Abstract
The ZnBr2 complex of the title compound has been studied by both structural and theoretical methods. Similar reactivities have been observed for the nitrone alone and the complex in 1,3-dipolar cycloadditions and nucleophilic additions.
Introduction
Lewis acid modulated reactions play a very important role in organic synthesis as they provide versatile intermediates, sometimes dictating the stereochemistry of the products [1]. Nitrones are substrates rather sensitive to Lewis-acid modulation in nucleophilic additions to chiral nitrones as we have amply demonstrated during the last few years [2]. For instance, it has been demonstrated in our laboratory that by employing either ZnBr2 or Et2AlCl, one can produce hydroxylamines with either syn or anti stereochemistry (with respect to the alfa group), respectively [3].
1,3-Dipolar cycloaddition reactions with nitrones are also susceptible to influence by the presence of Lewis acids. In fact, Lewis acids catalyzed nitrone cycloadditions have been extensively studied [4]. Kanemasa and Tsuruoka have suggested the participation of nitrone-MgBr2 complexes in some cycloaddition reactions with allylic alcohols [5]. The scope and limitations of chiral Mg(II) and Cu(II) complexes on the selectivity of cycloaddition of nitrones with alkenes have been studied by Jorgensen and coworkers [6]. Activation of nitrones towards cycloadditions with electron-rich alkenes by chiral Lewis acids have also been reported [7].
In the context of cycloaddition chemistry, we have recently reported the 1,3-dipolar cycloaddition of several hetaryl nitrones 1-5 with both electron-rich [8] and electron-deficient alkenes [9]. The asymmetric version of the cycloaddition reaction between C-(2-furyl)-N-benzyl nitrone 1 and acrylates has been used in the preparation of various protected derivatives of 4-hydroxy-pyroglutamic acids of synthetic utility [10].

With the aim of modulating the reactivity of hetaryl nitrones 1-5 we have started a project directed towoards an understanding of the properties of Lewis acid complexes with compounds 1-5. Stable complexes of nitrones have already been studied. Nitrone complexes of iron have been fully characterized and their acidic hydrolysis studied by Pierre and coworkers [11]. The same authors have also reported the electrochemical reduction of such complexes [12]. Several tin (IV) complexes with nitrones giving pentacoordinated metal compounds have been prepared [13]. Crist and coworkers [14] have prepared and characterized complexes of N-tert-butyl-C-(2-pyridyl) nitrone with Cu(II), Mn(II), Co(II), Ni(II), Fe(II) and Fe(III). The X-ray structures of some complexes have also been determined [15]. Boron chelates with some particular aryl nitrones have also been described [16].
In this communication we describe the structural and theoretical studies of both (Z)-N-benzyl-C-(2- pyridyl) nitrone and its ZnBr2 complex. Additionally, we also compare the reactivity of the nitrone alone and the isolated complex in cycloaddition reactions and a nucleophilic addition reaction.
Synthesis and Structural Analysis
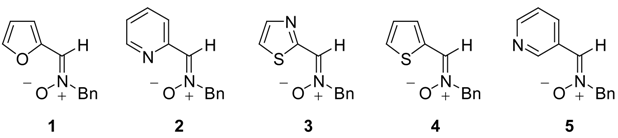
The N-benzyl-C-(2-pyridyl) nitrone 2 was prepared by condensation of pyridine-2-carbaldehyde [17] and N-benzylhydroxylamine [18] following our previously reported procedure [19]. Compound 2 was a crystalline stable product and showed a Z-configuration as demonstrated by NOE experiments and X-ray crystallography. Transparent blocks of 2 were grown at room temperature by slow evaporation of a 1:1 hexane/EtOAc mixture. X-ray diffraction data were obtained at 173 K and the structure is given in Figure 1. Selected data are given in Table 1.
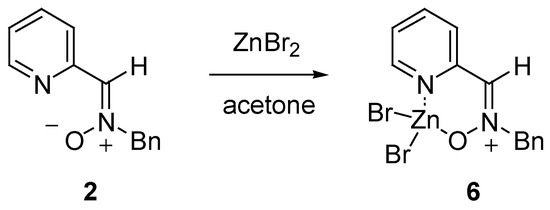
The reaction of 2 with ZnBr2 in acetone for 1 h gave the crystalline chelate 6 after precipitation with diethyl ether (Scheme 1). This compound displayed a signal for the azomethine proton at δ 5.40 ppm in contrast to the signal at δ 5.19 ppm displayed by nitrone 2 for the same proton. This result indicated the different orientation of the nitrone group induced by the complexation with the Lewis acid.
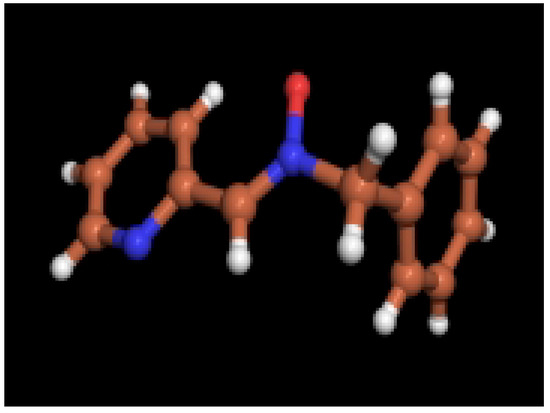
Figure 1.
X-ray structure of nitrone 2
Figure 2.
X-ray structure of complex 6
The complex 6 was crystallized as transparent blocks suitable for X-ray analysis from a 1:1 CH2Cl2/hexane mixture. The X-ray diffraction data were obtained at 243 K (due to some instability of the crystal at lower temperatures) and the structure is given in Figure 2. This structure provided an opportunity to compare the structure of 6 with 2. In fact, the features of the solid-state geometry of 6 summarized in Table 1, are discussed in comparison with the values for 2.
Scheme 1.
A coplanarity is seen, as in other hetaryl nitrones [20], between the planes of the heterocycle moiety and the nitrone function in both the nitrone alone and the chelate with ZnBr2. As expected, formation of the complex resulted in some bond length and bond angle differences between 2 and 6 (Table 1). It is worthwhile noting the change of the N2-C3-C4-N5 dihedral angle as a consequence of the formation of the chelate. In the complex the metal atom adopts an almost tetrahedral disposition the bond angles with bromine atoms being larger than those with nitrogen and oxygen atoms.
X-ray diffraction data
Selected X-ray acquisition data and crystallographic data for compounds 2 and 6 appear in Table 1 and Table 2, respectively. The final unit-cell parameters were obtained by least squares on the settings angles for 39 reflections with θmin/max = 9.49-24.75 deg. for 2 and 32 reflections with θmin/max = 10.35-24.56 deg. for 6. Intensity data were measured on a Siemens P4 diffractometer using the ω-2θ scan technique. The structures were solved by direct methods and all non-hydrogen atoms were refined anisotropically. The hydrogen atoms were located at calculated positions.

Table 1.
Selected X-ray acquisition data for 2 and 6.
| 2 | 6 | |
| Formula | C13H12N2O | C13H12Br2N2OZn |
| FW | 212.25 | 437.44 |
| Crystal system | orthorhombic | triclinic |
| Space group | Pbcn | P-1 |
| a (Å) | 11.033 (5) | 7.303 (2) |
| b (Å) | 11.107 (5) | 8.135 (3) |
| c (Å) | 17.414 (5) | 12.970 (6) |
| α (deg.) | 90 | 87.01 (3) |
| β (deg.) | 90 | 80.56 (3) |
| γ (deg.) | 90 | 84.60 (3) |
| V (Å3) | 2134.0 (15) | 756.2 (5) |
| Z | 8 | 2 |
| ρcalc (g/cm3) | 1.321 | 1.921 |
| F(000) | 896 | 424 |
| μ (Mo-Kα), cm-1 | 0.086 | 6.900 |
| crystal size, mm | 0.38x0.20x0.16 | 0.24x0.10x0.08 |
| measurement T (K) | 173 (2) | 243 (2) |
| θmax (deg.) | 25.11 | 25.0 |
| crystal decay (%) | 6.86 | 15.4 |
| total reflections | 2446 | 5169 |
| total unique reflections | 1886 | 2628 |
| Rmerge | 0.072 | 0.097 |
| reflections I>2σ(I) | 1083 | 1333 |
| No. parameters | 147 | 174 |
| R | 0.0474 | 0.1007 |
| Rw | 0.0847 | 0.2432 |
| GoF (S) | 1.084 | 1.162 |
| Siemens P4 diffractometer. Mo-Ka radiation (λ=0.71609Å), normal focus sealed tube, graphite monochromator. Values given for R, Rw and GoF are based on total unique reflections. Computing data collections: Siemens XSCANS [21]. Structure solution: SIR-97 [22]. Structure refinement: SHELXL-97 [23]. Molecular Graphics: PovChem v 2.1 [24] | ||
Theoretical Calculations
In order to assess the various factors contributing to the structural differences between 2 and 6 we have carried out ab initio energy calculations. The optimized structures for 2 and 6 are shown in Figure 3 and Figure 4.
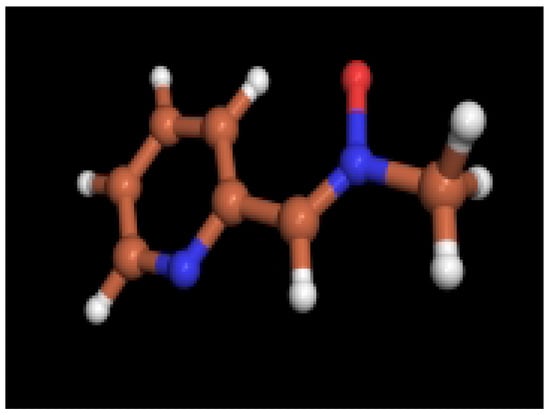
Figure 3.
Optimized structure (HF/3-21G) for 2
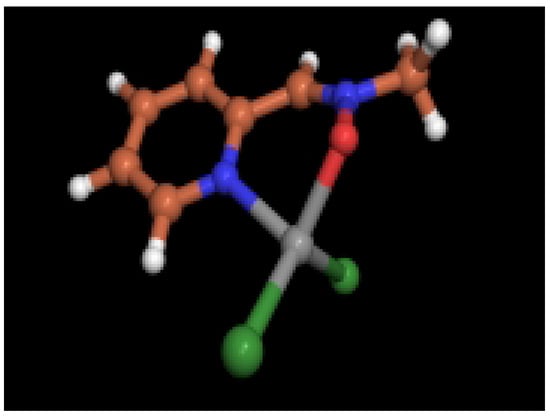
Figure 4.
Optimized structure (HF/3-21G) for 6
The molecular structures determined using ab initio calculations agree with those observed in the crystalline state although some minor differences were observed. For instance, theoretical calculations gave longer O1-N2 bonds and shorter N2-C3 bonds than those found in crystalline state, presumably due to the consideration of resonance for the nitrone function. Nevertheless, the modeled structures showed a good overlap with the X-ray structures (Figure 5 and Figure 6).
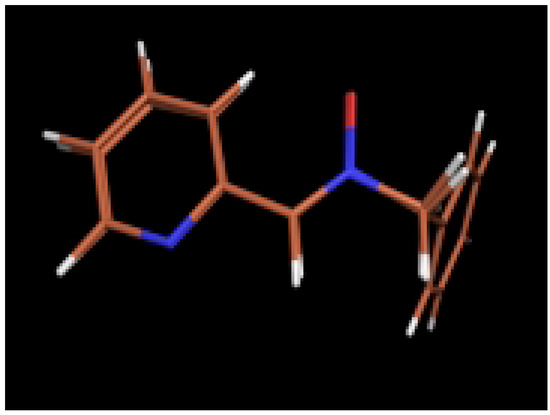
Figure 5.
Comparison of the modeled structure of nitrone 2 with the X-ray structure.
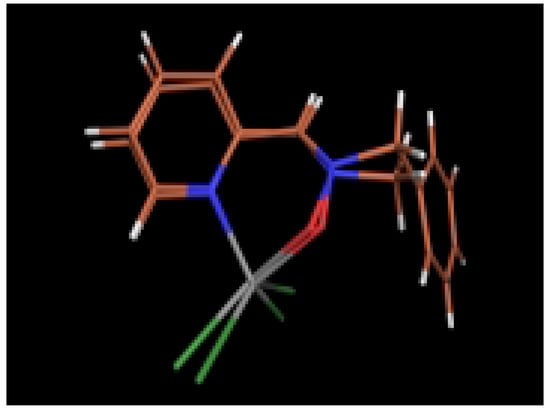
Figure 6.
Comparison of the modeled structure of complex 6 with the X-ray structure.
Ab initio calculations
Calculated structures were optimized at HF/3-21G level using Gaussian98 [25]. All internal coordinates were free. In both 2 and 6 the benzyl group has been replaced for a methyl group, and in the case of 6 the Br atoms have been replaced by Cl atoms. The X-ray data were used (after replacement of the indicated groups) to generate guessed structures which were pre-optimized at semiempirical level (PM3) using MOPAC97 as implemented in CS ChemOffice [26]. In the case of the nitrone alone a complete conformational analysis has been carried out in order to determine the preferred orientation of the nitrone function with respect to the pyridine ring.

Table 2.
Selected bond lengths (Å), bond angles (°) and dihedral angles (°) for 2 and 6.
