As part of a research programme targeting novel molecules as potential anti-inflammatory agents we synthesised 3-Chloro-5-methoxy-1-benzo[b]thiophene-2-sulphonylamide base on the reported anti-inflammatory activity of the structurally related molecule 3-isopropoxy-5-methoxy-N-(1H-1,2,3,4-tetraazol-5-yl)-1-benzothiophene-2-carboxamide [1,2].
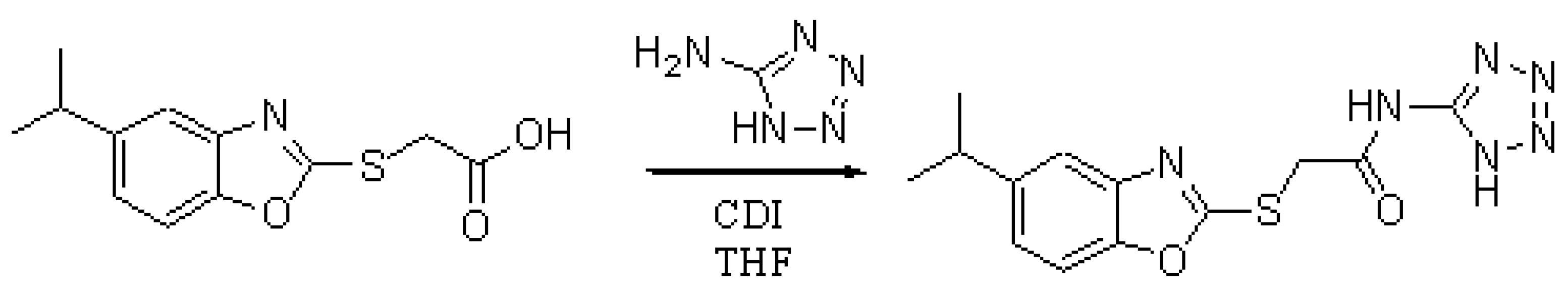
(5-Isopropyl benz-2-oxazole) acetic acid (150.0 mg, 0.60 mmol) was dissolved in anhydrous THF (5.0 mL) and CDI (107.0 mg, 0.66 mmol) was added and the reaction mixture was heated to reflux for 1.5 hours under an atmosphere of nitrogen. The reaction mixture was allowed to cool and 5-aminotetrazole (56.0 mg, 0.66 mmol) was added and the reaction mixture was heated to reflux for 2.5 hours. The reaction mixture was allowed to cool and the reaction mixture was poured into water (60.0 mL) and the aqueous solution was acidified with concentrated hydrochloric acid to form a precipitate which was collected by filtration and washed well with water and dried to afford (23.0 mg, 12.1 %) of the desired 5-isopropyl-N-(1H-1,2,3,4-tetrazol-5-yl) thiobenzo-2-oxazole acetamide as a colourless solid.
M.p. 223.5-225 °C.
MS: 319 (M + 1).+.
1H NMR (300 MHz, DMSO-d6): 1.20 (d, J = 6.90 Hz, 6H, CH(CH3)2), 2.94 (m, 1H, CH(CH3)2,), 4.44 (s, 2H, SCH2), 7.18 (dd, J = 1.53, J = 8.40 Hz, 1H, ArH), 7.48 (s, 1H, ArH), 7.52 (d, J = 8.40 Hz, 1H, ArH).
Anal. ca.cd. for C13H14N6O2S C 49.05, H 4.43, N 26.40: found C 48.76, H 4.49, N 26.25.
IR: 3100, 3050, 2900, 1600, 1500, 1490, 1450, 1290, 1250, 1100, 1090, 1050, 910, 740, 690.
HPLC retention time = 5.35 minutes . (10 % B/90 % D) to (90 % B/10 % D) over 20 minutes (B = 90 % CH3CN 10 % H2O) (D = 0.1N NH4OAc (pH = 4)) using Zorbax 4.6 mm x 250 mm.
Supplementary materials
Supplementary File 1Supplementary File 2References
- Connor, D. T.; Cetenko, M. D.; Mullikan, R. J.; Sorenson, P. C.; Unganst, R. J.; Weikert, R. L.; Adolphson, J. A.; Kennedy, D. O.; Thueson, C. D.; Wright, M. C.; Conroy, J. J. Med. Chem. 1992, 35(5), 958. [CrossRef]
- Wright, C. D.; Stewart, S. F.; Kuipers, P. J.; Hoffman, M. D.; Devall, L. J.; Kennedy, J. A.; Ferin, M. A.; Thueson, D. O.; Conroy, M. C. J. Leukocyte Biol. 1994, 55, 443.
- Sample availability: available from the authors and MDPI.
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/