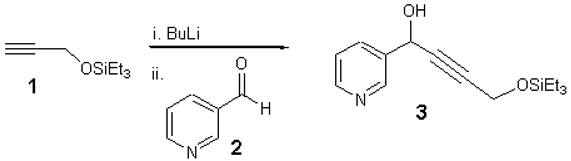
1-(Pyridin-3-yl)-4-(triethylsilyloxy)-2-butyn-1-ol
References
- Brimble, M. A.; Duncalf, L. J. Molecules 2000, 5, 162–166. [CrossRef]
Sample Availability: available from the authors and MDPI. |
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.
Share and Cite
Caprio, V.E.; Jones, M.W.; Brimble, M.A. 1-(Pyridin-3-yl)-4-(triethylsilyloxy)-2-butyn-1-ol. Molecules 2001, 6, M207. https://doi.org/10.3390/M207
Caprio VE, Jones MW, Brimble MA. 1-(Pyridin-3-yl)-4-(triethylsilyloxy)-2-butyn-1-ol. Molecules. 2001; 6(3):M207. https://doi.org/10.3390/M207
Chicago/Turabian StyleCaprio, Vittorio E., Michael W. Jones, and Margaret A. Brimble. 2001. "1-(Pyridin-3-yl)-4-(triethylsilyloxy)-2-butyn-1-ol" Molecules 6, no. 3: M207. https://doi.org/10.3390/M207
APA StyleCaprio, V. E., Jones, M. W., & Brimble, M. A. (2001). 1-(Pyridin-3-yl)-4-(triethylsilyloxy)-2-butyn-1-ol. Molecules, 6(3), M207. https://doi.org/10.3390/M207