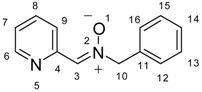

| Nitrone 2 | Complex 6 | |||
| X-ray data | HF / 3-21G | X-ray data | HF / 3-21G | |
| bond lenghts | ||||
| O1-N2 | 1.299 | 1.383 | 1.396 | 1.326 |
| N2-C3 | 1.300 | 1.268 | 1.261 | 1.228 |
| C3-C4 | 1.451 | 1.458 | 1.469 | 1.501 |
| C4-N5 | 1.365 | 1.337 | 1.318 | 1.318 |
| N2-C10 | 1.488 | 1.472 | 1.477 | 1.500 |
| O1-Zn | -------- | -------- | 1.993 | 1.930 |
| N5-Zn | -------- | -------- | 2.055 | 2.029 |
| Zn-Br1 | -------- | -------- | 2.321 | 2.287 |
| Zn-Br2 | -------- | -------- | 2.357 | 2.333 |
| bond angles | ||||
| O1-N2-C3 | 126.1 | 125.6 | 127.3 | 123.5 |
| N2-C3-C4 | 126.3 | 126.4 | 129.8 | 125.7 |
| C3-C4-N5 | 113.2 | 113.8 | 118.6 | 119.6 |
| O1-N2-C10 | 114.4 | 111.2 | 111.1 | 111.2 |
| C3-N2-C10 | 119.5 | 123.2 | 121.0 | 125.3 |
| O1-Zn-N5 | -------- | -------- | 89.7 | 87.5 |
| N2-O1-Zn | -------- | -------- | 118.1 | 111.1 |
| N5-Zn-Br1 | -------- | -------- | 108.1 | 105.3 |
| O1-Zn-Br2 | -------- | -------- | 113.7 | 126.1 |
| dihedral angles | ||||
| O1-N2-C3-C4 | 3.1 | 0.0 | 2.6 | 2.9 |
| N2-C3-C4-N5 | 175.6 | 180.0 | 13.0 | 23.7 |
| O1-N2-C10-C11 | 112.6 | -------- | -------- | 85.0 |
| N2-O1-Zn-N5 | -------- | -------- | 122.8 | 141.5 |
| O1-Zn-N5-C4 | -------- | -------- | 37.2 | 30.7 |
Reactivity
The reactivity of the complex was studied by condensing it with the dipolarophiles vinyl acetate 7 (Scheme 2) and methyl acrylate 9 (Scheme 3). To this respect we have recently reported [8] the reaction between nitrones 1-5 and vinyl acetate 7 to give the corresponding isoxazolidines. It might be expected a difference of reactivity between 2 and 6. However, only slight differences in both reactivity and selectivity were found. Data for the reactivity with the dipolarophiles are summarized in Table 3. The results of the same reaction with nitrone 2 are also given for comparison.
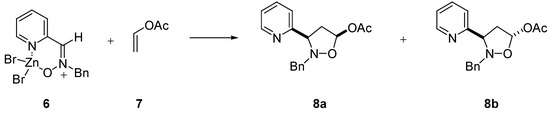
Scheme 2.
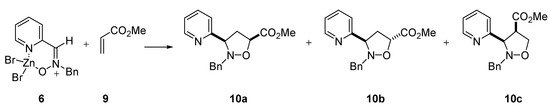
Scheme 3.
In both cycloadditions with vinyl acetate and allyl alcohol only the corresponding 3,5-regioisomers were detected, the cis adduct being obtained preferentially.

Table 3.
Reactivity of nitrone 2 and complex 6 in 1,3-dipolar cycloadditions
| 1,3-Dipole | dipolarophileb | time | cis : transc | yield (%) |
|---|---|---|---|---|
| 2 | 7 | 168 h | 85 : 15 | 93 |
| 2 | 9 | 72 h | 23 : 77d | 93 |
| 6 | 7 | 168 h | 89 : 11 | 92 |
| 6 | 9 | 72 h | 27 : 73e | 89 |
a All reactions were conducted in acetone at reflux. b 20 eq. were used. c The relative stereochemistry was determined by nOe experiments. d a 10% of compound 10c was obtained. e a 13% of compound 10c was obtained.
In the reaction with methyl acrylate, however, the trans adduct was obtained as the major compound. In this reaction one of the 3,4-regioisomers was also observed, although as a minor compound.
These results are in good agreement with previous data relating to the cycloaddition reactions of nitrones with electron-rich and electron-poor alkenes [8,9]. According to these data the 3,5- isoxazolidines are the major compounds and an exo-approach leading to cis adducts is preferred with electron-rich alkenes whereas the endo-approach leading to trans adducts is that preferred with electron-poor dipolarophiles.
We also compared the reactivity of the nitrone 2 and the complex 6 in a nucleophilic addition reaction. Thus, the nucleophilic addition of phenylmagnesium bromide, according to our previously described protocol [3c], led to the corresponding hydroxylamine. In this type of reaction no substantial differences were observed between the nitrone alone and the complex, yet (Scheme 4).
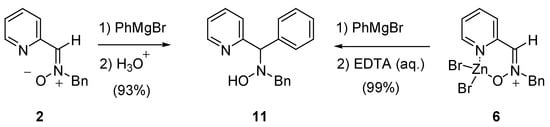
Scheme 4.
Conclusions
In summary, this preliminary result showed the possibility of using complexed nitrones as suitable 1,3-dipoles in cycloaddition reactions. Similar structures were found in the crystal structure and by the ab initio calculations both for nitrone 2 and ZnBr2-complex 6. The reactivity of those compounds were compared by condensing them with several dipolarophiles and a Grignard reagent. Only slight differences of reactivity were observed between the nitrone alone and the complex. Nevertheless, such a similar reactivity is rather promising since it open a window to the use of chiral auxiliaries linked to the metal atom (instead halogen atoms) in order to induce asymmetry in the studied reactions. Further investigations addressing this concept are now in progress in our laboratories.
Experimental
General
Reaction flasks and other glassware was heated in an oven at 130°C overnight and assembled in a stream of Ar. All solvents were dried by the usual methods. All reactions were monitored by TLC on silica gel 60 F254; the position of the spots were detected with 254 nm UV light or by spraying with one of the following staining systems: 50% methanolic sulfuric acid, 5% ethanolic phosphomolybdic acid and iodine. Preparative column chromatography was performed on columns of silica gel (60-240 mesh) and with solvents that were distilled prior to use. Preparative centrifugally accelerated radial thin-layer chromatography (PCAR-TLC) was performed with a Chromatotron® Model 7924 T (Harrison Research, Palo Alto, CA, USA); the rotors (1 or 2 mm layer thickness) were coated with silica gel Merck grade type 7749, TLC grade, with binder and fluorescence indicator (Aldrich 34,644-6) and the eluting solvents were delivered by the pump at a flow-rate of 0.5-1.5 mL min-1. Melting points were uncorrected. 1H and 13C-NMR spectra were recorded either on a Varian Unity or on a Bruker 300 instrument. Unles otherwiae mnoted chemical shifts are reported in ppm (δ) relative to CHCl3 (δ = 7.26) in CDCl3. Elemental analyses were performed on a Perkin Elmer 240B microanalyzer. Nitrone 2 was prepared as described previously by us [19].
Preparation of complex 6.
A solution of nitrone 2 (1.0 mmol) in acetone, was treated with ZnBr2 (1.0 mmol) under an inert atmosphere and the resulting mixture was stirred for 6 h. After this time diethyl ether was added until no more precipitation of a solid was observed. The resulting precipitate was filtered, washed with cold acetone and dried to give essentially pure complex 6 (0.437 g, 100%) as a white solid; mp.: 250-252 °C; 1H-NMR (acetone-d6) δ: 5.40 (s, 2H), 7.41-7.45 (m, 3H), 7.60-7.64 (m, 2H), 8.00 (t, 1H, J = 6.2 Hz), 8.07 (d, 1H, J = 7.7 Hz), 8.41 (dt, 1H, J = 1.7, 7.7 Hz), 8.76 (d, 1H, J = 4.5 Hz), 8.80 (s, 1H); 13C-NMR (acetone-d6) δ: 71.2, 128.4, 129.1 (2C), 129.3, 129.5, 130.0, 132.8, 137.8, 141.2, 149.6; Anal Calcd. for C13H12Br2N2OZn (437.45): C, 35.69; H, 2.76; N, 6.40. Found: C, 35.72; H, 2.86; N, 6.34.
General Procedure for the 1,3-dipolar cycloaddition of nitrone 2 and complex 6 with dipolarophiles 7 and 9.
The corresponding dipolarophile (20 mmol) was added to a solution of the corresponding 1,3-dipole (1 mmol) in acetone and the resulting solution was heated under an inert atmosphere (Ar) at reflux for the time indicated in Table 3. The mixture was cooled to ambient temperature and concentrated under reduced pressure. The cis:trans ratio of the residue was determined by 1H-NMR analysis and the relative stereochemistry of the adducts was determined by typical monodimensional NOE experiments. The crude material was purified by PCAR-TLC using a Chromatotron® (2 mm layer thickness).
cis-5-(Acetoxy)-2-benzyl-3-(2-pyridyl)isoxazolidine 8a. 1H-NMR δ: 1.99 (s, 3H), 2.58 (ddd, 1H, J = 2.4, 7.8, 13.7 Hz), 3.00-3.12 (m, 1H), 4.03 (s, 2H), 4.15 (t, 1H, J = 7.8 Hz), 6.40 (dd, 1H, J = 1.9, 6.3 Hz), 7.20-7.75 (m, 8H), 8.63 (ddd, 1H, J = 1.8, 4.8, 6.9 Hz); 13C-NMR δ: 21.2, 44.0, 60.8, 69.9, 95.5, 121.8, 122.7, 127.3, 128.2, 129.1, 136.2, 136.7, 149.1, 158.7, 170.4; Anal Calcd. for C17H18N2O3 (298.13):C, 68.44; H, 6.08; N, 9.39. Found: C, 68.31; H, 5.96; N, 9.16.
trans-5-(Acetoxy)-2-benzyl-3-(2-pyridyl)isoxazolidine 8b. 1H-NMR δ: 2.06 (s, 3H), 2.85 (dd, 1H, J = 2.9, 7.3 Hz), 4.03 (s, 2H), 4.28 (d, 1H, J = 13.2 Hz), 4.50 (t, 1H, J = 7.8 Hz), 6.35 (dd, 1H, J = 1.9, 6.5 Hz), 7.20-7.75 (m, 8H), 8.49 (ddd, 1H, J = 1.9, 5.1, 6.8 Hz); 13C-NMR δ: 21.2, 42.1, 62.4, 63.4, 97.2, 121.1, 121.2, 127.2, 132.1, 135.1, 136.2, 136.7, 149.2, 158.8, 170.1; Anal Calcd. for C17H18N2O3 (298.13): C, 68.44; H, 6.08; N, 9.39. Found: C, 68.59; H, 6.21; N, 9.44.
cis-2-Benzyl-5-(metoxycarbonyl)-3-(2-pyridyl)isoxazolidine 10a. 1H=NMR δ: 2.81 (ddd, 1H, J = 5.1, 6.8, 12.9 Hz), 3.02 (ddd, 1H, J = 8.3, 9.3, 12.9 Hz), 3.64 (s, 3H), 3.99 (s, 2H), 4.15 (dd, 1H, J = 6.8, 8.3 Hz), 4.67 (dd, 1H, J = 5.1, 9.3 Hz), 7.16-7.19 (m, 1H), 7.19-7.26 (m, 2H), 7.27-7.31 (m, 1H), 7.39 (dd, 2H, J = 2.1, 6.7 Hz), 7.57 (d, 1H, J = 6.8 Hz), 7.62 (dt, 1H, J = 1.7, 7.2 Hz), 8.47 (ddd, 1H, J = 1.8, 4.9, 6.7 Hz); 13C-NMR δ: 40.1, 51.9, 60.4, 70.3, 75.1, 121.7, 122.5, 127.1, 128.1, 128.3, 136.7, 137.0, 148.9, 159.5, 172.0; Anal Calcd. for C17H18N2O3 (298.13):C, 68.44; H, 6.08; N, 9.39. Found: C, 68.30; H, 6.18; N, 9.32.
trans-2-Benzyl-5-(metoxycarbonyl)-3-(2-pyridyl)isoxazolidine 10b. 1H-NMR δ: 2.88 (td, 1H, J = 7.3, 12.5 Hz), 2.98 (ddd, 1H, J = 5.6, 8.5, 12.5 Hz), 3.70 (s, 3H), 3.94 and 4.24 (2d, 2H, J = 13.4 Hz), 4.33 (dd, 1H, J = 5.6, 7.3 Hz), 4.55 (dd, 1H, J = 7.3, 8.5 Hz), 7.14-7.20 (m, 1H), 7.20-7.27 (m, 2H), 7.28-7.32 (m, 1H), 7.40 (dd, 2H, J = 1.7, 8.3 Hz), 7.57 (d, 1H, J = 8.3 Hz), 7.62 (dt, 1H, J = 1.7, 7.8 Hz), 8.47 (ddd, 1H, J = 1.7, 4.9, 7.8 Hz); 13C-NMR (CDCl3) δ: 39.2, 51.9, 61.7, 69.5, 76.1, 121.5, 122.2, 127.0, 128.0, 128.8, 136.4, 137.2, 148.8, 159.1, 172.1; Anal Calcd. for C17H18N2O3 (298.13): C, 68.44; H, 6.08; N, 9.39. Found: C, 68.56; H, 5.89; N, 9.42.
cis-2-Benzyl-4-(metoxycarbonyl)-3-(2-pyridyl)isoxazolidine 10c. 1H-NMR δ: 3.75 (s, 3H), 3.98 (td, 1H, J = 5.3, 8.3 Hz), 4.01 and 4.09 (2d, 2H, J = 13.4 Hz), 4.26 (t, 1H, J = 8.3 Hz), 4.35 (dd, 1H, J = 5.3, 8.3 Hz), 4.51 (d, 1H, J = 5.3 Hz), 7.14-7.22 (m, 1H), 7.22-7.33 (m, 3H), 7.37 (dd, 2H, J = 1.6, 7.2 Hz), 7.50 (d, 1H, J = 7.8 Hz), 7.60 (dt, 1H, J = 1.6, 7.7 Hz), 8.53 (ddd, 1H, J = 1.6, 4.8, 7.7 Hz); 13C-NMR δ: 52.2, 54.4, 60.2, 68.7, 73.0, 122.3, 122.4, 127.2, 128.2, 129.0, 136.4, 137.0, 149.2, 159.0, 173.0. Anal Calcd. for C17H18N2O (298.13): C, 68.44; H, 6.08; N, 9.39.. Found: C, 68.32; H, 6.14; N, 9.45.
N-Benzyl-N-(phenyl-pyridin-2-yl-methyl)-hydroxylamine 13
To a cooled (0°C) solution of the electrophile (nitrone 2 or complex 6, 1 mmol) in THF (10 mL) was added a solution of PhMgBr (1 ml of a 3.0 M solution in THF, 3 mmol). The resulting solution was stirred for 2 h at which time a saturated solution of NH4Cl was added. The reaction mixture was diluted with diethyl ether (20 mL) and the organic layer was separated. The aqueous layer was extracted twice with diethyl ether; the organic extracts were combined, washed with brine, dried (MgSO4) and evaporated under vacuum. The crude material was purified by PCAR-TLC using a Chromatotron® (1 mm layer thickness); mp.: 110-112 °C; 1H-NMR δ: 3.70 (d, 1H, J = 13.8 Hz), 3.90 (d, 1H, J = 13.8 Hz), 5.03 (s, 1H), 6.10 (s, 1H), 7.11-7.65 (m, 13H), 8.52 (d, 1H, J = 4.4 Hz); 13C-NMR δ: 61.4, 73.0, 122.1, 122.7, 127.2, 127.7, 128.2, 128.5, 128.6, 129.3, 136.8, 138.0, 140.0, 148.9, 161.5. Anal Calcd. for C19H18N2O (290.36): C, 78.59; H, 6.25; N, 9.65. Found: C, 78.56; H, 6.28; N, 9.62.
Acknowledgements
The authors gratefully acknowledge the financial support given by the DGES (Project PB97-1014. Madrid. Spain) and by DGA (Project P079/99-C, Aragon. Spain).
References and Notes
- (a) Kobayashi, S.; Busujima, T.; Nagayama, A. Novel Classification of Lewis-Acids on the Basis of Activity and Selectivity. Chem. Eur. J. 2000, 6, 3491–3494, and references cited therein. [Google Scholar] [CrossRef] (b) Haughton, L.; Williams, J.M.J. Catalytic Applications of Transition-Metals in Organic-Synthesis. J. Chem. Soc. Perkin Trans. 1 2000, 3335–3349. [Google Scholar] [CrossRef]
- For a recent account see: Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Nucleophilic additions to chiral nitrones: New approaches to nitrogenated compounds. Synlett 2000, 442–454. [Google Scholar]
- See inter alia: (a) Merino, P.; Castillo, E.; Franco, S.; Merchan, F.L.; Tejero, T. Enantiodivergent approach to D- and L-secondary N-hydroxy-α-amino acids by using N-benzyl-2,3-O- isopropylidene-D-glyceraldehyde nitrone as an effective N-hydroxyglycine cation equivalent. J. Org. Chem. 1998, 63, 2371–2374. [Google Scholar] (b) Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Diastereoselective nucleophilic addition of acetylide to N-benzyl-2,3-O-isopropylidene-D- glyceraldehyde nitrone (BIGN). Stereodivergent synthesis of β-hydroxy-α-(hydroxyamino)- and β- hydroxy-α-aminoacids. Tetrahedron: Asymmetry 1997, 8, 3489–3496. [Google Scholar] (c) Merino, P.; Castillo, E.; Franco, S.; Merchan, F.L.; Tejero, T. Nucleophilic additions of Grignard reagents to N-benzyl-2,3- O-isopropylidene-D-glyceraldehyde nitrone (BIGN). Synthesis of (2S,3R)) and (2S,3S)-3- phenylisoserine. Tetrahedron 1998, 54, 12301–12322. [Google Scholar]
- Kanemasa, S.; Uemura, T.; Wada, E. Lewis-acid catalyzed nitrone cycloadditions to bidentate and tridentate alpha,beta-unsaturated keones. High-rate acceleration, absolutely enoselective and regioselective reactions. Tetrahedron Lett. 1992, 33, 7889–7892. [Google Scholar] Kanemasa, S.; Oderaotoshi, Y. Asymmetric cyloaddition reactions catalyzed by transition-metal complexes. New guidelines for structural design of chiral catalyst. J. Synth. Org. Chem. Jpn. 1998, 56, 368–377. [Google Scholar]
- Kanemasa, S.; Tsururoka, T. Magnesium bromide promoted E/Z isomerization of carbonyl- conjugated nitrones ad highly stereoselective and regioselective cycloadditions to allylic alcohol dipolarophiles. Chem. Lett. 1995, 49, 49–50. [Google Scholar] [CrossRef]
- (a) Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. Control of diastereoselectivity and enantioselectivity in metal-catalyzed 1,3-dipolar cycloaddition reactions of nitrones with alkenes. Experimental and theoretical investigations. J. Org. Chem. 1996, 61, 346–355. [Google Scholar] (b) Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. Molecular-sieve dependent absolute stereoselectivity in asymmetric catalytic 1,3-dipolar cycloaddition reactions. J. Org. Chem. 1998, 63, 5483–5488. [Google Scholar] (c) Jensen, K.B.; Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. Improvement of taddolate-TiCl2-catalyzed 1,3- dipolar nitrone cycloaddition reactions by substitution of the oxazolidinone auxiliary of the alkene with succinimide. J. Org. Chem. 1997, 62, 2471–2477. [Google Scholar]
- Simonsen, K.B.; Bayon, P.; Hazell, R.G.; Gothelf, K.V.; Jorgensen, K.A. Catalytic enantioselective inverse-electron demand 1,3-dipolar cycloaddition reactions of nitrones with alkenes. J. Am. Chem. Soc. 1999, 121, 3845–3847. [Google Scholar] [CrossRef]
- Merino, P.; Anoro, S.; Merchan, F.L.; Tejero, T. 1,3-Dipolar cycloadditions of N-benzyl furfuryl nitrones with electron-rich alkenes. Molecules 2000, 5, 132–152. [Google Scholar] [CrossRef]
- (a) Tejero, T.; Dondoni, A.; Rojo, I.; Merchan, F.L.; Merino, P. 1,3-Dipolar cycloaddition of C-(2- thiazolyl)nitrones to chiral acrylates. Synthesis of enantiopure α-amino-2-alkylthiazoles and 5- formylpyrrolidin-2-ones. Tetrahedron 1997, 53, 3301–3318. [Google Scholar] (b) Merino, P.; Anoro, S.; Rojo, I.; Merchan, F.L.; Tejero, T. 1,3-Dipolar cycloaddition between hetaryl nitrones and methyl acrylate: Theoretical study and application to the synthesis of functionalized pyrrolidines. Heterocycles 2000, 53, 861–875. [Google Scholar]
- Merino, P.; Anoro, S.; Franco, S.; Merchan, F.L.; Tejero, T.; Tuñon, V. 1,3-Dipolar cycloaddition of furfuryl nitrones with acrylates. A convenient approach to protected 4-hydroxypyroglutamic acids. J. Org. Chem. 2000, 65, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Moinet, C.; Toupet, L. Synthesis, structure and hydrolysis of some ((eta(5)- cyclopentadienyl)(eta(6)-arene)iron(II))(PF6) complexes bearing a imine or a nitrone function in α position of the arene ligand. J. Organomet. Chem. 1997, 527, 51–64. [Google Scholar] [CrossRef]
- Pierre, F.; Stricker, A.; Moinet, C.; Sinbandhit, S.; Toupet, L. Electrochemical reduction of some ((eta(5)-cyclopentadienyl)(eta(6)-arene)iron(II))(PF6) complexes bearing an imine or a nitrone function in benzylic position of the arene ligand. J. Organomet. Chem. 1998, 553, 253–267. [Google Scholar] [CrossRef]
- Alallaf, T.A.K.; Abdulrahman, A. Diorganotin (IV) dichloride complexes of some N- arylfurfuralnitrones. Synth. React. Inorg. Metal-Org. C 1997, 27, 985–996. [Google Scholar] [CrossRef]
- (a) Villamena, F.A.; Dickman, M.-H.; Crist, D.R. Nitrones as ligands in complexes of Cu(II), Mn(II), Co(II), Ni(II), Fe(II), and Fe(III) with N-tert-butyl-α-(2-pyridyl)nitrone and 2,5,5- trimethyl-1-pyrroline-N-oxide. Inorg. Chem. 1998, 37, 1446–1453. [Google Scholar] [CrossRef] (b) Villamena, F.A.; Crist, D.R. Metal-nitrone complexes. Spin-trapping and solution characterization. J. Chem. Soc. Dalton, Trans. 1998, 4055–4064. [Google Scholar] [CrossRef]
- Dickmann, M.-H.; Ward, J.P.; Villamena, F.A.; Crist, D.R. Bis(μ-(N-((methylthio)- phenylmethylene)-methanamine-N-oxide)-O-O)bis(bis-(1,1,1,5,5,5-hexa-fluoro-pentane-2,4- dionato-O,O') nickel(II)) Acta Cryst. Sect. C Cryst. Struct. Commun. 1998, 54, 929–930. [Google Scholar] [CrossRef]
- Kliegel, W.; Metge, J.; Rettig, S.J.; Trotter, J. Structural Studies of Organoboron Compounds Lxviii - C-(2-Hydroxyaryl)-N-(2-Hydroxyphenylmethyl)Nitrones as Regioselective Bidentate Ligands in Boron Chelate Formation - Crystal and Molecular-Structures of a Diphenylboron Complex and Its Parent Ligand. Can. J. Chem. 1998, 76, 1082–1092. [Google Scholar] [CrossRef]
- Purchased from Aldrich (P6,200-3) and distilled prior to use.
- Borch, R.F.; Berstein, M.D.; Durst, M.D. The cyanoborohydridoborate anion as a selective reducing agent. J. Am. Chem. Soc. 1971, 93, 2897–2904. [Google Scholar] [CrossRef]
- Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synthesis of N-benzyl nitrones. Synth. Commun. 1994, 24, 2537–2550. [Google Scholar] [CrossRef]
- Merino, P.; Anoro, S.; Tejero, T.; Laguna, M.; Cerrada, E.; Moreno, A. unpublished results.
- Siemens XSCANS. X-Ray Single Crystal Analysis System. Copyright (C) 1992 by SIEMENS. Siemens analytical X-ray instruments Inc. Madison, Wisconsin. USA.
- SIR-97. A Package for Crystal Structure Solution by Direct Methods and Refinement. Giacovazzo et al. 1997.
- SHELXL-97. Program for the Refinement of Crystal Structures. Sheldrick, G. M. 1997.
- PovChem v. 2.1. Copyright (C) 1999 by Paul A. Thiessen.
- Gaussian 98, Revision A.3, Frisch, J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery Jr., J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al- Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian, Inc.: Pittsburgh PA, 1998.
- CS ChemOffice. Copyright (C) 1997 by Cambridge Soft Corporation. Cambridge, MA. USA.
- Samples Availability: Samples of compounds 2 and 6 are available from MDPI.
© 2001 by Molecular Diversity Preservation International (MDPI)