14 g (19%); M.p. 150-151 °C; Calc.for C19H20N4S (336.45): 67.83% C, 5.99% H, 16.65% N, 9.53% S; found: 67.75% C, 5.92% H, 16.59% N, 9.52% S; FTIR: 3427.65 (NH), 2957.7, 2918.7 (CH), 1620.29 (C=N); 1H-NMR: 12.26 (1H, s, NH), 8.95 (1H, s, NH), 8.41-7.61 (9H, m, ArH), 3.78 (2H, t, NHCH2) (JA,B= 6.57 Hz), 2.32-2.25 (1H, m, CH(CH3)2), 1.18 (6H, d, CH(CH3)2) (JA,B= 6.57 Hz); 13C-NMR: 180.18 (C=S), 158.83 (Cq), 156.12 (Cq), 151.41 (Cq), 137.52 (Cq), 134.57 (CHAr), 131.18 (CHAr), 129.75 (CHAr), 128.83 (CHAr), 128.31 (CHAr), 127.80 (CHAr), 120.68 (CHAr), 112.60 (Cq), 54.38 (NHCH2), 28.16 (NCH2CH), 20.75 (CH3).
1-Cyclohexyl-3-(2-phenylquinazolin-4-yl) thiourea (7e).
(NH2R= cyclohexylamine); Yield: 0.25 g (30%); M.p. 154-155 °C; Calc.for C21H22N4S (362.49): 69.58% C, 6.12% H, 15.46% N, 8.84% S; Found: 69.41% C, 6.12% H, 15.38% N, 8.74% S; FTIR: 3441.7, 3425.8 (NH), 1619.3 (C=N); 1H-NMR: 12.08 (1H, b, NHCH), 8.75 (1H, s, NH), 8.32-7.54 (9H, m, ArH), 4.28-4.38 (1H, m, NHCH), 2.34-2.27 (2H, m, CH2), 1.91-1.71 (4H, m, 2CH2), 1.55- 1.38 (4H, m, 2CH2); 13C-NMR: 178.54 (C=S), 158.90 (Cq), 156.07 (Cq), 151.45 (Cq), 137.69 (Cq), 134.55 (CHAr), 131.15 (CHAr), 129.77 (CHAr), 128.92 (CHAr), 128.40 (CHAr), 127.80 (CHAr), 120.65 (CHAr), 112.66 (Cq), 55.49 (CH), 32.78 (CH2), 32.78 (CH2), 25.87 (CH2), 25.17 (CH2).
1-Phenyl-3-(2-phenylquinazolin-4-yl)thiourea (7f).
(NH2R= aniline); Yield: 0.35 g (43%); M.p. 165-166oC; Calc.for C21H16N4S (356.44): 70.76% C, 4.52% H, 15.72% N, 8.99% S; Found: 70.58% C, 4.52% H, 15.64% N, 8.83% S; FTIR: 3436.3, 3418.7 (NH), 1617.3 (C=N); 1H-NMR: 14.26 (1H, s, NHPh), 8.93 (1H, s, NH), 8.35-7.29 (14H, m, ArH); 13C-NMR: 178.62 (C=S), 158.57 (Cq), 155.96 (Cq), 151.64 (Cq), 144.85(Cq), 138.49 (Cq), 137.36 (Cq), 134.85 (CHAr), 131.31 (CHAr), 129.93 (CHAr), 129.24 (CHAr), 128.39 (CHAr), 128.03 (CHAr), 126.88 (CHAr), 124.13 (CHAr), 120.65 (CHAr), 112.62 (Cq); MS, m/z (Ir/%): 356 (6), 323 (7), 276 (9), 264 (12), 263 (51), 222 (13), 221 (100), 205 (95), 178 (8), 135 (49), 118 (17), 104 (7), 102 (20), 93 (21), 91 (6), 77 (57), 51 (25).
1-(4-Methylphenyl)-3-(2-phenylquinazolin-4-yl) thiourea (7g).
(NH2R= p-toluidine); Yield: 0.31 g (38%); M.p. 179-180oC; Calc. for C22H18N4S (370.47): 71.33% C, 4.90% H, 15.12% N, 8.65% S; Found: 71.21% C, 4.89% H, 15.11% N, 8.62% S; FTIR: 3422.5 (NH), 1619.9(C=N); 1H-NMR: 14.16 (1H, s, NHPh), 8.92 (1H, s, NH), 8.36-7.29 (13H, m, ArH), 2.40 (3H, s, CH3); 13C-NMR: 178.62 (C=S), 158.57 (Cq), 155.99 (Cq), 151.52 (Cq), 144.85 (Cq), 138.49 (Cq), 137.30 (Cq), 136.83 (Cq), 134.84 (CHAr), 131.32 (CHAr), 129.82 (CHAr), 129.09 (CHAr), 128.38 (CHAr), 128.03 (CHAr), 124.18 (CHAr), 120.67 (CHAr), 112.63 (Cq), 21.33 (CH3); MS, m/z (Ir/%): 337 (4), 264 (18), 263 (62), 222 (33), 221 (85), 206 (19), 205 (100), 149 (54), 148 (18), 118 (15), 107 (71), 106 (58), 91 (29), 77 (29).
1-(3-Methylphenyl)-3-(2-phenylquinazolin-4-yl) thiourea (7h).
(NH2R= m-toluidine); Yield: 0.36 g (43%); M.p. 157-158oC; Calc. for C22H18N4S (370.47): 71.33% C, 4.90% H, 15.12% N, 8.65% S; Found: 71.32% C, 4.90% H, 15.10% N, 8.65% S; FTIR: 3405.2 (NH), 1618.7 (C=N); 1H-NMR: 14.22 (1H, s, NHPh), 8.91 (1H, s, NH), 8.38-7.11 (13H, m, ArH), 2.41 (3H, s, CH3); 13C-NMR: 178.53 (C=S), 158.56 (Cq), 155.84 (Cq), 151.38 (Cq), 144.76(Cq), 138.49 (Cq), 137.30 (Cq), 136.83 (Cq), 134.86 (CHAr), 131.37 (CHAr), 129.87 (CHAr), 129.06 (CHAr), 128.46 (CHAr), 128.05 (CHAr), 127.66 (CHAr), 124.64 (CHAr), 121.07 (CHAr), 120.67 (CHAr), 112.63 (Cq), 21.69 (CH3).
1-(2-Methylphenyl)-3-(2-phenylquinazolin-4-yl) thiourea (7I).
(NH2R= o-toluidine); Yield: 0.42 g (51%); M.p. 157-158oC; Calc. for C22H18N4S (370.47): 71.33% C, 4.90% H, 15.12% N, 8.65% S; Found: 71.03% C, 4.64% H, 14.92% N, 8.41% S; FTIR: 3325.2 (NH), 1623.59 (C=N); 1H-NMR: 14.22 (1H, s, NHPh), 8.91 (1H, s, NH), 8.38-7.11 (13H, m, ArH), 2.41 (3H, s, CH3); 13C-NMR: 178.53 (C=S), 158.56 (Cq), 155.84 (Cq), 151.38 (Cq), 144.76(Cq), 138.49 (Cq), 137.30 (Cq), 136.83 (Cq), 134.86 (CHAr), 131.37 (CHAr), 129.87 (CHAr), 129.06 (CHAr), 128.46 (CHAr), 128.05 (CHAr), 127.66 (CHAr), 124.64 (CHAr), 121.07 (CHAr), 120.67 (CHAr), 112.63 (Cq), 21.69 (CH3).
1-(4-Methoxyphenyl)-3-(2-phenylquinazolin-4-yl) thiourea (7j).
(NH2R= p-anisidine); Yield: 0.34 g (40%); M.p. 168-169oC; Calc. for C22H18N4OS (386.47): 68.37% C, 4.69% H, 14.50% N, 8.30% S; Found: 68.24% C, 4.57% H, 14.49% N, 8.23% S; FTIR: 3428.2 (NH), 2934.9 (CH), 1616.8 (C=N); 1H-NMR: 14.09 (1H, s, NHPh), 8.91 (1H, s, NH), 8.35- 6.98 (13H, m, ArH), 3.86 (3H, s, OCH3); 13C-NMR: 178.78 (C=S), 158.39 (Cq), 155.98 (Cq), 152.49 (Cq), 151.55 (Cq), 141.78 (Cq), 137.32 (Cq), 134.86 (CHAr), 131.4(Cq), 131.32 (CHAr), 129.87 (CHAr), 129.09 (CHAr), 128.35 (CHAr), 128.03 (CHAr), 125.78 (CHAr), 120.67 (CHAr), 114.48 (CHAr), 113.45 (Cq), 112.63 (Cq), 55.75 (OCH3).
1-(4-nitrophenyl)-3-(2-phenylquinazolin-4-yl) thiourea (7k).
(NH2R= p-nitroaniline); Yield: 0.48 g (53%); M.p. 165-166oC; Calc. for C21H15N5O2S (401.44): 62.83% C, 3.77% H, 17.45% N, 7.99% S; Found: 62.66% C, 3.58% H, 17.24% N, 7.84% S; FTIR: 3333.0 (NH), 1615.7 (C=N); 1H-NMR (CF3COOD): 8.87-7.94 (13H, m, ArH); 13C-NMR: 179.93 (C=S), 161.56 (Cq), 160.34 (Cq), 147.97 (Cq), 145.71 (Cq), 142.34 (Cq), 142.01 (CHAr), 138.41 (CHAr), 133.84 (CHAr), 132.67 (CHAr), 131.27 (Cq), 130.90 (CHAr), 127.36 (CHAr), 126.21 (CHAr), 125.73 (CHAr), 122.65 (CHAr), 114.20 (Cq).
1-(3-nitrophenyl)-3-(2-phenylquinazolin-4-yl)thiourea (7l).
(NH2R= m-nitroaniline); Yield: 0.39 g (41%); M.p. 174-175oC; Calc. for C21H15N5O2S (401.44): 62.83% C, 3.77% H, 17.45% N, 7.99% S; Found: 62.74% C, 3.64% H, 17.32% N, 7.87% S; FTIR: 3315.6 (NH), 1620.8 (C=N); 1H-NMR (CF3COOD): 8.87-7.75 (13H, m, ArH); 13C-NMR: 180.60 (C=S), 161.55 (Cq), 160.60 (Cq), 150.54 (Cq), 142.41 (Cq), 142.34 (Cq), 142.07 (CHAr), 140.60 (Cq), 138.47 (CHAr), 133.90 (CHAr), 132.91 (CHAr), 132.82 (CHAr), 132.76 (CHAr), 131.27 (Cq), 130.97 (CHAr), 125.91 (CHAr), 124.93 (CHAr), 122.71 (CHAr), 121.58(CHAr), 114.30 (Cq).
1-(4-Chlorophenyl)-3-(2-phenylquinazolin-4-yl) thiourea (7m).
(NH2R= p-chloroaniline); yield: 048 g (55%); M.p. 187-188oC; Calc.for C21H15ClN4S (390.89): 64.53% C, 3.87% H, 9.07% Cl, 14.33% N, 8.20% S; found: 64.34% C, 3.69% H, 9.01% Cl, 14.21% N, 8.16% S;; FTIR: 3430.3, 3339.3 (NH), 1620.40 (C=N); 1H-NMR (CF3COOD): 8.74- 7.48 (13H, m, ArH); 13C-NMR: 180.40 (C=S), 161.44 (Cq), 160.73 (Cq), 142.28 (Cq), 141.98 (CHAr), 138.41 (CHAr), 137.24 (Cq), 136.92 (Cq), 133.80 (CHAr), 132.63 (CHAr), 131.07 (Cq), 130.88(CHAr), 127.84 (CHAr), 125.86 (CHAr), 122.58 (CHAr), 114.27 (Cq).
1-(1-Naphthyl)-3-(2-phenylquinazolin-4-yl) thiourea (7n).
(NH2R= 1-naphthylamine); Yield: 0.41 g (45%); M.p. 168-169oC; Calc. for C25H18N4S (406.50): 73.87% C, 4.46% H, 13.78% N, 7.89% S; Found: 73.83% C, 4.46% H, 13.77% N, 7.84% S; FTIR: 3250.1 (NH), 3055.0, 2943.4 (CH), 1619.4 (C=N); 1H-NMR: 14.27 (1H, s, NHPh), 9.16 (1H, s, NH), 8.28-7.29 (19H, m, ArH); 13C-NMR: 180.79 (C=S), 164.76 (Cq), 158.76 (Cq), 156.24 (Cq), 151.73 (Cq), 141.70 (Cq), 137.02 (Cq), 134.90 (CHAr), 134.61(Cq), 131.19 (CHAr), 129.97 (CHAr), 129.02 (CHAr), 128.87 (CHAr), 128.55 (CHAr), 128.24 (CHAr), 128.05 (CHAr), 127.16 (CHAr), 126.64 (CHAr), 125.65 (CHAr), 124.71 (CHAr), 122.41 (CHAr), 120.19 (CHAr), 112.74 (Cq).
1-(2-Naphthyl)-3-(2-phenylquinazolin-4-yl) thiourea (7o).
(NH2R= 2-naphthylamine); Yield: 0.55 g (66%); M.p. 179-180oC; Calc. for C25H18N4S (406.50): 73.87% C, 4.46% H, 13.78% N, 7.89% S; Found: 73.74% C, 4.38% H, 13.61% N, 7.80% S; FTIR, /cm-1: 3429.1, 3374.2 (NH), 3057.6 (CH), 1618.4 (C=N); 1H-NMR (CF3COOD): 8.72-7.63 (19H, m, ArH); 13C-NMR (CF3COOD): 179.30 (C=S), 161.29 (Cq), 160.30 (Cq), 142.09 (Cq), 141.83 (CHAr), 138.41 (CHAr) 136.08 (Cq), 135.59 (Cq), 135.02 (Cq) , 133.68 (CHAr), 132.59 (CHAr), 131.86 (CHAr), 131.05 (Cq), 130.99 (CHAr), 130.67 (CHAr), 129.89 (CHAr), 129.63 (CHAr), 126.46 (CHAr), 125.68 (CHAr), 124.82 (CHAr), 122.41 (CHAr), 114.07 (Cq).
1-(2-phenylquinazolin-4-yl)-3-(2-pyridyl) thiourea (7p).
(NH2R= 2-aminopyridine); Yield 0.33 g (41%); M.p. 207-208oC; Calc.for C20H15N5S (357.43): 67.21% C, 4.23% H, 19.59% N, 8.97% S; Found: 67.04% C, 3.97% H, 19.22% N, 8.83% S; FTIR, /cm-1: 3348.7 (NH), 3059.4 (CH), 1621.7 (C=N); 1H-NMR (DMSO-d6): 8.71-7.27 (13H, m, ArH); 13C-NMR: 179.57 (C=S), 161.05 (Cq), 155.96 (Cq), 151.64 (Cq), 144.85(Cq), 138.49 (Cq), 135.90 (Cq), 137.73 (CHAr), 134.08 (CHAr), 130.36 (CHAr), 127.98 (CHAr), 127.69 (CHAr), 126.76 (CHAr), 123.26 (CHAr), 119.93 (CHAr), 114.25 (Cq); MS, m/z (Ir/%): 357 (14), 324 (7), 264 (30), 263 (49), 222 (21), 221 (100), 205 (82), 178 (8), 136 (55), 118 (28), 104 (13), 102 (29), 94 (27), 78 (56), 77 (38), 51 (27).
1-(1-Adamantyl)-3-(2-phenylquinazolin-4-yl) thiourea (7q).
(NH2R= 1-adamantanamine hydrochloride); Yield: 0.29g (31%); M.p. 202-203 °C; Calc.for C25H26N4S (414.50): 72.43% C, 6.32% H, 13.52% N, 7.73% S; Found: 72.31% C, 6.27% H, 13.41% N, 7.54% S; FTIR, /cm-1: 3445.3 (NH), 2909.9 (CH), 2854.9 (CH), 1622.5 (C=N); 1H- NMR: 11.77 (1H, s, NH), 8.53 (1H, s, NH), 8.27-7.43 (9H, m, ArH), 2.53-2.41 (6H, m, 3CH2), 2.26-2.09 (6H, m, 3CH2), 1.84-1.68 (3H, m, 3CH); 13C-NMR: 177.48 (C=S), 156.07 (Cq), 149.83 (Cq), 137.91 (Cq), 134.63 (CHAr), 131.02 (CHAr), 129.81 (CHAr), 128.70 (CHAr), 127.68 (CHAr), 120.67 (CHAr), 112.75 (Cq), 55.91 (NHCAd), 41.10 (CH2), 36.67 (CH2), 29.97 (CH); MS, m/z (Ir/%): 414.2 (4), 380.3 (2), 264 (7), 263 (21), 222 (32), 221 (75), 205 (65), 193 (15), 151 (16), 135 (100), 118 (17), 94 (45), 77 (29).
1-(3-Homoadamantyl)-3-(2-phenylquinazolin-4-yl) thiourea (7r).
(NH2R= 3-homoadamantanamine hydrochloride); Yield: 0.3 g (33%); M.p. 217-218 °C; Calc.for C26H28N4S (428.59): 72.86% C, 6.58% H, 13.07% N, 7.48% S; Found: 72.43% C, 6.48% H, 13.01% N, 7.24% S; FTIR, /cm-1: 3436.2 (NH), 2909.9 (CH), 2849.9 (CH), 1620.4 (C=N); 1H- NMR: 11.98 (1H, s, NH), 8.64 (1H, s, NH), 8.36-7.54 (9H, m, ArH), 2.61-1.53 (15H, m, Ad); 13C- NMR: 176.92 (C=S), 159.19 (Cq), 156.03 (Cq), 151.30 (Cq), 137.86 (Cq), 134.45 (CHAr), 131.03 (CHAr), 129.71 (CHAr), 128.71 (CHAr), 127.72 (CHAr), 120.73 (CHAr), 112.65 (Cq), 60.98 (CH2), 43.15 (CH2), 37.84 (CH2), 35.56 (CH2), 31.22 (CH), 31.18 (CH2), 27.82 (CH); MS, m/z (Ir/%): 428.2 (3), 264 (12), 263 (34), 222 (48), 221 (86), 205 (83), 165 (12), 149 (100), 118 (13), 94 (23), 77 (29).
1-(1-Adamantylmethyl)-3-(2-phenylquinazolin-4-yl) thiourea (7s).
[NH2R= 1-(1-adamantylmethylamine hydrochloride)]; Yield: 0.33 g (34%); M.p. 197-198 °C; Calc. for C26H28N4S (428.53): 72.86% C, 6.59% H, 13.07% N, 7.48% S; Found: 72.86% C, 6.57% H, 13.04% N, 7.39% S; FTIR, /cm-1: 3450.3 (NH), 2909.9 (CH), 2849.5 (CH), 1620.0 (C=N); 1H- NMR: 12.04 (1H, s, NH), 8.90 (1H, s, NH), 8.31-7.51 (9H, m, ArH), 3.69 (2H, d, NHCH2) (JA,B= 6.14 Hz), 1.96-1.56 (15H, m, Ad); 13C-NMR: 180.75 (C=S), 156.08 (Cq), 151.43 (Cq), 138.70 (Cq), 137.76 (Cq), 134.63 (CHAr), 131.04 (CHAr), 129.78 (CHAr), 128.78 (CHAr), 127.85 (CHAr), 120.77 (CHAr), 112.67 (Cq), 58.74 (NHCH2), 40.85 (CH2 Ad), 36.95 (CH2 Ad), 34.82 (CqAd), 28.38 (CH); MS, m/z (Ir/%): 428 (9), 395 (5), 264 (15), 263 (24), 222 (41), 221 (49), 205 (64), 165 (11), 149 (12), 135 (100), 118 (9), 93 (19), 77 (23).