Abstract
N-Bromosulphonamides, synthesized via direct bromination of sulphonamides, react with several types of arene substrates in the presence of KSCN to afford aryl thiocyanates. The method appears to be generally applicable to benzenoid substrates with a wide range of substituents, such as N,N-dimethylaniline, p-xylene, anisole, mesitylene and cumene.
Introduction
Direct introduction of sulfur into aromatic rings is an interesting objective. Two general strategies are commonly utilized for this purpose: sulfonation and thiocyanation. Though the latter would be more useful for our purpose, electrophilic thiocyanation methods described so far are often limited in scope [1], their main disadvantages being that the reagents used are either highly toxic or present serious disposal problems (or both).
The rather unstable thiocyanogen, (SCN)2, has often been used, generated usually in situ from copper or lead thiocyanates [2,3,4] or from molecular bromine and an alkali metal thiocyanate [1a]. Procedures involving the somewhat more reactive thiocyanogen chloride or iodide, XSCN, have also been described [3,5]. These methods call for the use of molecular halogen as co-reagent, or call for other sources of Cl2 such as SbCl5 or C6H5ICl2. Recently, a number of different reaction conditions based upon the reaction of N-bromosuccinimide (NBS) with alkali metal thiocyanates have been investigated. Based on these literature precedents, we decided to react N-bromosulfonamides with some aromatic compounds under mild conditions in the presence of KSCN.
Result and Discussion
Several methods have been used to prepare arylthiocyanates, as discussed previously. In our attempts to develop a milder, more convenient procedure, we have studied a number of different conditions based upon the reaction of N-bromosulfonamides (A) or (B) with alkali method thiocyanates. While we have not identified the species responsible for carrying out the thiocyanation, we can rule out thiocyanogen since reactions carried out under conditions known to generate (SCN)2 failed to yield any product. It seems likely that N-bromosulphonamides (A or B), generated by the following reactions, is the actual thiocyanating agents involved.
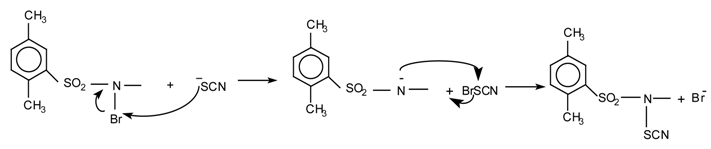
The N-bromosulfonamides have been found to be best reagents in combination with potassium thiocyanate in methanol or acetic acid as solvent. The reagents are stable and can be reused many times. The results obtained are summarized in the Table below.
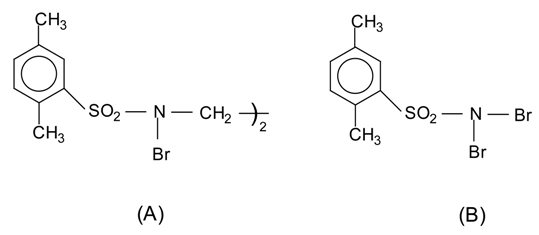

Table.
Preparation of aryl thiocyanates
| Substrate | Temp(°C)/ time(h) | Solvent | N-bromo reagent | Product | % Yield |
|---|---|---|---|---|---|
| N,N-Dimethylaniline | 0/3 | MeOH | A | 1 | 98 |
| Anisole | 25/4 | HOAc | B | 2a | 275 |
| Mesitylene | 0/2 | MeOH | A | 3 | 96 |
| Cumene | 0/2 | MeOH | B | 4 | 80 |
| Thiophene | 25/4 | HOAc | A | 5 | 70 |
| Benzene | 25/2 | HOAc | A,B | − | − |
a 2-Thiocyanato-1-methoxy-benzene was also formed (20%)
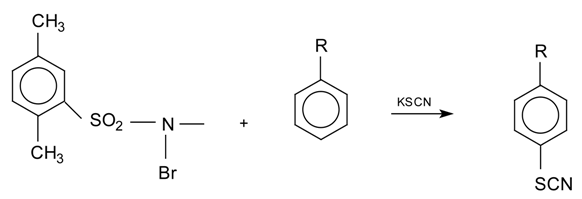
The results described in here have considerable potential for the efficient generation of aromatic thiocyanates. The general reaction is the following:
Experimental
General
Anhydrous conditions were found to be necessary to produce high yields of the desired thiocyanated reaction products. Methanol was dried by refluxing (0.5 h) and distilling from magnesium turnings. KSCN was dried in oven (126°C)[6]. The N,N′-dibromo-N,N′-bis(2,5-dimethylbenzenesulphonyl) ethylenediamine and N,N-dibromo-2,5-dimethylbenzenesulphonamide used were freshly recrystallized from hot ethanol and dried over P2O5 for 40 h prior to use [7]. All liquid substrates used were redistilled beforehand. The starting materials were dried and redistilled. IR spectra were run on a Shimadzu 435-U-04 IR spectrometer and 1H-NMR spectra (90 MHz) on a JEOL FT-NMR spectrometer, chemical shifts being measured relative to TMS (int, 1H).
N,N-Dimethyl-4-thiocyanatoaniline(1): A solution of N,N′-dibromo- N,N′-bis(2,5-dimethylbenzenesulphonyl)ethylenediamine (A); (6.81g, 0.0123 mol) and KSCN (3.18g, 0.0328 mol) in dry methanol (20 mL) was stirred at 25 °C for 30 min then N,N-dimethylaniline (0.496g, 0.0041 mol) was added and the reaction mixture further stirred at 0°C for 3h[8]. After removal of most of the methanol, water (30 mL) was added and the solution made alkaline with aq. 20% NaOH. It was filtered, extracted with CH2Cl2 (3×25 mL), the organic layer dried (MgSO4) and then evaporated to yield the crude product. Extraction with boiling heptane (20 ml), hot filtration and cooling offered 1 as yellow needles, mp. 73- 74°C. IR (KBr): ν 3110, 2900, 2110, 1600, 1500, 760 cm-1; 1H-NMR (D2O): δ 2.97 (s, 6H, -CH3), 6.76(d, 2H, H-2, H-6), 7.3(d, 2H, H-3, H-5) [6].
1-Methoxy-4-thiocyanatobenzene (2): A solution of dry KSCN (2.425 g, 0.025 mol) and N,N-dibromo(2,5-dimethyl)-benzenesulphonamide (B) (2.285 g,, 0.0125 mol) in glacial acetic acid (30 mL) was stirred for 30 min at room temperature. Anisole (0.562 g, 0.0052 mol) was then added to the red solution and the mixture stirred an hour at 35°C. After removal of the solvent under reduced pressure, CH2Cl2 (30 mL) was added, the resulting brown solid was washed with additional CH2Cl2 (3×25 mL). The combined washings were then washed successively with aq 20% NaOH and water, dried (MgSO4) and concentrated to yield a brown solid mp. 33-44°C. IR(KBr): ν 3050, 2910, 2160, 1620 cm-1.1H- NMR (acetone-d6): δ 3.6(s, 3H, -CH3 ), 6.7-7.2 (d, 4H, ring hydrogens); 13C-NMR (acetone): δ 59.72 (s, CH3), 112.5 (s, CN), 119.3-135 (ring carbons). Anal. Found: C, 58.16; H, 4.27; N, 8.48; S, 19.41. Calcd. For C8H7ONS: C, 58.50; H, 4.30; N, 8.51; S, 19.52.
1,3.5-Trimethyl-4-thiocyanatobenzene(3): Brown viscous oil, IR (KBr): ν 3297, 2931, 2073, 1600 cm-1; 1H-NMR (acetone-d6): δ 2.36 (s, 3H, -CH3), 2.98 (s, 6H, -CH3), 7.20 (s, 2H, ring hydrogens); 13C-NMR (acetone): δ 42.60 (s, -CH3), 45.20 (s, -CH3), 119.75 (s, -CN), 132-136 (ring carbons). Anal. Found: C, 67.75; H, 6.25; N, 7.90; S, 18.09. Calcd. For C10H11NS: C, 67.43; H, 6.42; N, 7.64; S, 18.31.
1-Isopropyl-4-thiocyanatobenzene (4): Thick yellow oil, IR(KBr): ν 3120, 2910, 2186, 1600 cm-1; 1H-NMR (acetone-d6): δ 2.55 (d, 6H, -CH3), 2.96 (m, 1H, -CH), 7.27-7.68 (d, 4H, ring hydrogens); 13C-NMR (acetone): δ 40.31 (s, -CH3), 46.65 (s, -CH), 130 (s, -CN), 133.58-136.75 (ring carbons). Anal. Found: C, 67.75; H, 6.25; N, 7.90; S, 18.09. Calcd. For C10H11NS: C, 67.43; H, 6.42; N, 7.64; S, 18.31.
2-Thiocyanatothiophene (5): Yellow oil, bp 134-136°C/12 mm, IR(KBr): ν 3100, 2160, 1385, 1220, 850, 712 cm-1; 1H-NMR (CDCl3): δ 8.07 (dd, 1H, H-5), 7.86 (dd, 1H, H-3), 7.53 (dd, 1H, H-4) [6].
References and Notes
- (a) Brewster, R. Q.; Schroeder, W. Org. Synth. Coll. Vol. II 574. (b) Soderback, E. Ann. Chem. 1919, 419, 217. (c) Kaufman, H. P.; Weber, E. Arch. Pharm. 1929, 267, 201. (b) Kelly, T. R.; Kim, T. R.; Curtis, A. D. M. J. Org. Chem. 1993, 58, 5855.
- Kaufmann, H. P.; Kuchler, K. Ber. 1934, 67, 944.
- Bacon, R. G. R.; Guy, R. G. J. Chem. Soc. 1960, 318.
- Wood, J. L. Org. React. 1946, 3, 240.
- (a) Uemura, S.; Onoe, A.; Okazaki, H.; Okano, M. Bull. Chem. Soc. Jpn. 1975, 48, 619. (b) Woodgate, P. D.; Lee, H. H.; Rutledge, P. S.; Cambie, R. C. Tetrahedron Lett. 1976, 1531.
- Dean, T. F.; De Stefano, V.; Ian, Still, W. J. Synth. Commun. 1995, 25, 1277.
- Khazaei, A.; Shirdarreh, A. Synth. Commun. 1999, 29, 4079.
- Treatment of N,N-dimethyaniline under otherwise identical conditions for 4 h at 25°C gave N,N′- dimethyl-2,4-dithiocyanatoaniline (95%), mp. 75-76°C (CH2Cl2/hexane); spectral data (FTIR, 1H- NMR) were in accord with expected values.
- Samples Availability: Not available
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes